Chapter 21
Infectious Diseases of Amphibians
It Isn’t Just Redleg Anymore
Some infectious diseases such as chytridiomycosis and Ranavirus infection have been the focus of intensive research efforts to understand their potential role in the global decline of amphibian species.1 The emergence of these diseases in the last decade has led to the recognition that the knowledge base for amphibian infectious diseases is limited but has also increased the interest in comprehensively investigating and describing amphibian mortality events.
As an example, in the recent past, it was acceptable to give any amphibian with red skin a diagnosis of redleg syndrome, which was a result of a gram-negative bacterial infection (bacterial dermatosepticemia), but it is now known that a variety of infectious diseases including ranaviruses, chytridiomycosis, fungal infections, and chlamydophilosis, among others, can result in similar clinical syndromes that are indistinguishable without ancillary diagnostic testing. In retrospect, it is likely that many amphibian mortality events attributed to redleg syndrome were, in fact, misdiagnosed because of the gross appearance of their lesions and bacterial culture of Aeromonas hydrophila, which is easily isolated from animals dying of other diseases.2–4 This example emphasizes the need for careful and complete investigation of amphibian infectious diseases.
Bacterial Diseases
Chlamydophilosis
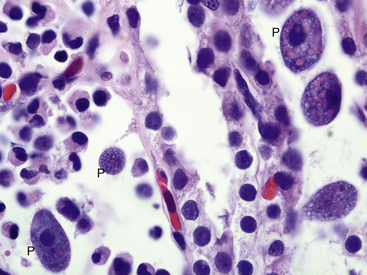
Infections with obligate intracellular bacteria in the family Chlamydiaceae in captive African Clawed Frogs (Xenopus sp.) were first described in the 1980s.5,6 Although Chlamydophila psittaci was identified in early cases, subsequent reports of chlamydophilosis in amphibians have implicated Chlamydophila pneumoniae, best known as a common respiratory pathogen in humans. These reports have included an observation of disease in a free-ranging Giant Barred Frog from Australia, suggesting that chlamydophilosis may not simply be a disease of captivity.7–10 A survey of tissues from both captive and wild frogs in Switzerland revealed by immunohistochemical analysis and molecular methods evidence of a variety of chlamydiae including C. pneumoniae, Chlamydia suis, and Chlamydophila abortus.11 No lesions of chlamydophilosis were observed in the Swiss frogs, which suggests that amphibians could be a previously unrecognized reservoir for chlamydial organisms. This author has identified infections with C. pneumoniae in critically endangered Wyoming Toads (Anaxyrus [Bufo] baxteri), demonstrating that infections can be a concern in programs rearing amphibians for reintroduction into the wild.
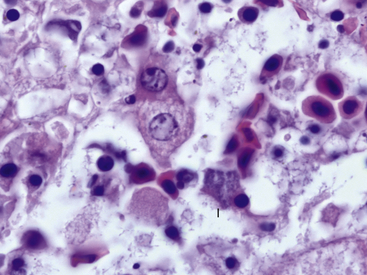
Clinically, chlamydophilosis can have a variety of presentations including sudden death, nonspecific clinical signs such as lethargy, and a resemblance to redleg syndrome. Histologic lesions also vary and can include hepatic necrosis, interstitial pneumonia, interstitial nephritis, epicarditis, and myocarditis. Inflammatory cell infiltrates have been described as nonsuppurative, mononuclear, or lymphohistiocytic. In most cases, affected cells such as hepatocytes and macrophages are observed to contain fine granular basophilic cytoplasmic inclusions suggestive of chlamydial infection (Figure 21-1). Recently, definitive diagnosis and classification of chlamydophilosis at necropsy of amphibians has been aided by the use of immunohistochemical analysis with Chlamydiaceae family–specific anti-LPS monoclonal antibodies and by polymerase chain reaction (PCR) and DNA sequencing of chlamydial 16sRNA and ompA genes. Diagnostic tests for chlamydial infection in clinically ill amphibians could include PCR; however, the clinician should be aware that these tests have not been validated for this purpose. Treatment with tetracycline group antibiotics has been suggested.12
Ochrobactrum anthropi-Associated Spinal Arthropathy
A case series of Australian Cane Toads (Chaunus [Bufo] marinus) with pyogranulomatous vertebral osteomyelitis and arthritis has recently been reported.13 Pure cultures of O. anthropi, a gram-negative bacterium found in soil and related to organisms in the Brucella group, were obtained from some of the affected animals. This is the first published report of an infectious spinal arthropathy in amphibians. The author has observed histologically similar lesions in the spine of Wyoming Toads and Mountain Yellow-legged Frogs (Rana muscosa) with mycobacteriosis; routine staining of pyogranulomatous spinal lesions with Fite’s acid-fast stain is suggested.
Protozoal and Protozoal-Like Diseases
Mesomycetozoal Diseases
Protozoa in the Phylum Neomonada, Class Mesomycetozoea have been recognized as pathogens of aquatic animals for some time but had been difficult to classify definitively as either protozoa or fungi. Based on molecular phylogeny, organisms in the Mesomycetozoea are now considered to be protozoa arising from near the animal–fungal divergence. Pathogens within the class include Dermocystidium and Ichthyophonus and Ichthyophonus-like organisms in fish and amphibians, Psorospermium in crayfish, and Rhinosporidium seeberi in mammals and birds.14
Mesomycetozoal organisms in the genera Dermocystidium, Dermosporidium, and Dermomycoides cause spherical to U-shaped raised skin lesions in a variety of amphibians and especially anurans in the genera Rana and Bufo. Histologically, lesions consist of dermal cystlike lesions containing myriad spherical 7 μm to 12 μm endospores that are sometimes associated with a granulomatous inflammatory response. Infections have not been associated with mortality. Recent molecular and morphologic studies have suggested that enough differences exist between the better studied Dermocystidium of fish and the organisms observed in amphibians to place the amphibian organisms into the new genera Amphibiocystidium and Amphibiothecum.15–17
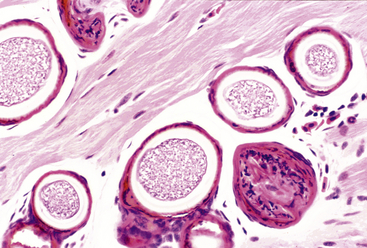
Organisms that morphologically resemble Ichthyophonus hoferi in fish are best known for causing granulomatous myositis with associated body swellings and occasionally cutaneous ulceration in Red-spotted Newts (Notophthalmus viridescens) (Figure 21-2). Similar organisms and lesions are increasingly recognized in frogs including American Bullfrogs (Rana catesbeiana), Green Frogs (Rana clamitans), Wood Frogs (Rana sylvatica), Pickerel Frogs (Rana palustris), and Spring Peepers (Pseudacris crucifer). Infections are frequently an incidental finding; however, heavy infections can result in death of individual animals and sometimes have been associated with mortality events.3 Clinical signs in lethally infected frogs include lethargy, poor response to prodding, and thin body condition without the prominent caudal body swellings observed in newts.3 Histologically, the skeletal muscle of affected animals contains spherical to elongate spores that vary between 40 μm and more than 200 μm in diameter. Both active and passive spores are described, and passive spores possess a thick (up to 12-μm) triple-contoured wall. Unlike Ichthyophonus in fish, infections in amphibians seem to be limited to skeletal muscle, and disseminated systemic infections have not been reported. The life cycle is unknown; however, an association with the leech Placobella picta has been proposed and needs experimental support.18 Additional experimental studies of life cycle and molecular phylogeny are required for the Ichthyophonus-like organisms in amphibians.
Alveolate Protozoal Disease
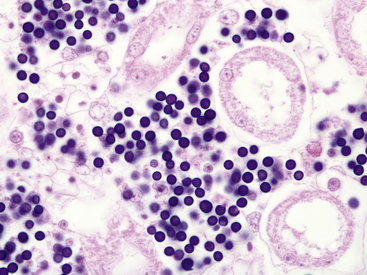
A newly identified organism identified by 18S rRNA sequencing as being most closely related to unspecified alveolate protozoa from freshwater lakes and marine hydrothermal vents and has been associated with mass die-offs of ranid tadpoles in the United States.19–21 Species affected include Southern Leopard Frogs (Rana sphenocephala), Mink Frogs (Rana septentrionalis), Green Frogs, Mississippi Gopher Frogs (Rana sevosa), and American Bullfrogs. Disease has only rarely been reported from postmetamorphic animals.22 Clinically affected Southern Leopard Frogs have enlarged abdomens, swim erratically, and can be easily captured by hand.20 Gross findings in affected tadpoles include white discoloration and enlargement of the liver, spleen, and kidneys. Histologically, organs are infiltrated by large numbers of spherical, 6-μm to 9 μm-diameter, basophilic organisms, and minimal or no evidence of a host inflammatory response is present (Figure 21-3). The organisms morphologically resemble mesomycetozoan organisms and were originally described as Dermocystidium-like.3 Diagnosis is by observation of organisms on organ impression smears or by histopathologic examination. Molecular diagnostic methods may be available through individual researchers but are not commercially obtainable at this time.
Cryptosporidiosis
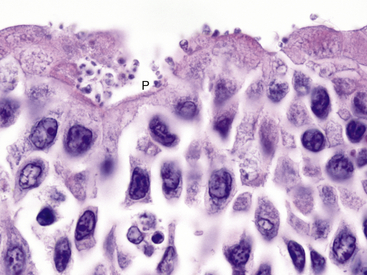
Coccidian parasites in the genus Cryptosporidium are important pathogens of mammals, birds, reptiles, and fish, but until a recent case report in a captive African Clawed Frog (Xenopus laevis), clinically significant infections had not been well-documented in amphibians. The affected frog was seen with a history of chronic weight loss and emaciation and on histologic examination was found to have a proliferative gastritis associated with large numbers of superficial 0.5-μm to 3-μm spherical protozoal organisms.23 Characteristic features of the genus Cryptosporidium were observed by transmission electron microscopy and confirmed the diagnosis. The specific species of Cryptosporidium was not determined. Experimental attempts to infect some amphibians with Cryptosporidium parvum and Cryptosporidium serpentis have been unsuccessful .24 Cryptosporidium fragile has recently been described from the stomach of Black-Spined Toads (Duttaphrynus melanostictus imported from Malaysia.25 The author has observed an unidentified Cryptosporidium (confirmed by transmission electron microscopy) infecting the ventral ciliated epithelium of the tongue in captive Wyoming Toads without apparent clinical signs (Figure 21-4). Additional surveys are required to determine the distribution and significance of cryptosporidial infections in amphibians.
Ciliate Protozoal Diseases
Intravascular ciliate protozoa have recently been reported as a major cause of death in captive specimens of the critically endangered Kihansi Spray Toad (Nectophrynoides asperginis).26 Lesions in affected animals included lymphatic obstruction and lymphangectasia with regional edema and cellulitis, obstruction of hepatic sinusoids by protozoa with associated hepatitis, and obstruction of glomerular capillaries by protozoa. The species of ciliate involved was not reported, and although a cutaneous route of infection was suspected, this could not be confirmed.
Infections with tissue-invasive ciliate protozoa such as Tetrahymena corlissi have been mentioned to occur in amphibians, but lesions are not as well-documented as they are in fish.27 Ciliate protozoa tentatively identified as a Tetrahymena sp. have recently been observed in association with necrotizing kidney lesions in Panamanian Golden Frogs (Atelopus zeteki) from this author’s institution (Figure 21-5). Presumptively, most of these infections represent opportunistic invasion by free-living environmental protozoa. It is important to note that ciliate and opalinid (can morphologically resemble ciliates) protozoa are frequent components of the normal intestinal fauna of many amphibian species.28,29 Treatment is not usually indicated for protozoa observed on routine fecal screening for parasites.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree