John K. House, Consulting Editor Alison A. Gunn Gemma Chuck Sheila M. McGuirk Maternal shedding of enteric pathogens increases around the time of parturition. In intensive dairy production systems, calf pathogen exposure and subsequently the risk of morbidity and mortality can be reduced by removing the calf from the dam after birth and placing the calf in a clean, dry, sheltered environment. Neonatal calves are cold tolerant when dry and sheltered from drafts. Wet drafty conditions lead to depletion of energy reserves and hypothermia. Hypothermia compromises colostral transfer and increases vulnerability of newborns to contagious and opportunistic pathogens. Under cold conditions calves generate heat by shivering and metabolism of brown adipose tissue. The calf is born with short-lived, stored energy in the form of muscular glycogen.1 Colostrum is a critical source of concentrated energy to meet the metabolic demands of thermogenesis. Ingestion and absorption of colostral immunoglobulins is essential for neonatal ruminants to thrive. Cows, sheep, and goats have an epitheliochorial type of placenta that does not allow passage of immunoglobulin; hence, neonates are born virtually agammaglobulinemic, rendering them susceptible to infection. Ingestion of colostrum provides maternal immunoglobulins that enterocytes selectively absorb by pinocytosis during the first 24 hours after birth. The ability of the enterocytes to absorb immunoglobulins decreases rapidly soon after birth, resulting in “closure” of the intestines by 24 to 36 hours postpartum. The immune system of the neonate starts to develop from birth, with endogenous immunoglobulin G (IgG) reaching functional levels at 3 to 6 weeks of age.2 The uptake of maternal leucocytes by the calf also plays a significant role in the development of the neonatal immune system. Inadequate colostrum consumption increases the risk of disease. Passive transfer achieving serum IgG concentrations 10 mg/mL or greater at 24 to 48 hours is associated with reduced risk for morbidity and mortality.3–5 Provision of adequate good-quality colostrum also results in improved daily weight gain and feed conversion efficiency both preweaning and postweaning, although the effect of this on joining dates and age at first calving varies among studies.5–7 Both serum IgG at 24 to 48 hours and the provision of adequate good-quality colostrum have been associated with increased milk production in the first and second lactations.6,8 The major factors influencing the effectiveness of passive transfer of immunoglobulins in calves are (1) the concentration of immunoglobulins in colostrum fed, (2) the volume of colostrum ingested, and (3) the age of the calf at first colostral feeding. Colostrogenesis occurs several weeks before calving under the influence of prolactin and other lactogenic hormones. Colostrum is nutrient dense and has a higher level of energy and protein than is found in milk. Bovine colostrum contains approximately 45 mg/mL9 of immunoglobulin and 106 leukocytes/mL.10 IgG1 is transported from blood in endosomes through the polarized mammary epithelial cell by a process known as transcytosis.11 The IgG1 is bound by the Fc-receptor of the neonate (FcRn) at the basal side and transferred to the apical side under the control of intracellular proteins termed small GTPases.12 The FcRn receptor consists of two subunits: an integral membrane α-chain (Fc fragment of IgG, receptor, transporter, alpha [FcGRT]) and a β-light chain (β2 microglobulin [β2M]). In vitro, the expression of FcGRT and several of the small GTPases controlling the transfer of IgG are upregulated by estrogen and progesterone, and it is thought that this mechanism results in the marked increase in transport of IgG1 observed in the 4 weeks prior to calving.12,13 Immunoglobulins account for more than 95% of the whey protein at calving, and IgG1 accounts for approximately 80% of this amount.14 IgG2, IgA, and IgM account for 10%, 7%, and 5%, respectively. The amount of IgA remains constant throughout all lactations, whereas the colostrum of cows in their first lactation contains less IgG1, IgG2, and IgM. Immunoglobulin levels in mature milk are much lower, comprising only 7% of the total whey protein, with an increase in the relative contribution of IgA. Older cows have a tendency to a higher ratio of IgG1 to IgG2.15 This is consistent with a lower level of IgG1 in the serum of heifers. The transport of IgG into bovine colostrum has been associated with a transient decrease in serum total protein, globulin, and IgG concentrations during the preparturient period, particularly in dairy cows. Up to 500 g of IgG1 a week are removed from blood during the most active period of colostrum formation.16 Passively derived immunoglobulins enhance neonatal immunity via functioning as neutralizers and opsonins. The association between failure of passive transfer (FPT) of immunoglobulins and neonatal infection has been well established in the calf.17,18 Colostral leukocytes participate in regulation of the neonate’s immune response. Comparison of the immune response of calves fed leukocyte-replete or leukocyte-depleted colostrum indicates that colostral leukocytes enhance humoral immunity and phagocyte function.19–23 Following experimental Escherichia coli infection, calves fed leukocyte-replete colostrum recovered more quickly and shed fewer bacteria than calves fed leukocyte-depleted colostrum.10 Transfer of cellular immunity via colostral leukocytes has also been demonstrated in sheep.24 Colostral leukocytes are destroyed when colostrum is frozen, pasteurized, or fermented. Colostrum also contains hormones (growth hormone, relaxin, prolactin, insulin, and glucagon), essential fatty and amino acids, minerals, trace elements, and (pre-)vitamins (especially beta-carotene and vitamins A, B, D, and E) (Table 19-1), as well as cytokines, growth factors (members of the IgF system, including IgF binding proteins [IgFBP]), and nonspecific antimicrobial factors such as lactoferrin. Colostrum is a significant source of copper to the newborn calf but not of selenium or zinc.25 TABLE 19-1 Composition of Colostrum of Holstein Cows46* * Data from 55 samples. SE, Standard error. Colostrum has been shown to have many roles apart from establishment of the humoral neonatal immune system. Lactose intake is insufficient to maintain plasma glucose concentrations, and colostrum feeding increases plasma glucose, most likely due to stimulation of gluconeogenesis. Newborn calves are deficient in vitamin A and have a low lactoferrin status. Both are found in elevated quantities in colostrum compared with milk. Lactoferrin is an iron-binding glycoprotein, which is a potent antimicrobial factor in the milk cistern and in the alimentary tract of newborn animals. Digestive functions develop during fetal life and undergo the largest morphologic and functional changes during the first 48 hours of extrauterine life under the influence of insulin-like growth factor-1 (IgF1), lactoferrin, and other bioactive substances, many of which are present in significantly higher concentration in colostrum than milk. These factors are key regulators in the development of the gastrointestinal tracts of bovine neonates, including stimulation of mucosal and submucosal growth, brush-border enzymes, intestinal DNA synthesis, increased duodenal villus size, apoptosis, protein synthesis and degradation, and digestion and absorption. They also exert systemic effects outside the GIT on metabolism and endocrine systems, vascular tone and hemostasis, activity and behavior, and systemic growth.26–28 Optimal transfer of immunoglobulin will occur when calves are administered 7.5% to 10% of body weight of first milking colostrum within 2 hours of birth followed by an additional feed of 5% of body weight at 12 hours (Table 19-2). The initial feed should provide the calf with a minimum of 100 g of IgG, and at least 150 g of IgG within 2 hours of birth are required to ensure 90% of calves have optimal passive transfer.29 Dairy calves should be administered colostrum by nipple bottle or esophageal tube, since increased FPT occurs in dairy calves that are left on their dam.9,30,31 This is due to the reduced volume of colostrum, and therefore mass of IgG, consumed by the calf if left to suckle naturally during the first 24 hours.5 Conversely, separation of beef calves from the dam is not practical and may result in mismothering. Generally failure of passive transfer is less of a problem in beef cattle, since the lower volume of colostrum produced results in an elevated concentration of IgG, and supplementation is only recommended following dystocia. TABLE 19-2 Liters of Colostrum Required to Achieve Adequate Passive Transfer (Blood IgG Level ≥ 13.4 mg/mL) for Different Birth Weights, Absorption Rates and Colostrum Qualities * Calves should be fed 7.5% of body weight or, at a minimum, the volume shown. IgG levels would be enhanced by 5% of body weight 6 to 12 hours later. † Calves should be fed 7.5% of body weight and an equivalent volume 6 to 12 hours later. ‡ It is unlikely that adequate passive transfer will be achieved. Calculations of total IgG required and apparent efficiency of absorption are based on Chigerwe et al (2008)29,160 using blood volume calculations from Quigley et al (1998).161 The correlation between the Brix refractometer reading and serum IgG levels was calculated using Quigley et al (2013).49 This table was compiled with the assistance of Zoe Vogels from The Vet Group, Timboon, Victoria. It is assumed that the minimum volume fed would be 5% of body weight even where good quality colostrum is available. It should be noted that below a Brix of 22%, it is not possible to achieve adequate passive transfer with a single feed of colostrum. FPT occurs when calves do not absorb sufficient immunoglobulins. Only small amounts of IgG and IgM to unknown antigens are made by the fetus, and they offer no protection against disease. It has been estimated that approximately 31% of preweaning mortality events occurring in the first 3 weeks of life can be attributed to failure of passive transfer.32 The problem in mixed-source calf-rearing properties is significantly worse.3 A high percentage (19.2% to 38%) of dairy calves are still found with significant FPT.30,31,33 Some debate exists as to the serum IgG concentration that signifies FPT. Calves are generally defined as having FPT if the calf serum IgG concentration is less than 10 mg/mL when sampled between 24 and 48 hours of age.34 It has been suggested that serum IgG concentration below 13.4 mg/mL constitutes FPT.29,35 Ensuring that every calf has adequate passive transfer is challenging and rates of 10% are probably indicative of good management practices.29 There is a large variation in colostrum immunoglobulin concentrations between individual cows and a variety of influences: genetic, environmental, and physical on the uptake of colostral antibodies by the calf. Although calves born to beef cows with lower colostrum immunoglobulin concentration had an increased risk of mortality,36 numerous other studies in beef and dairy calves have failed to demonstrate an association between the colostral immunoglobulin levels and the serum immunoglobulin of the calf at 48 hours of age.37–41 Administration of a known volume and concentration of colostrum via oroesophageal feeder at a specific time after birth to dairy calves has been shown to have more linear correlation with calf serum IgG,29,42 but administration of sufficient colostrum to provide 150 g of IgG within 4 hours of birth can still result in FPT. At the herd level, colostrum quality is affected by breed, parity, and climate. For individual cows the biggest determinants are colostral volume and time from calving until milking.43,44 Colostrum concentrations of Holstein-Friesian cows varied from 20 g/L of immunoglobulin to more than 110 g/L of IgG1 in cows from a single herd.43 First milking colostrum samples collected within 4 hours of milking from cows at 11 dairies in Pennsylvania varied from 9 to 166 g/L of IgG1.45 The mean concentration of IgG1 in first milking colostrum in three large studies of dairy cows varied between 37.5 ± 30.2 and 48.2 ± 28.9 g/L.43,45,46 Colostrum IgG concentration declines with time after calving even if the cow is not milked.43,44,47,48 However, several studies have demonstrated that this decline is minimal until the cow has been calved 8 to 9 hours.43,47 There is no increase in colostral volume if postpartum milking is delayed, so it appears that the declining IgG is not due to dilution.44,48,49 During the colostrum phase, mammary cells have leaky tight junctions,50,51 so it is likely that once parturition occurs, the active transcytosis of IgG1 ceases and it is passively reabsorbed. Studies of colostrum and calf immunoglobulin levels in specific beef breeds have failed to demonstrate consistent differences between specific breeds, and many studies have shown evidence of variations within breed lines. In dairy breeds, colostrum from Holstein cows was statistically lower in total IgG than colostrum from Jersey, Ayrshire, and Guernsey cows.52,53 Prepartum dairy cows have a significantly greater decline in serum IgG compared with beef cows13; however, the colostrum of dairy cows has lower concentrations of IgG because of the much larger volume. Some studies have shown very low volumes of colostrum (<2 L) in beef cows.54,55 The volume of colostrum produced and the concentration of immunoglobulin in the colostrum is markedly affected by differences in the onset of lactogenesis, and the resulting dilution of colostral immunoglobulins—hence it is likely that much of the variation observed among breeds, lines, and cows is an effect of colostrum volume. First and second calving beef and dairy cows often have a lower immunoglobulin concentration than cows of third or greater parity.39,43,53,56,57 Similarly, calves born to beef heifers and second parity cows have a significantly lower mean concentration of serum IgG compared with that of calves born to mature cows.56,58,59 Genetic variance has been found in the genes encoding the FcRn receptor involved in transcytosis of IgG1 into the mammary gland. In Holstein-Friesian cows, haplotype 5 of the FcGRT subunit has been shown to be significantly associated with a high colostrum IgG, and FcGRT haplotype 2 shows a similar trend.60 FPT in beef calves from 16 breeds has been associated with FcGRT haplotype 3; conversely, calves with the FcGRT haplotype 2 were less likely to have high levels of serum IgG.61 FPT in these calves is also associated with one of eight haplotypes of the β2M subunit of the FcRn receptor.62 It is likely that variation in this receptor is impacting the concentration of IgG1 in colostrum, rather than the uptake of IgG1 by the calf; neonatal uptake does not involve the FcRn receptor. An estimated 10% of Holstein cows are capable of high mass transfer of IgG1, resulting in an IgG mass more than 1 standard deviation above the mean.45 Variation in the FcRn receptor is probably contributing to this variation, along with other genetic or hormonal regulation of transport. Opportunity may exist to enhance the concentrations of immunoglobulins in colostrum by genetic manipulation of IgG transport in the mammary gland. However, it should be remembered that serum IgG concentrations in the periparturient cow are already decreased as a result of IgG transport into colostrum13; further, decrease in immunoglobulins may exacerbate the immunosuppression that occurs during the peripartum period. Numerous studies have failed to demonstrate an effect of often severe protein and/or energy restriction for up to 3 months before calving on colostrum immunoglobulin levels.63–66 Protein restriction may result in an increase in IgG levels and a concurrent decrease in colostral volume, making the colostrum more viscous and difficult to suckle, especially in heifers, resulting in decreased IgG levels in the calves.58,67 This is likely to be a direct volume response as shown by other studies of colostral IgG concentration.13,43 There was no significant effect of dietary restriction on colostral IgM in any of the studies. Selenium supplementation has been shown to increase the IgG levels in the colostrum of selenium-deficient cows,68 but not lambs. IgG uptake by the calf may also be affected by serum copper levels.69 Twins have not been demonstrated to affect colostrum IgG concentration in beef cows36,56; however, another study of sheep, goats, and dairy cows demonstrated an increase in total protein, whey protein, and IgG concentration.70 Short dry periods of 28 to 35 days do not affect colostrum IgG levels, but when the dry period was eliminated completely, there was a decrease.71–73 Elimination of the dry period did not affect colostrogenesis in terms of cellular and fluid composition, and it is likely that the reduced quality is due to the lack of a period of secretion and accumulation. The mean colostral IgG of these cows remained close to 50 g/L so that 3 L of colostrum would provide the calf with adequate IgG. Treatment of cows with internal teat sealant at drying off has no effect on transfer of colostral antibodies following calving, but will help prevent leaking of colostrum prior to calving and the consequential reduction of the quality of the colostrum harvested.74 Few studies have looked at the effects of periparturient disease on IgG levels in colostrum. A decrease in colostral IgG has been observed in cows with periparturient infections and clinical mastitis,36 and where colostral IgG was not measured, calves of cows with periparturient mastitis had a lower serum IgG.56 The reported effect of subclinical mastitis is also inconsistent. At the quarter level a persistent infection at the time of calving, detected by serial cultures, results in a decrease in total volume of colostrum produced but no variation in IgG1 concentration, compared with a matched quarter on the same cow.75 At the cow level, an increased risk of colostrum with an IgG content of less than 30 g/L is found in cows with a somatic cell count greater than 50,000 cells/mL, measured at the herd recording after calving.57 At the herd level, cows from farms with an average count less than 200,000 in the month prior to sampling produced colostrum with higher levels of IgG2, tocopherol, vitamin A, potassium, and total solid levels; however, no significant variation was noted in IgG1.46 Environmental temperatures have an effect on colostral IgG concentrations with several large studies in dairy cattle demonstrating an increase in colostral IgG concentration in the summer57,76,77; however, at the extreme, heifers exposed to heat stress had decreased colostral IgG concentration.78 Photo period has no effect on colostral IgG concentration or volume.44 The key influences on immunoglobulin absorption by the neonatal calf are the concentration of immunoglobulins in the colostrum, volume fed, and time between birth and colostrum feeding. When small volumes are fed, the method of feeding can also affect the final serum IgG level. Early ingestion of colostrum is crucial because IgG is absorbed by the intestinal epithelial cells of the newborn ruminant, and the ability of enterocytes to transport immunoglobulin declines linearly from 2 to 20 hours and completely ceases (intestinal closure) between 24 and 36 hours.79 Serum IgG concentrations peak around 32 hours and then decline until the production of antibodies by the neonate exceeds the decay of passively acquired antibodies. Any delay in first feeding of colostrum results in decreased absorption of immunoglobulin and a lower serum IgG.29,80 Absorption of immunoglobulins occurs along the whole length of the small intestine in calves, with the amount transported increasing from the duodenum to the ileum,81 whereas in lambs and kids there is virtually no absorption from the duodenum and the majority occurs in the jejunum.82,83 IgG and other macromolecules are mainly absorbed by micropinocytosis, although some receptor-mediated endocytosis has been demonstrated; however, this does not involve the neonatal Fc receptor FcRn.81,82,84 Preferential absorption of homologous IgG has been observed in pigs; however, recent studies in kids and lambs have shown that similar IgG transfer can be achieved by feeding bovine colostrum, although in lambs the apparent efficiency of absorption is lower.85–87 The age of the calf when it receives its first feed and the amount of immunoglobulins received will influence the time of closure of the intestinal permeability to colostral immunoglobulins and the final serum immunoglobulin levels of the calf.88–92 Cessation of absorption occurs by 24 hours in calves that receive a full feed of colostrum within the first 4 hours after birth. When the colostrum volume is less than 2 L, the gut will remain permeable for a longer time and the rate of absorption will increase in response to a subsequent feed. If the calf is older than 12 hours when it receives its first feed, there is a significant increase in the possibility of the calf being agammaglobulinemic. It is likely that the time of closure is related to the immunoglobulin concentration of the colostrum as well as the volume fed. Delay in colostrum feeding will also result in lower plasma insulin and IgF1 for up to 48 hours after first colostrum intake and reduce plasma concentrations of β-carotene, retinol, and α-tocopherol for nearly a month after birth.93,94 The IgG concentration of the colostrum has the greatest effect on the mass of IgG fed and will determine the effectiveness of transfer of passive immunity when dairy calves are fed a fixed amount of colostrum. Therefore, it is important to implement routine measurement of colostrum IgG content on dairy farms. Provision of a high concentration of IgG in the initial feeding results in a much higher calf serum IgG than feeding increased volumes of lower-quality IgG.95 When different volumes and concentrations of colostrum have been fed to calves after birth, increased volumes of high-quality colostrum resulted in a 50% increase in the calf serum IgG concentration at 24 hours, whereas the magnitude of effect of increased volume was much lower when low immunoglobulin colostrum was fed. Calves should be fed 150 g of IgG within 4 to 6 hours of birth and earlier whenever practical. The volume at each feed should be determined after measuring colostrum IgG levels and is more important to optimize where lower-quality colostrum is the only source. There is no difference in the IgG concentration at 48 hours when 40-kg calves are administered either 3 or 4 L of colostrum with equivalent immunoglobulin levels regardless of IgG concentration.29,42 Similarly with lower-quality colostrum, higher serum IgG levels will be achieved when two feeds of 2 L are given at 2 and 6 hours postpartum than if 4 L are given in one feed.95 The pathophysiology underlying these observations is not fully understood, but 22% of the variation in the effective absorption of colostrum is attributed to abomasal emptying rate, and it is likely that the excessive mechanical distention of the abomasum with 4 L of colostrum delays outflow.42,95,96 Therefore, feeding regimens should be based around feeding 7.5% of body weight liters within 4 hours of birth, or a minimum of 150 g if availability is restricted and quality is higher than 50 g/L. Calf serum IgG levels will be significantly boosted by a further feed of 5% of body weight of colostrum within 12 hours of birth,95 and if less than 150 g of colostrum was fed at the initial feeding because of limitations of quality or volume, then a further 7.5% should be fed.35 Emphasis should be placed on feeding colostrum with the highest concentration of colostrum at the initial feed when the effectiveness of absorption is higher. Many farmers are currently feeding 10% to 12% of body weight as a single initial feed. Although this will provide good levels of IgG where colostrum quality is adequate, it may result in moderate depression and discomfort in the calf.97 Good-quality colostrum is a precious resource, and more efficient use is possible by knowing colostrum quality, limiting the initial feed to 7.5% of body weight, and placing emphasis on a second feed of good-quality colostrum before the calf reaches 12 hours of age. Method of feeding also has a significant effect on colostrum intake. A high proportion of calves left to suckle their dams shows evidence of failure of passive transfer, compared with those artificially fed by teat or esophageal feeder.9,98,99 This is due to the reduced volume of colostrum, and therefore mass of IgG, consumed by the calf if left to suckle naturally during the first 24 hours. Therefore, it should be strongly recommended that dairy calves be administered colostrum containing 150 g IgG within 2 hours of birth.5 Teat-feeding stimulates a reflex closure of the esophageal groove allowing colostrum to pass directly to the abomasum, where it clots before moving to the small intestine for absorption. When an esophageal feeder is used, this reflex does not occur and the colostrum passes to the reticulorumen.100,101 Promotion of the advantages of closure of the esophageal groove has resulted in the use of a nipple bottle as the more common method of administering colostrum. However, several studies have demonstrated that there is no difference in immunoglobulin concentrations at 48 hours between calves that sucked larger volumes of colostrum from a nipple bottle and those that were administered a similar quantity via an esophageal feeder, although there may be a delay of approximately 3 hours before the serum IgG increases in the tube-fed calves.97,102–104 This delay is not due to the deposition of colostrum in the reticulorumen, since all except 400 mL will immediately pass into the abomasum because of the pressure gradient resulting from the placement of the reticulorumen dorsal to the abomasum in the neonatal calf.105 Instead, the delay is likely to be due to delayed abomasal emptying as a result of distention and changes in physiologic and neuronal control mechanisms.96,106 Calves fed by a nipple bottle will commonly not ingest sufficient colostrum to minimize the risk of FPT and should be encouraged to ingest the maximum volume they will voluntarily ingest within 1 to 4 hours of age.35 Calves should be fed as soon as they are standing; delaying up to 6 hours after birth or provision of a heat lamp will not increase the amount of colostrum suckled.35,107 Calves that have a high degree of vigor during the first hour of life and during colostrum suckling will consume more colostrum from the bottle, as will larger calves.107 Bottle-fed calves that do not ingest their full allocation of colostrum at either their first or second colostrum feed should be targeted for immediate esophageal feeding, and the total remaining should be fed. In order to minimize trauma when an esophageal feeder is used, the calf should be standing with its rump against a solid wall or corner. The feeder used should have a total volume of at least 3 L so that intubation only has to occur once. The calf should be encouraged to swallow prior to insertion by sucking a finger to produce saliva or by feeding a few mouthfuls of colostrum. The tube should be introduced without physical exertion, and the head should be positioned slightly downward to allow swallowing as the tube is inserted into the esophagus. Before feeding, the knob at the end of the tube should be palpated on the left-hand side dorsal to the trachea to ensure that positioning is correct. At this point the calf head may be tilted slightly upward as the bottle is elevated to administer the colostrum. On beef properties, routine administration of colostrum to the newborn calf is disruptive and delays the time until first sucking108 but will decrease the incidence of FPT.37,109 Feeding colostrum to high-risk calves is likely beneficial, most commonly after dystocia. Beef cows often produce smaller colostrum volumes, and where volumes less than 1.5 L are fed, use of a nipple bottle is recommended, since serum IgG at 24 hours is higher compared with calves administered colostrum with an esophageal feeder.110 This most likely occurs because a greater percentage of the total colostrum is deposited into reticulorumen. Where dairy colostrum was fed, this was also shown to be a biosecurity risk. Calves that have experienced dystocia are more likely to have failure of passive transfer.5,58,59,111 Decreased vitality of the neonate and, in many cases edema of the head and tongue, result in a slower time to stand and inadequate colostrum intake as a consequence. Calves born to primiparous cows are more endangered than those born to multiparous cows. Respiratory acidosis did not result in a decreased level of IgG at 37 to 48 hours in studies where blood gases were measured promptly using arterial blood, although delayed absorption was observed in one study.112,113 The effects of twinning are equivocal, with two large studies, both in beef herds, demonstrating opposite effects of twinning on calf serum IgG. This is likely to be related to management, since the study that showed an increase in IgG with twinning moved all cows with twins to a barn for care and observation.56,111 It has been shown that the conformation of the dam’s udder and ventral abdomen has a significant effect on the average teat seeking time prior to first suckling. There was nearly half an hour delay in the time to suckle.114 The overall effect on calf IgG was not measured, but a separate study demonstrated a significantly lower serum IgG in cows with pendulous udders.115 Protein restriction in the last third of gestation may affect uptake of IgG by the calf,116 although IgM concentration was unaffected. This finding was attributed to the selective absorption of IgM in newborn calves, which is highly efficient when colostrum intake is low. IgM is the primary immunoglobulin that provides protection to the neonatal calf during the first few days of life, and it has been suggested that the efficient absorption of IgM is a compensatory measure that provides immunity to calves even when they are hypogammaglobulinemic.117,118 There is a seasonal variation in the IgG levels of calves and efficiency of absorption after colostrum feeding, being lower in the winter in cold climates and lower in the summer in hot climates.38,119–122 Colostrum immunoglobulin concentration is reduced in hot and cold weather,76–78 and this is exacerbated by calves also being less willing to suckle in extremes of temperature. Heat stress results in smaller calves that may be less vigorous,121,123 and one study has shown that calves subjected to a cold and wet environment had a slower rate of colostral absorption,124 although the serum immunoglobulin at 24 hours was not significantly different from the control calves. In that study the calves exposed to cold temperatures were fed colostrum via an esophageal feeder. The effect would be exacerbated in cold-stressed calves, since they are less likely to suckle voluntarily. There is some evidence that IgG levels in calves at 24 and 36 hours have low to moderate heritability. The breed of the sire and dam has some effect, but there is variation within lines of the same breed.39,41,59,125 Haplotypes of both the FcGRT and β2M genes coding for the FcRn receptor are associated with FPT in neonatal beef calves, as discussed previously.61,62 Since this receptor is not involved in the uptake of IgG by the calf, it is likely that the risk factor for FPT is the colostrum quality of the dam. Colostrum collection should occur as close to parturition as possible. Fresh cows should be removed from the calving area and milked out at least twice daily to help ensure optimal colostrum quality. Colostrum collected from cows who have leaked milk prior to calving, have signs of ill health, or have evidence of mastitis should not be fed to neonatal calves. Not only will there be a compromise in colostrum quality, but there is also increased potential for the presence of infectious pathogens, such as mycobacterium paratuberculosis, Salmonella spp., Mycoplasma bovis, Staphylococcus aureus, bovine diarrhea virus, and bovine leukemia virus.126–128 Neonatal enteric pathogens such as cryptosporidium may also be present.129,130 Consequently, unpasteurized colostrum is a potential risk factor for calf diarrhea and neonatal septicemia.131,132 Unpasteurized colostrum is also a biosecurity risk, and the use of colostrum from cows from a different property should not be recommended. Risk will be increased significantly if colostrum is pooled as organisms are disseminated, subsequently infecting a larger number of calves.132–134 This is exacerbated further if storage is poor, resulting in pathogen proliferation.131 Colostrum with high bacterial levels not only present a significant disease risk, but also affect absorption of IgG by direct blocking of uptake and transport of immunoglobulin molecules across intestinal epithelial cells or by binding free immunoglobulin in the gut lumen.34,135 Pooling of colostrum will also result in reduced IgG concentrations because of the overrepresentation of high-volume, low-immunoglobulin colostrum and consequently is associated with an increased level of FPT.30,136 When pooling is necessary for pasteurization, the colostrum IgG should be estimated using a Brix refractometer and low-quality colostrum excluded. Microbial contamination of colostrum compromises colostral quality and subsequently calf health. Bacterial contamination may occur during harvest, storage, or administration.126,131,137 Possible sources of contamination include cow skin, milking cup liners, hoses, milking buckets, storage, and feeding utensils. Contamination of colostrum with pathogens during harvest can be avoided by careful and hygienic teat preparation in fresh cows prior to applying cups.126 This may involve washing and drying of teats and application of a premilking teat disinfectant.138,139 Thorough and effective cleaning and disinfection of collection, storage, and feeding equipment is essential. It is important that separate, clearly marked esophageal feeders are used for feeding colostrum and are not used for sick calves, since this may also be a significant disease risk. Milk feeding equipment should not have cracks or scratches that allow for buildup of bacteria. Equipment should be rinsed in lukewarm water, and then actively scrubbed in water hotter than 50° C (122° F) containing detergent and bleach, or dry chlorinated detergent, to remove any residue. Bottle brushes should be used to clean esophageal feeders, nipples, and feeding bottles. Finally, they should be rinsed in water containing acid or an acid rinse and then allowed to completely dry before stacking or storage. When concern exists regarding hygiene practices, plating 10 µL of colostrum onto a blood plate and incubating at 37° C (98.6° F) overnight provides a simple crude assessment of microbial contamination of colostrum being fed to calves and allows identification of the origin of any process failure. Fresh colostrum fed to calves should have a total bacteria count less than 100,000 colony-forming units/mL and less than 10,000 coliforms/mL.126,132 Short-term storage of colostrum is necessary in order to deliver known good-quality colostrum to neonatal calves within hours of birth to reduce the risk of failure of passive transfer. When colostrum is stored at ambient temperatures, bacterial proliferation leads to fermentation, thus reducing total solids, protein, fat, and lactose content. Refrigeration of colostrum delays bacterial growth. Producers should aim to feed refrigerated colostrum within 2 days of collection. Storage of colostrum in the refrigerator for more than 24 hours will result in a decreased amount of IgG absorbed, and this may lead to FPT if stored for more than 48 hours.140 The addition of 10 mL of 50% potassium sorbate solution per liter of colostrum at the time of collection to colostrum (0.5% wt/vol), in combination with refrigeration, inhibits bacterial growth for 4 days.131 Pasteurization of colostrum is an effective way to control colostrum bacterial load, reducing the risk of infectious disease transmission from cow to calf.141 Degradation of immunoglobulins may be a problem with large-batch colostrum pasteurizers. Calves fed large-batch pasteurized colostrum have significantly lower serum IgG levels than those fed unpasteurized colostrum.34 However, pasteurization of small batches of colostrum (57 L) at 67° C (152.6° F) or less caused a minimal decrease in the immunoglobulin concentration of colostrum compared with pasteurization of large batches (95 L), or batches at higher temperatures (76° C [168.8° F]) and has little effect on the subsequent serum immunoglobulin in calves at 24 hours of age.142,143 At this temperature Mycobacterium paratuberculosis may still survive.144,145 Heat treatment of colostrum (at 60° C [140° F] for 60 minutes) at a commercial level has been shown to effectively reduce colostrum microbial counts while maintaining IgG concentration.146 Calves fed such colostrum were also found to have a reduced morbidity.147 This critical temperature appears to significantly reduce the bacterial load while not denaturing the IgG or changing the viscosity of colostrum.148 Longer-term preservation of colostrum can be achieved by freezing or lyophilization without change in the immunoglobulin and nutritional content.149–151 However, the cellular content of colostrum is destroyed by freezing, which may have longer-term effects on the development of the immune system.152 In kids, the feeding of lyophilized colostrum resulted in higher IgG levels than feeding frozen colostrums.153 Colostrum should not be thawed in a microwave, since the defrosting of a large volume in this way results in uneven heating and clots.102 It should be stored in 1- to 2-L aliquots in flat plastic bags and allowed to thaw at room temperature in a fridge or in a rocking heated water bath with a water temperature no more than 40° C (104° F). The quality of colostrum has traditionally been measured by visual inspection or by the use of a colostrometer (a hydrometer). The level of IgG in colostrum cannot be accurately predicted based on its appearance,154 hence the need for more objective instruments. The colostrometer measures the specific gravity of a liquid, which in the case of colostrum is associated with the protein content. Globulins contribute greatly to this protein, so the colostrometer has been used to determine the immunoglobulin content of colostrums.155 Although they are relatively quick to use on farm, colostrometers have limited sensitivity (0.32) and specificity (0.97) for detection of low-quality colostrums.156 Consequently, two of every three low-quality colostrum samples were incorrectly classified as acceptable. Colostrometer readings are also significantly affected by temperature,157 and for a known quality of colostrum, the colostrometer can differ by 0.8 g/L for every 1° C (1.8° F) change in temperature. The specific gravity of colostrum can also differ among breeds and can be influenced by lactation number, month of calving, year of calving, and protein yield in the previous lactation.77 Field experience has shown that colostrometers are most accurate for diagnosing samples of moderate or inferior quality but may indicate erroneously high readings for samples in the superior range.40 It should be noted that the markings on a colostrometer are calibrated for Holstein cows. However, as a comparative field test, it can still be a useful tool, where calves are force-fed colostrum. In a non-interventionist situation, the dam’s colostral immunoglobulin is not a good predictor of the level of immunity that the calf will attain.37,38 From a practical perspective, colostrometers are very fragile because they are made of glass, which can render them unsuitable in a farm environment. Cow-side immunoassay kits for measuring colostral IgG are available and have been shown to have an acceptable sensitivity and specificity for this purpose.158 However, their lack of availability on a global scale limits their use along with financial constraints. Optical and digital Brix refractometers can be used to measure the quality of colostrum using fresh and frozen samples.159 The optical Brix refractometer demonstrated a specificity of 85% and sensitivity of 90.5% using a cutoff of 22% Brix. A cutoff of 22% Brix was deemed to equate to 50 g/L of immunoglobulin using radial immunodiffusion (RID); thus samples greater than 22% Brix were considered to be of adequate quality.159 In another study, the results showed a cutoff point of 21% was estimated in samples greater than 50 g/L, as measured by RID.49 The benefits of the Brix refractometer are that they are not affected by ambient temperature and they are robust, easy, and economical to use at an on-farm level.160 It has been found that high-quality colostrum samples can yield a “fuzziness” through the viewfinder of a Brix refractometer. Generally this happens in samples greater than 22%. A smaller sample size on the prism can help with clarity in these high-quality samples. The poorer the quality of colostrum, the clearer the value is to read. Brix refractometers are inexpensive, easily available, and robust, and as such should be standard dairy farm equipment. Farmers should be advised to purchase a refractometer that only measures degrees Brix with a scale from 0% to 32% so that they are easy to read. To get an accurate reading, it is necessary to press the cover plate firmly over the prism while looking through the viewfinder, since some thick samples will cause the cover plate to lift off the prism. It is also important that each sample is placed on a clean, dry prism.
Initial Management and Clinical Investigation of Neonatal Disease

Environmental Management
Colostrum Management
Item
Mean
SE
Fat (%)
6.70
4.16
Protein (%)
14.92
3.32
Lactose (%)
2.49
0.65
Total solids (%)
27.64
5.84
Ash (%)
0.05
0.01
IgG1 (mg/mL)
34.96
12.23
IgG2 (mg/mL)
6.00
2.82
IgA (mg/mL)
1.66
0.99
IgM (mg/mL)
4.32
2.84
Lactoferrin (mg/mL)
0.82
0.54
Retinol (µg/g)
4.90
1.82
Tocopherol (µg/g)
2.92
3.65
β-Carotene (µg/g)
0.68
0.63
Vitamin E (µg/g of fat)
77.17
33.51
Thiamin (µg/mL)
0.90
0.28
Riboflavin (µg/mL)
4.55
0.31
Niacin (µg/mL)
0.34
1.57
Vitamin B12 (µg/mL)
0.60
0.35
Pyridoxal (µg/mL)
0.15
0.07
Pyridoxamine (µg/mL)
0.21
0.07
Pyridoxine (µg/mL)
0.04
0.07
Ca (mg/kg)
4716.10
1898.00
P (mg/kg)
4452.10
1706.29
Mg (mg/kg)
733.24
286.07
Na (mg/kg)
1058.93
526.02
K (mg/kg)
2845.89
1159.89
Zn (mg/kg)
38.10
15.90
Fe (mg/kg)
5.33
3.09
Cu (mg/kg)
0.34
0.14
S (mg/kg)
2595.67
904.97
Mn (mg/kg)
0.10
0.11
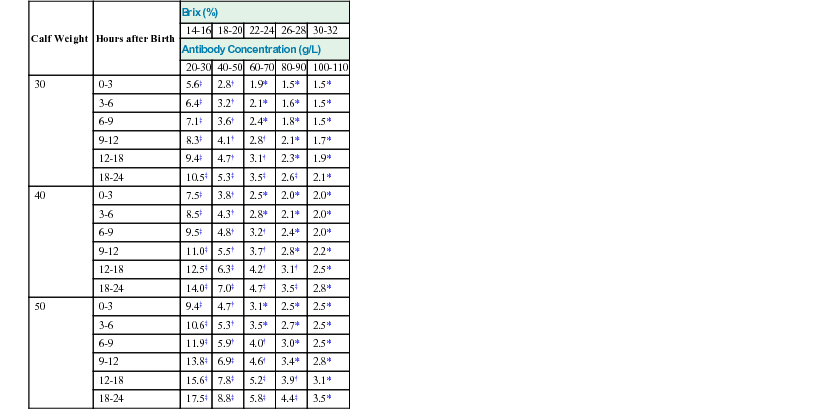
Provision of Adequate Colostrum
Calf Weight
Hours after Birth
Brix (%)
14-16
18-20
22-24
26-28
30-32
Antibody Concentration (g/L)
20-30
40-50
60-70
80-90
100-110
30
0-3
5.6‡
2.8†
1.9*
1.5*
1.5*
3-6
6.4‡
3.2†
2.1*
1.6*
1.5*
6-9
7.1‡
3.6†
2.4*
1.8*
1.5*
9-12
8.3‡
4.1†
2.8†
2.1*
1.7*
12-18
9.4‡
4.7†
3.1†
2.3*
1.9*
18-24
10.5‡
5.3‡
3.5‡
2.6‡
2.1*
40
0-3
7.5‡
3.8†
2.5*
2.0*
2.0*
3-6
8.5‡
4.3†
2.8*
2.1*
2.0*
6-9
9.5‡
4.8†
3.2†
2.4*
2.0*
9-12
11.0‡
5.5†
3.7†
2.8*
2.2*
12-18
12.5‡
6.3‡
4.2†
3.1†
2.5*
18-24
14.0‡
7.0‡
4.7‡
3.5‡
2.8*
50
0-3
9.4‡
4.7†
3.1*
2.5*
2.5*
3-6
10.6‡
5.3†
3.5*
2.7*
2.5*
6-9
11.9‡
5.9†
4.0†
3.0*
2.5*
9-12
13.8‡
6.9‡
4.6†
3.4*
2.8*
12-18
15.6‡
7.8‡
5.2‡
3.9†
3.1*
18-24
17.5‡
8.8‡
5.8‡
4.4‡
3.5*
Failure of Passive Transfer
Risk Factors for Inadequate Colostrum Uptake
Factors Affecting Colostrum Quality
Factors Affecting Colostrum Uptake by the Calf
Time and Method of FIRST Feeding.
Other Factors Influencing Colostrum Uptake.
Management to Ensure Adequate Passive Transfer (Boxes 19-1 and 19-2)
Ensuring Adequate Quality
Colostrum Harvesting, Handling, and Storage.
Monitoring Colostrum Immunoglobulin Quality.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Initial Management and Clinical Investigation of Neonatal Disease
Chapter 19
