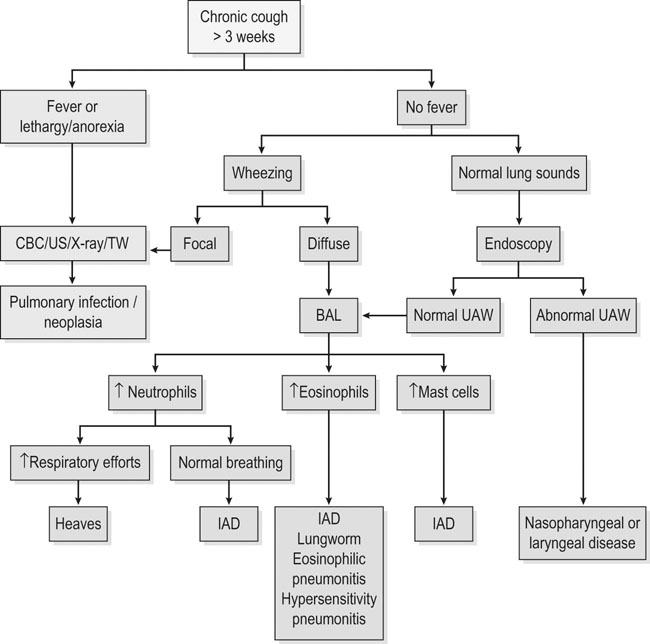
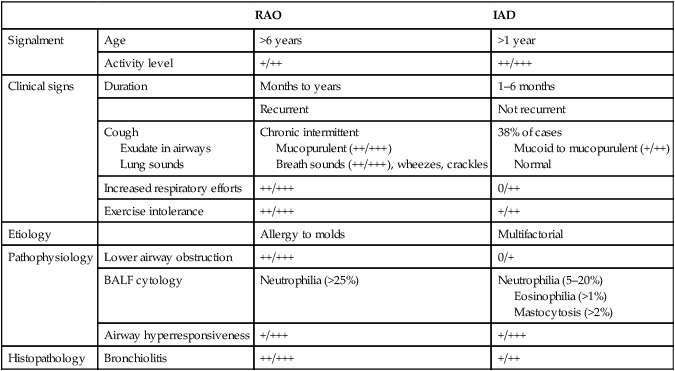
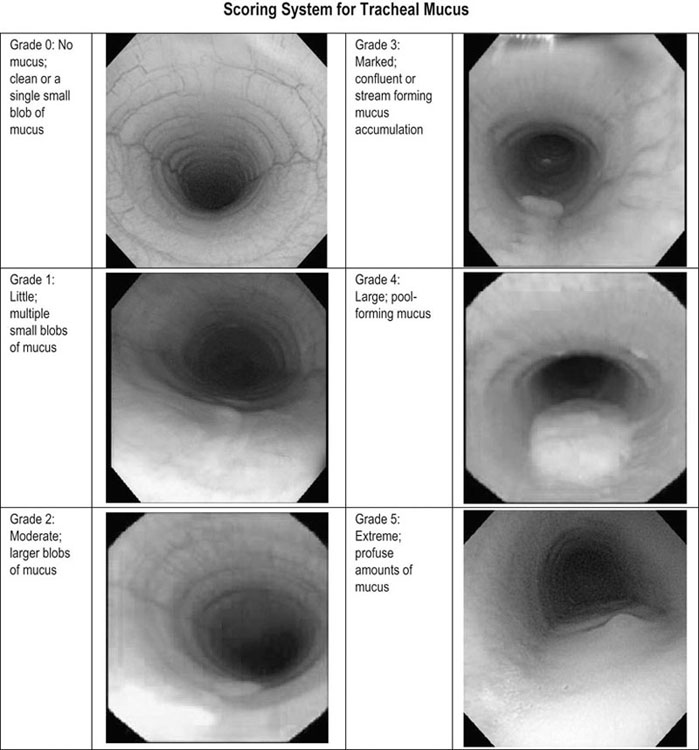
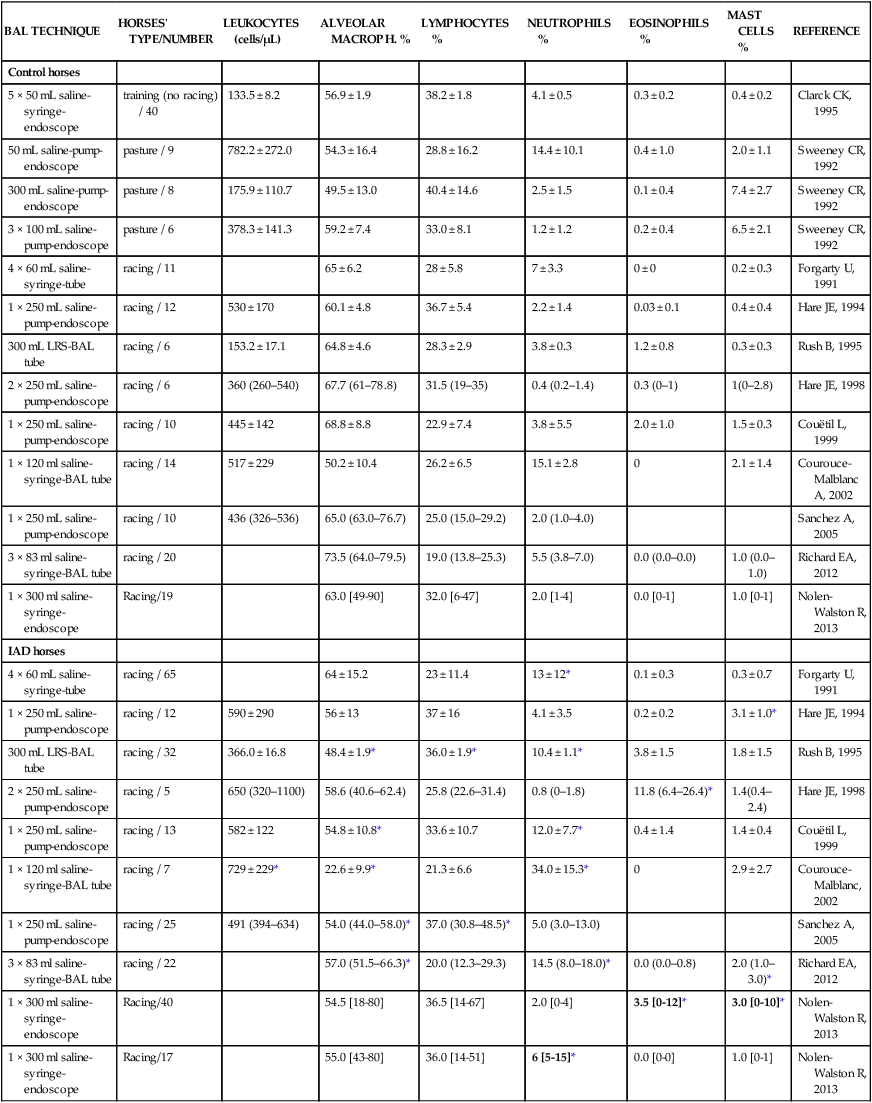
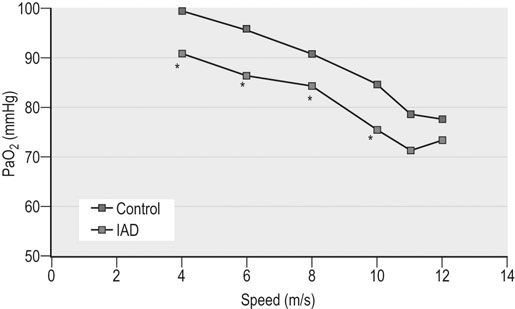
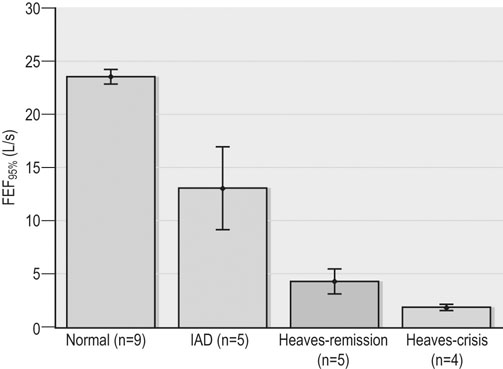
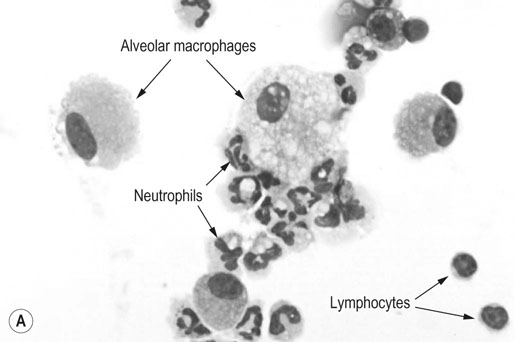
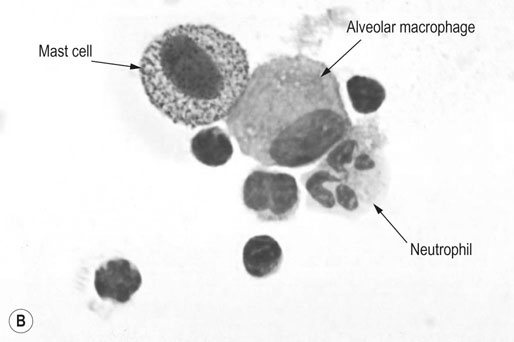
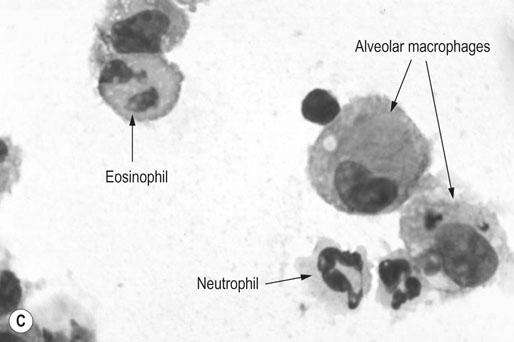
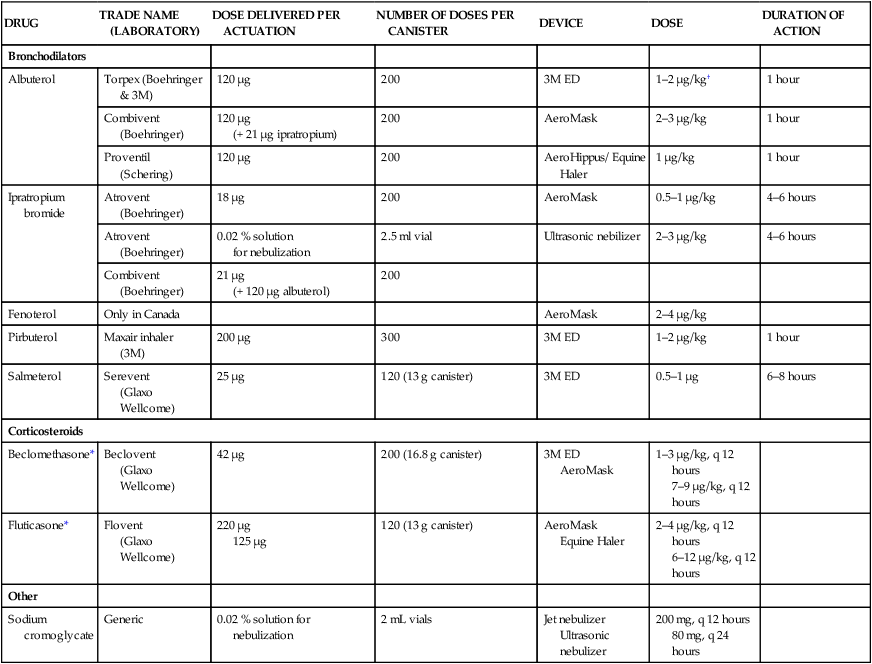
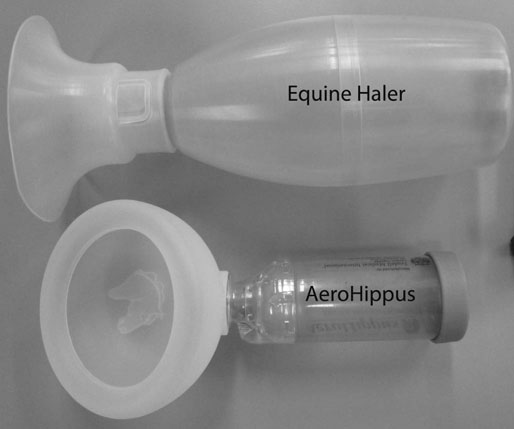
Respiratory and musculoskeletal diseases are the two most common causes of training interruption and poor performance in athletic horses.1–5 Inflammatory airway disease (IAD) is a chronic respiratory syndrome affecting horses of any age, gender or breed that is characterized clinically by excess tracheobronchial mucus, cough and decreased performance.13 The negative impact of IAD on performance has been documented both in racehorses and sport horses.2,6–8 The prevalence of IAD as determined by excess tracheal mucus is high both in racehorses (13–22%),7,9,10 sports horses (31%)8 and pleasure horses (20%).11 Recurrent airway obstruction (RAO) or heaves is another common chronic respiratory disease presenting similarities with IAD. But heaves only affects mature-to-older horses and is characterized by more severe clinical signs and exercise intolerance that may abate at times; nonetheless, horses are affected for life. • IAD is associated with poor performance, cough and increased respiratory secretions. • Young athletic horses are commonly affected with IAD. • Airway endoscopy, bronchoalveolar lavage fluid cytology and exercise testing are valuable diagnostic tools for IAD. • Horses with IAD exhibit various degrees of airflow obstruction and airway hyperresponsiveness. • Etiology of IAD appears to be multifactorial, with environmental dusts playing an important role. • Therapy is aimed at decreasing environmental dusts and controlling airway inflammation. • Aerosol therapy with corticosteroids and bronchodilators is effective Inflammatory airway disease (IAD) is a respiratory syndrome characterized by mild lower airway inflammation and excess mucus that is commonly encountered in young athletic horses and recognized as a separate entity from recurrent airway obstruction (RAO).12,13 In the majority of cases, RAO and IAD may be differentiated based on clinical grounds (Table 28.1). It is likely that both conditions represent a continuum of respiratory diseases where mild-to-moderate airway inflammation is represented by horses with IAD and where some horses might progress to severe inflammation and develop RAO. However, there is currently no evidence suggesting which IAD phenotype or risk factors predispose a horse with IAD to develop RAO. In addition, the majority of horses with IAD recover fully after proper therapy. It is also worth noting that IAD is common worldwide, whereas RAO is virtually absent in countries like Australia and South Africa despite reports of high prevalence of IAD. Table 28.1 Comparison between recurrent airway obstruction (RAO) and inflammatory airway disease (IAD) in horses Low/mild: +, medium/moderate: ++; high/severe: +++. Horses with IAD typically have a history of cough, increased respiratory secretions and decreased performance or delayed recovery after exercise.14–16 Foals and older horses can also suffer from IAD.17–19 In these cases, the diagnosis is often reached by ruling out infectious causes and other non-infectious respiratory diseases, such as RAO. The possibility of IAD should be considered in horses with cough and tracheobronchial mucopurulent exudate that do not respond, or relapse, after antimicrobial therapy. In such cases, further diagnostic tests should be pursued, in particular bronchoalveolar lavage. Duration of clinical signs of IAD is seven to nine weeks on average with a range between 4 and 22 weeks, which is longer than most infectious respiratory diseases.15,20,21 In a study involving 170 Thoroughbred horses in training over a two-year period, it was estimated that horses had some degree of IAD during a third of their time spent in training and each affected horse had an average of two episodes of IAD during the study.15 IAD is more common in yearlings and two-year-old racehorses with the incidence and prevalence decreasing significantly between the ages of two to four years.21–23 Horses of any breed can be affected including Quarter Horse, Warmblood, Appaloosa, Thoroughbred cross and American Saddlebred.9,15,16,18,24,25 The most common complaints reported by owners of athletic horses, other than racehorses, with IAD are chronic cough, exercise intolerance, poor willingness to perform and prolonged recovery after exercise.8,16 These horses can be involved in a variety of activities such as barrel racing, show-jumping, three-day event, dressage, or pleasure riding. In some cases, owners report a history of respiratory disease affecting several horses in the barn and all horses recovered except for the one with IAD, which continues to cough intermittently. There is a strong association between poor performance and IAD, as defined by increased amount of tracheal mucus, both in racehorses and sports horses.7–9 Detection of more than a few blobs of mucus in the trachea after a race is associated with poor performance in both Standardbreds and Thoroughbreds. More pronounced accumulation of mucus that forms a continuous stream in the trachea has been associated with poor performance in horses competing in dressage and show-jumping. The most common clinical signs associated with IAD are increased respiratory secretions, cough, and decreased performance.8,11,14,21,26 Pharyngeal lymphoid hyperplasia is positively correlated with tracheal mucus accumulation in racehorses but not in performance or pleasure horses.7,8,11 This difference is likely due to the fact that racehorses tend to be younger and pharyngeal lymphoid hyperplasia is most pronounced yearlings and two-year-old horses and then it decreases rapidly with increasing age. Estimation of the quantity of mucus present in the trachea by endoscopy reveals that horses free of respiratory disease have either no mucus or a few isolated flecks and horses with IAD have large blobs of mucus up to a continuous stream of variable width (score 0 or 1, Fig. 28.1).8,19,23 The prevalence of tracheal exudate increases after strenuous exercise27 although this finding is not consistent.28 The amount of tracheal mucus in performance and pleasure horses can be positively, but weakly, correlated with age or not associated with age.19,29 Cough is only present in 38 % of horses with IAD; however, 85% of coughing horses have IAD.15,30 Daily observation of racehorses in training showed that coughing is not noted 62% of the time during which they have IAD and that there is a strong associations between coughing, the amount of mucus present in the upper airways, and pharyngeal lymphoid hyperplasia.15,22,30 Also, a strong association exists between coughing, isolation of bacteria, and the degree of inflammation in tracheal wash fluid.23,30 Nevertheless, bacteriological examination of tracheal wash samples reveals that 35–58% of horses with IAD do not contain significant amounts of bacteria. On the other hand, there is a strong association between presence of cough and BAL neutrophilia.31 Other clinical signs of respiratory disease such as nasal discharge and fever do not appear to be associated with IAD,15,21 although one study found a 4.5-fold increased odds of nasal discharge in horses with IAD.32 Interestingly, the prevalence of nasal discharge is low in racehorses (4% of cases), except in yearlings (>30%).21 Thoracic auscultation is usually normal; however, some horses with IAD can exhibit increased breath sounds or wheezes. Horses with severe IAD might have a slightly increased respiratory rate and abdominal contraction on expiration. For the most part, IAD is subclinical and can be undetected unless coughing is present or tracheal exudate is detected by endoscopy.18 Endoscopic examination of the respiratory tract is a simple, well tolerated by most horses and a valuable diagnostic tool for IAD. Horses with normal respiratory tract have at most a few flecks of mucus visible in the trachea by endoscopy.29,33 Increased mucopurulent exudate in the tracheobronchial tree is detected in the majority of horses with IAD but tracheal exudate is non-specific and is also present in the majority of pulmonary diseases other than IAD.33 A semi-quantitative mucus scoring system using a scale between 0 and 5 has been developed and validated for use in the horse (Fig. 28.1).19,34 This scale is useful to grade severity of IAD since a positive association has been found between tracheal mucus score, BAL neutrophilia and poor performance.8,19,35 Tracheal septum thickness increases with age but is not greater in IAD as compared to healthy horses.19,36 Cytologic examination of respiratory secretions collected from the tracheobronchial tree is important when trying to confirm a diagnosis of pulmonary disease. Tracheal secretions can be collected by direct aspiration or wash of tracheal lumen via transcutaneous catheterization or through an endoscope.37–39 Normal tracheal fluid cytology usually contains neutrophils representing less than 20% of total nucleated cells.40,41 Several studies have shown an association between tracheal neutrophilia (>20%), coughing and IAD.21,30,42,43 A relationship between tracheal mucus accumulation and neutrophilia was found by some investigators but not by others.43,44 However, cytological examination of tracheal mucus in horses free of respiratory disease is highly variable, with neutrophil percentages ranging between 3 and 83% of total nucleated cells45,46 and it correlates poorly with pulmonary histopathology.45,47 Furthermore, mucus score but, not the proportion of neutrophils in tracheal wash, correlates with performance in racehorses as well as in sports horses.8,35 Therefore, the preponderance of evidence suggests that neutrophil accumulation in tracheal lumen represents a physiological response to contamination of the respiratory tract by inhaled particulates or bacteria in otherwise healthy horses. In some cases, contamination can overwhelm local defense mechanisms (mucociliary clearance, immune response) and result in clinical signs such as cough and excess mucus accumulation. In contrast, neutrophils represent a small proportion of total nucleated cells present in BAL fluid with most studies reporting neutrophils as <5% of cells in healthy horses (Table 28.2). Furthermore, BAL cytology reportedly correlates well with histopathology of the lungs48 and does not correlate with tracheal wash cytology.46,49 A BAL can be easily performed in field conditions using either a flexible endoscope (≥2 meters long) or an equine BAL catheter at least 2.5 meters long and 10 mm diameter with inflatable cuff at the end (Bivona, Gary, IN; Cook, Bloomington; IN, Jorgensen Laboratories, Loveland, CO). The technique has been described in detail previously.50,51 It is important to always use the same technique during a BAL because the volume of fluid used as well as the number of boluses administered have a significant effect on cell count and differential (see Table 28.2). Fluid samples should be processed within 1–2 hours or stored at 4°C if sample shipping to the laboratory is to be delayed. Normal BAL fluid should appear slightly turbid with a layer of white foam on the surface (surfactant). Between 50 and 90% of the volume infused is expected to be retrieved. Table 28.2 Bronchoalveolar lavage (BAL) fluid cytology in control horses and in horses with inflammatory airway disease (IAD) and technique used to perform BAL *Significantly different from controls (P < 0.05). Data expressed as mean ± SD or median (1st quartile – 3rd quartile). Measurement of lung function can be done in a variety of ways including assessment of gas exchanges, determination of lung volumes (spirometry), and evaluation of the movement of air in and out the respiratory tract (lung mechanics). Racehorses with IAD exhibit more pronounced exercise-induced hypoxemia than healthy controls during standardized run to fatigue on a high-speed treadmill (Fig. 28.2).52–54 Both groups of horses had comparable PaCO2, suggesting that impaired gas exchanges were likely a result of ventilation–perfusion mismatching and not hypoventilation. Spirometry is useful in severe obstructive diseases such as RAO but not in milder diseases like IAD. On the contrary, lung mechanics allows quantification of the degree of airflow obstruction present in horses’ airways. Airflow obstruction is the basic mechanism responsible, at least in part, for some of the manifestation of IAD such as exercise intolerance and cough. The advantage of lung mechanics is quantification of airflow obstruction, which is often subclinical in horses with IAD. Measurement of the degree of airway obstruction allows the clinician to determine the severity of the disease process and provides objective means of assessing response to therapy. The disadvantage is that sophisticated equipment and a sound understanding of respiratory physiology are required. Therefore, tests of lung mechanics are currently used mainly in the research arena and in some referral clinics. However, one commercially available test (Open Pleth™, Ambulatory Monitoring, Inc., Ardsley, NY) allows lung function testing in the field, in particular measurement of airway responsiveness.55,56 There are four main methods used in the horse to evaluate lung mechanics: 1. measurement of pleural pressure changes in relation to airflow at the nose during normal (tidal) breathing or ‘conventional lung mechanics’, 2. measurement of airflow during forceful exhalation or ‘forced expiration’, 3. evaluation of the pressure–flow relationship while an oscillating source of flow is applied to the respiratory system during tidal breathing (‘forced or impulse oscillometry’), 4. measurement of thoracic and abdominal volume changes by plethysmography in relation to airflow at the nose during tidal breathing (‘flowmetrics’). Conventional lung mechanics has been the first test of lung mechanics adapted to the horse 57 and is still commonly used in research.58–60 However, this test has not been used extensively in clinical settings because it does not permit detection of airway obstruction until it is severe and clinical signs are then evident.18,61 Recently, some researchers showed that the addition of dead space to the respiratory system increases CO2 rebreathing and ventilation which is accompanied by a significant rise in lung resistance in horses with IAD as compared to healthy controls.62 Forced expiration (FE) can be performed in standing, sedated horses.63 As in people, FE is a sensitive test of airflow obstruction where a reduction in forced expiratory flow during late FE (i.e. FEF95%) is an early indicator of mild airway obstruction such as in IAD (Fig. 28.3).18 Forced or impulse oscillometry techniques have the advantage of being non-invasive and have been used both in research and clinical cases. Horses with IAD often show frequency dependence of resistance with higher values obtained at the lower frequencies (<10 Hz) suggestive of heterogeneous airway obstruction.64,65 Finally, flowmetrics or inductance plethysmography (Open Pleth™, Ambulatory Monitoring, Inc., Ardsley, NY, Fig. 28.4) is non-invasive, portable in the field and shows satisfactory correlation with standard lung mechanics.56,66 Another means of detecting airway obstruction is by testing airway reactivity in response to inhaled irritant such as histamine. Exaggerated airway narrowing in response to an irritant is called airway hyperresponsiveness. Airway reactivity can be quantified using the four tests of lung mechanics described above.63,66–68 Airway hyperresponsiveness is a prominent feature of IAD in horses with increased BALF eosinophil and mast cell counts.31,65,68,69 The sensitivity of forced oscillometry and flowmetrics is enhanced when used to detect airway hyperresponsiveness. This increased bronchoconstriction in response to inhaled irritants plays an important role in the pathogenesis of the cough and presumably exercise intolerance. Hematology and serum biochemistry of horses with IAD are usually within normal limits. Cytologic specimens of BALF are prepared by centrifugation and processed with Wright’s stain. Total cell count in healthy horses is usually less than 400 cells/µl (Table 28.2). Differential cell counts should be determined by examination of at least 200 cells per slide and preferably 500 cells. Cytological analysis of BAL fluid allows recognition of 3 main types of inflammatory profiles in IAD (Table 28.2).50 The most commonly encountered profile is characterized by an increased total nucleated cell count with mild neutrophilia (5–20 % cells), lymphocytosis, and monocytosis (Fig. 28.5A).14,16,18,31 Two other cytological profiles characterized by increased percentages of mast cells (>2%, Fig. 28.5B) and eosinophils (>1%, Fig. 28.5C) are also observed in some horses with IAD.31,69,70 Some horses can also present with a combination of BALF abnormalities such as elevated neutrophils and mast cells, mast cells and eosinophils or all three cell types.71,72 In contrast, BAL of horses with RAO shows moderate-to-severe neutrophilia (>20% cells), lymphopenia, and decreased alveolar macrophages.18,73,74 Cytology of BALF collected from horses with RAO in clinical remission can be normal if there has been sufficient time free of exposure to inciting allergens.75 Some RAO cases can be in clinical remission but still exhibit some degree of pulmonary neutrophilia and, therefore, can be difficult to differentiate from IAD. One way to discriminate RAO from IAD is by performing a hay challenge and monitoring clinical signs of respiratory disease, which should develop within a few hours to a few days in RAO-affected horses.59,76 Horses with IAD exposed to moldy hay exhibit a worsening of coughing and pulmonary neutrophilia; however, they do not develop increased respiratory effort or nostrils flaring like RAO-affected horses do. Although practical, this challenge might not be feasible in client-owned horses and in such situations, pulmonary function testing is indicated. Tracheal secretions can be collected by direct wash of tracheal lumen with sterile saline via a catheter introduced through the endoscope’s working channel or by percutaneous transtracheal aspiration. If microbiologic culture of the fluid is being pursued, tracheal wash (TW) should be performed using a sterile, multiple lumen guarded catheter (double or triple lumen) introduced through the endoscope working channel or via percutaneous catheterization of the trachea in mid-cervical region using a sterile technique. The latter technique requires sedation, whereas the former one can be conducted using only simple restraint such as application of a nose twitch. An abundant commensal flora inhabits nasal passages and nasopharynx (e.g. Streptococcus zooepidemicus). Therefore, TW via the instrument channel of the endoscope without a multiple lumen guarded catheter will result in contamination of the sample and will be inappropriate for microbiologic culture and render any such results uninterpretable.77 Furthermore, coughing during the procedure increases the chances of oropharyngeal contamination of the sample. A smear of the TW sample should be made as soon as possible after collection. Total nucleated cell count in healthy horses is usually less than 500 cells/µL with few to no red blood cells.11,78 Differential count should be determined based on 200–300 consecutive cells. Cells in TW are more difficult to identify and count than in BAL because they often are more degenerate and are trapped in abundant mucus strands. Normal TW fluid should contain a majority of macrophages (30–70%), low numbers of epithelial cells (higher if collected with catheter through the endoscope), <20% neutrophils, <10% lymphocytes, <1% eosinophils and very few mast cells (<0.2%).11,49,77,78 Importantly, several studies have documented the fact that there is no correlation between TW and BAL cytology, indicating that one is not a substitute for the other.49,79 Tracheal wash is the sample of choice when suspecting an infectious etiology and BAL is preferred otherwise because of the high correlation with lung lesions (as determined by histopathology). Horses that cough during training more than 4 times in a 10-min period are more likely to have >20% neutrophils in TW, large numbers of bacteria (>103 cfu/mL) and intracellular bacteria.40 The likelihood of isolating bacteria from tracheal wash samples collected from racehorses in training is strongly associated with the TW inflammation and mucus accumulation, in particular in young horses.15,23,30 But there is no apparent association between TW neutrophilia and mucus in older racehorses.44 Isolation of more than 103 colony-forming units of pathogenic Streptococcus spp. is also strongly associated with coughing.30 Therefore, tracheal inflammation and high bacterial counts are common in racehorses but only excess tracheal mucus is associated with decreased performance, not TW neutrophilia.7 Histopathological examination of the lungs can be conducted ante-mortem by collecting tissue samples using transcutaneous lung biopsy needles and endobronchial biopsies forceps via the endoscope, or at post-mortem examination. Horses with IAD present similar histopathology findings as horses with RAO but with less severe and chronic changes.48 Typical morphological findings include peribronchiolitis, bronchiolitis, bronchiolar epithelial hyperplasia, goblet cell metaplasia, luminal mucus, and in some cases alveolitis.80,81 Severity of histopathologic changes assessed by a scoring system appears to be negatively correlated to various indices of respiratory and cardiovascular function in horses with IAD.80 Consequently, as pulmonary lesions become more severe, horses become more exercise intolerant. However, histopathology findings obtained from transcutaneous lung biopsies or endobronchial biopsies are highly variable and the potential diagnostic benefit should be weighed against the potential risks associated with transthoracic biopsy such as hemorrhage and pneumothorax. In horses presenting with clinical signs including cough, increased respiratory secretions, and poor performance, detailed history, physical examination, and diagnostic tests should help eliminate differential diagnoses (Fig. 28.5B). Using a validated questionnaire administered to clients is useful to identify horses with heaves;82 however, the same questionnaire has been unable to differentiate IAD from healthy horses.16 Horses with IAD are not febrile and presence of a fever suggests infectious respiratory diseases such as viral diseases, bronchopneumonia, pleuropneumonia, or pulmonary abscess. Hematology findings can be helpful to confirm a suspicion of respiratory infection; however, they are often non-specific. Leukocytosis with neutrophilia is commonly found with bacterial respiratory infections and during the acute phase of a bacterial infection, increased numbers of immature neutrophils (‘left shift’) may be observed. Neutrophilia can also accompany non-infectious inflammatory diseases (such as caused by toxins), neoplasia, and mycotic and parasitic infections. Hematologic changes during the early phase of a viral respiratory infection, such as influenza, are often characterized by normocytic, normochromic anemia, lymphopenia or lymphocytosis, and sometimes neutropenia.83,84 Neutrophilia can follow within a week of initial clinical signs, particularly in cases of secondary bacterial infection. Monocytosis can develop during the recovery phase of a viral infection. Coughing is often chronic, intermittent (>3 weeks) in horses with IAD. Main differential diagnoses are mild cases of heaves (RAO), and parasitic pneumonitis (Fig. 28.6). Horses with RAO typically display exercise intolerance and increased respiratory efforts during periods of disease exacerbation; however, these signs can be subtle during periods of disease remission. In those cases, pulmonary function testing or more simply, moldy hay challenge, will help reaching a definitive diagnosis. Neutrophilia is commonly observed in BAL fluid from horses with RAO and IAD.14,73,85 The neutrophilia is usually more pronounced (>20%) with RAO than IAD; however, there is significant overlap between diseases.16,18,86 Eosinophilic inflammation can be associated with IAD, parasitic pneumonitis (Parascaris equorum, Dictyocaulus arnfieldi),87 hypersensitivity pneumonitis, fungal pneumonia, and cutaneous habronemiasis.70,88,89 Clinical signs of parasitic pneumonitis are non-specific. Fecal flotation (Baermann technique) is often not diagnostic of P. equorum infection because migration through the lungs occurs during the pre-patent period.90 D. arnfieldi follows a complete cycle in donkeys, mules, and asses; however, the infection is usually not patent in horses. Therefore, the Baermann fecal flotation is not useful either. P. equorum pneumonitis is more commonly detected in foals less than 6 month of age. D. arnfieldi usually occurs in horses in contact with infected donkeys and rarely by ingesting larvae excreted by infected horses.91 A presumptive diagnosis can be reached when respiratory secretions reveal eosinophilic inflammation with sometimes evidence of parasite eggs or larvae, exposure to donkeys exists, and anthelmintic therapy results in clinical improvement.90 Increased numbers of metachromatic cells (mast cells, basophils) are common in horses with IAD but have not been associated with other types of respiratory disease.70,92 Treatment of IAD should combine environmental changes and medical therapy. The goals of medical therapy are to control airway inflammation and relieve airflow obstruction using mainly corticosteroids and bronchodilators or antimicrobials when infection is suspected. There are few peer-reviewed clinical trials published, especially concerning aerosol therapy. Therefore, most of the drugs and dosages recommended are based on studies performed on horses with RAO; however, good clinical response of IAD has been observed using those guidelines (Table 28.3). Both systemic and aerosolized drugs are effective; however, the potential for adverse effects and prolonged elimination times is greater with systemic administration. The advantages of aerosol therapy are ease of administration and safety. The disadvantages are cost of delivery of devices and drugs (metered-dose inhalers). Non-steroidal anti-inflammatory and anti-histamine drugs are ineffective for the treatment of IAD. Table 28.3 Therapeutic aerosols used for the treatment of inflammatory airway disease (IAD) and recurrent airway obstruction (RAO)* 3M ED: aerosol delivery device for equine developed by 3M Animal Care Products (no longer commercially available), AeroMask: aerosol delivery device for equine developed by Trudell Medical International. *Dosages reported in this table were taken from studies using CFC propellant. Current aerosols are packaged with HFA propellant that require lower dosages for a given effect when using certain types of corticosteroids (e.g. beclomethasone, see discussion about aerosol therapy for further explanation) †Approved for use in horses at a dose of 360–720 µg per horse no more than four times per day. A large proportion of racehorses suspected of respiratory disease because of poor performance, cough, or nasal discharge are treated with antimicrobials usually without supportive evidence of bacterial infection.93,94 However, there is currently no controlled study reporting the efficacy of antimicrobials in the treatment of IAD. Available data show no association between response to treatment and the type of antimicrobial used, duration of treatment or time elapsed between repeated examinations.93 Only 50% of racehorses diagnosed with IAD based on excess tracheal mucus respond favorably to a single course of antibiotic therapy.93 Therefore, there is currently no evidence-based data supporting the use of antimicrobials in horses with IAD. Two clinical trials have been reported concerning the use of inhaled corticosteroids for IAD and only one in the peer-reviewed literature.71,95 In a randomized cross-over study, seven horses diagnosed with IAD based on criteria provided in a consensus statement 13 were treated for two weeks with inhaled fluticasone (3000 µg, q 12 h) using an equine aerosol delivery device (AeroHippus™, Trudell Medical International, London, Ontario, Canada, Fig. 28.7) or with intramuscular dexamethasone (0.05 mg/kg q 24 h).71 After two weeks of therapy, the only significant change detected was a relative decrease in BALF lymphocytes and none of the other BALF cell types were affected by treatment. A second study used a randomized, controlled cross-over design with 8 horses diagnosed with IAD based on elevated tracheal mucus and neutrophil percentages (>20%) and horses were treated for two weeks with either inhaled fluticasone (1000 µg q 12 h), ipratropium bromide (250 µg q 12 h), a combination of the two drugs or a placebo (four horses per treatment group) using a different type of aerosol delivery device (Equine Haler™, Equine HealthCare ApS, Denmark, Fig. 28.7).95 Results showed that two weeks of inhaled fluticasone with or without ipratropium significantly decreased tracheal neutrophilia (−18.5% and −22%, respectively) as compared to ipratropium alone (−7.9%) or placebo (+14.2%) and most of the improvement occurred during the first week. Since tracheal cytology does not necessarily reflect severity of lesions in lungs, results from this study should be interpreted with caution and large field trials are needed before evidence-based recommendations can be made. Clinical experience suggests that a combination of environmental measures aimed at decreasing dust exposure coupled with inhalation therapy using the same drugs effective against RAO is also beneficial for IAD (Table 28.3). As a general rule, the low end of the dose range recommended for RAO seems appropriate for IAD cases. Currently available aerosol delivery devices used to administer drugs packaged in metered-dose inhalers (MDI) provide similar clinical efficacy (AeroHippus™ and Equine Haler™, Fig. 28.7).96 Improved clinical signs, decreased airway hyperresponsiveness, and reduced pulmonary inflammation are usually detectable within two weeks of therapy. Bronchodilators are indicated to relax airway smooth muscle and relieve airflow obstruction. Two main classes of inhaled bronchodilators have been used in the horse: beta2-agonists (e.g. albuterol, clenbuterol) and anticholinergic drugs. Horses with IAD have a mild degree of airway obstruction that could be secondary to airway inflammation or bronchospasm.62,65,97 Another common cause of airway obstruction is bronchial hyperresponsiveness which is particularly evident in horses with IAD associated with increased concentrations of eosinophils or mast cells in BAL lavage fluid.31,98 Bronchodilators should not be used as the sole therapy for a prolonged period of time because they do not suppress airway inflammation. However, evidence from ex-vivo and in-vivo studies suggest that clenbuterol (0.8 µg/kg PO or IV, q 12 h) has mild anti-inflammatory properties and reduces airway responsiveness to an irritant challenge in healthy, IAD- and RAO-affected horses.99–103 The bronchoprotective effect of clenbuterol decreases after 2 weeks of therapy due to tachyphylaxis.99 In general, bronchodilators should be administered prior to inhalation therapy with corticosteroids to optimize lung deposition of corticosteroids.104 Sodium cromoglycate (cromolyn) improves clinical signs and decreases bronchial hyperresponsiveness when administered to horses with IAD characterized by a high mast cell count in BAL fluid (Table 28.3).70 However, it is ineffective for the treatment of IAD with other inflammatory profiles.
Inflammatory diseases of the lower airway of athletic horses
Introduction
Inflammatory airway disease (IAD, small airway disease, small airway inflammatory disease, lower airway disease, bronchiolitis)
Recognition
History and presenting complaint
RAO
IAD
Signalment
Age
>6 years
>1 year
Activity level
+/++
++/+++
Clinical signs
Duration
Months to years
1–6 months
Recurrent
Not recurrent
Cough
Exudate in airways
Lung sounds
Chronic intermittent
Mucopurulent (++/+++)
Breath sounds (++/+++), wheezes, crackles
38% of cases
Mucoid to mucopurulent (+/++)
Normal
Increased respiratory efforts
++/+++
0/++
Exercise intolerance
++/+++
+/++
Etiology
Allergy to molds
Multifactorial
Pathophysiology
Lower airway obstruction
++/+++
0/+
BALF cytology
Neutrophilia (>25%)
Neutrophilia (5–20%)
Eosinophilia (>1%)
Mastocytosis (>2%)
Airway hyperresponsiveness
+/+++
+/+++
Histopathology
Bronchiolitis
++/+++
+/++
Physical examination
Special examination
BAL TECHNIQUE
HORSES’ TYPE/NUMBER
LEUKOCYTES (cells/µL)
ALVEOLAR MACROPH. %
LYMPHOCYTES %
NEUTROPHILS %
EOSINOPHILS %
MAST CELLS %
REFERENCE
Control horses
5 × 50 mL saline-syringe-endoscope
training (no racing) / 40
133.5 ± 8.2
56.9 ± 1.9
38.2 ± 1.8
4.1 ± 0.5
0.3 ± 0.2
0.4 ± 0.2
Clarck CK, 1995
50 mL saline-pump-endoscope
pasture / 9
782.2 ± 272.0
54.3 ± 16.4
28.8 ± 16.2
14.4 ± 10.1
0.4 ± 1.0
2.0 ± 1.1
Sweeney CR, 1992
300 mL saline-pump-endoscope
pasture / 8
175.9 ± 110.7
49.5 ± 13.0
40.4 ± 14.6
2.5 ± 1.5
0.1 ± 0.4
7.4 ± 2.7
Sweeney CR, 1992
3 × 100 mL saline-pump-endoscope
pasture / 6
378.3 ± 141.3
59.2 ± 7.4
33.0 ± 8.1
1.2 ± 1.2
0.2 ± 0.4
6.5 ± 2.1
Sweeney CR, 1992
4 × 60 mL saline- syringe-tube
racing / 11
65 ± 6.2
28 ± 5.8
7 ± 3.3
0 ± 0
0.2 ± 0.3
Forgarty U, 1991
1 × 250 mL saline-pump-endoscope
racing / 12
530 ± 170
60.1 ± 4.8
36.7 ± 5.4
2.2 ± 1.4
0.03 ± 0.1
0.4 ± 0.4
Hare JE, 1994
300 mL LRS-BAL tube
racing / 6
153.2 ± 17.1
64.8 ± 4.6
28.3 ± 2.9
3.8 ± 0.3
1.2 ± 0.8
0.3 ± 0.3
Rush B, 1995
2 × 250 mL saline-pump-endoscope
racing / 6
360 (260–540)
67.7 (61–78.8)
31.5 (19–35)
0.4 (0.2–1.4)
0.3 (0–1)
1(0–2.8)
Hare JE, 1998
1 × 250 mL saline-pump-endoscope
racing / 10
445 ± 142
68.8 ± 8.8
22.9 ± 7.4
3.8 ± 5.5
2.0 ± 1.0
1.5 ± 0.3
Couëtil L, 1999
1 × 120 ml saline-syringe-BAL tube
racing / 14
517 ± 229
50.2 ± 10.4
26.2 ± 6.5
15.1 ± 2.8
0
2.1 ± 1.4
Courouce-Malblanc A, 2002
1 × 250 mL saline-pump-endoscope
racing / 10
436 (326–536)
65.0 (63.0–76.7)
25.0 (15.0–29.2)
2.0 (1.0–4.0)
Sanchez A, 2005
3 × 83 ml saline-syringe-BAL tube
racing / 20
73.5 (64.0–79.5)
19.0 (13.8–25.3)
5.5 (3.8–7.0)
0.0 (0.0–0.0)
1.0 (0.0–1.0)
Richard EA, 2012
1 × 300 ml saline-syringe-endoscope
Racing/19
63.0 [49-90]
32.0 [6-47]
2.0 [1-4]
0.0 [0-1]
1.0 [0-1]
Nolen-Walston R, 2013
IAD horses
4 × 60 mL saline- syringe-tube
racing / 65
64 ± 15.2
23 ± 11.4
13 ± 12*
0.1 ± 0.3
0.3 ± 0.7
Forgarty U, 1991
1 × 250 mL saline-pump-endoscope
racing / 12
590 ± 290
56 ± 13
37 ± 16
4.1 ± 3.5
0.2 ± 0.2
3.1 ± 1.0*
Hare JE, 1994
300 mL LRS-BAL tube
racing / 32
366.0 ± 16.8
48.4 ± 1.9*
36.0 ± 1.9*
10.4 ± 1.1*
3.8 ± 1.5
1.8 ± 1.5
Rush B, 1995
2 × 250 mL saline-pump-endoscope
racing / 5
650 (320–1100)
58.6 (40.6–62.4)
25.8 (22.6–31.4)
0.8 (0–1.8)
11.8 (6.4–26.4)*
1.4(0.4–2.4)
Hare JE, 1998
1 × 250 mL saline-pump-endoscope
racing / 13
582 ± 122
54.8 ± 10.8*
33.6 ± 10.7
12.0 ± 7.7*
0.4 ± 1.4
1.4 ± 0.4
Couëtil L, 1999
1 × 120 ml saline-syringe-BAL tube
racing / 7
729 ± 229*
22.6 ± 9.9*
21.3 ± 6.6
34.0 ± 15.3*
0
2.9 ± 2.7
Courouce-Malblanc, 2002
1 × 250 mL saline-pump-endoscope
racing / 25
491 (394–634)
54.0 (44.0–58.0)*
37.0 (30.8–48.5)*
5.0 (3.0–13.0)
Sanchez A, 2005
3 × 83 ml saline-syringe-BAL tube
racing / 22
57.0 (51.5–66.3)*
20.0 (12.3–29.3)
14.5 (8.0–18.0)*
0.0 (0.0–0.8)
2.0 (1.0–3.0)*
Richard EA, 2012
1 × 300 ml saline-syringe-endoscope
Racing/40
54.5 [18-80]
36.5 [14-67]
2.0 [0-4]
3.5 [0-12]*
3.0 [0-10]*
Nolen-Walston R, 2013
1 × 300 ml saline-syringe-endoscope
Racing/17
55.0 [43-80]
36.0 [14-51]
6 [5-15]*
0.0 [0-0]
1.0 [0-1]
Nolen-Walston R, 2013

Laboratory examination
Necropsy examination
Diagnostic confirmation
Treatment and prognosis
Therapeutic aims
DRUG
TRADE NAME
(LABORATORY)
DOSE DELIVERED PER ACTUATION
NUMBER OF DOSES PER CANISTER
DEVICE
DOSE
DURATION OF ACTION
Bronchodilators
Albuterol
Torpex (Boehringer & 3M)
120 µg
200
3M ED
1–2 µg/kg†
1 hour
Combivent
(Boehringer)
120 µg
(+ 21 µg ipratropium)
200
AeroMask
2–3 µg/kg
1 hour
Proventil
(Schering)
120 µg
200
AeroHippus/ Equine Haler
1 µg/kg
1 hour
Ipratropium bromide
Atrovent
(Boehringer)
18 µg
200
AeroMask
0.5–1 µg/kg
4–6 hours
Atrovent
(Boehringer)
0.02 % solution
for nebulization
2.5 ml vial
Ultrasonic nebilizer
2–3 µg/kg
4–6 hours
Combivent
(Boehringer)
21 µg
(+ 120 µg albuterol)
200
Fenoterol
Only in Canada
AeroMask
2–4 µg/kg
Pirbuterol
Maxair inhaler
(3M)
200 µg
300
3M ED
1–2 µg/kg
1 hour
Salmeterol
Serevent
(Glaxo Wellcome)
25 µg
120 (13 g canister)
3M ED
0.5–1 µg
6–8 hours
Corticosteroids
Beclomethasone*
Beclovent
(Glaxo Wellcome)
42 µg
200 (16.8 g canister)
3M ED
AeroMask
1–3 µg/kg, q 12 hours
7–9 µg/kg, q 12 hours
Fluticasone*
Flovent
(Glaxo Wellcome)
220 µg
125 µg
120 (13 g canister)
AeroMask
Equine Haler
2–4 µg/kg, q 12 hours
6–12 µg/kg, q 12 hours
Other
Sodium cromoglycate
Generic
0.02 % solution for nebulization
2 mL vials
Jet nebulizer
Ultrasonic nebulizer
200 mg, q 12 hours
80 mg, q 24 hours
Therapy
Medical therapy
Other therapies
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree