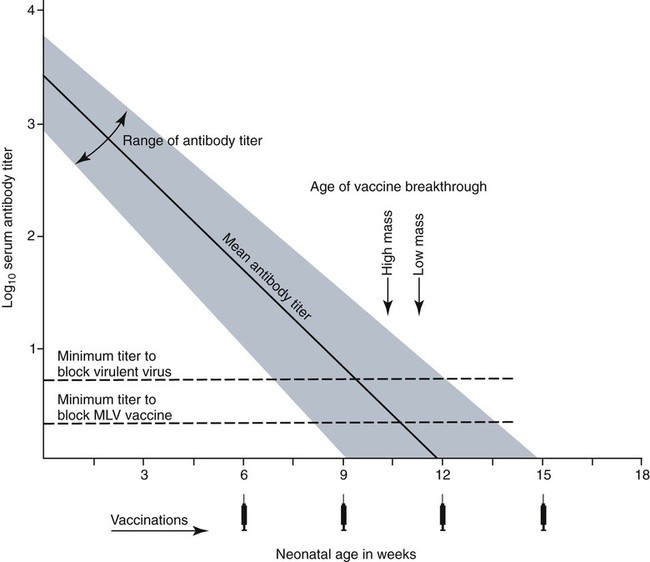
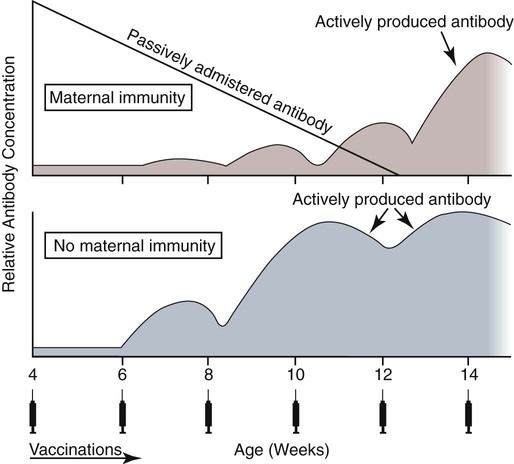
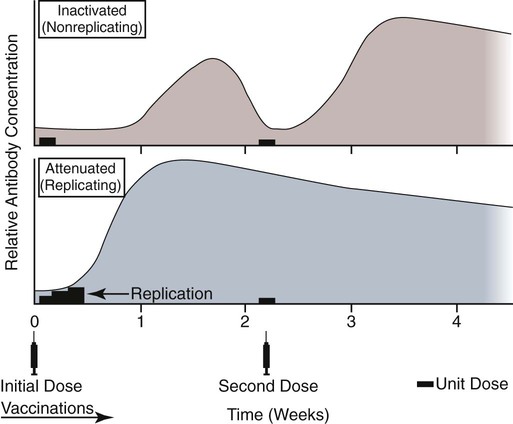
Immunoprophylaxis involves enhancement of a specific immune response in an animal by exposing it to vaccines. Once stimulated, the animal should be able to protect itself after subsequent exposure to the same infectious agent. This response can be actively induced, either through natural exposure, or by administration of vaccines containing microorganisms, their components, or their metabolic by-products.367,739 Immunity can also be passively transferred by administering humoral or cellular factors obtained from a previously sensitized donor. Sometimes passive immunotherapy is also indicated during an infection, usually early in the course of illness. Because of the relatively small number of surface antigens present on viruses and bacteria needed to stimulate an immune response, immunoprophylaxis is relatively successful in preventing disease caused by infection with these organisms. In contrast, fungal, protozoal, and metazoan pathogens and neoplasms contain more complex antigenic determinants, which makes immunoprophylaxis more difficult. Instead, these infections are often controlled with nonspecific immunotherapy. Immunotherapy, unlike immunoprophylaxis, is the attempt to nonspecifically increase the immune response in an already infected animal. See Chapter 2 for a discussion of immunotherapy and available treatment options. Despite the difficulty in producing protective immunity against multiantigenic organisms, vaccines are being developed for some of these infection of dogs and cats (see later discussion). Animals also have innate immune defensive mechanisms; however, this is not the focus of this chapter. For information about innate immunity, consult the respective organ system chapters in this book and other sources.509,631 To understand passive immunoprophylaxis, one must understand the concepts involving maternal immunity. When the dam is exposed to antigens of pathogenic organisms or administered vaccines, antibodies are produced in her circulation. Because dogs and cats have endotheliochorial placentas, only 2% to 18% of the total maternally derived antibody (MDA) is transferred in utero from an immune dam to the fetus. This small amount of antibody protects colostrum-deprived puppies or kittens for a very short time after birth. Instead, the majority of the neonatal MDAs are provided through breast milk, with the highest concentrations occurring in the colostrum. Although the highest concentrations of IgG and IgM are found in colostrums, low levels persist throughout the entire nursing period. While nursing in the first days of life, the newborn absorbs these antibodies into its systemic circulation. This action occurs in the duodenum and jejunum through membrane receptors on the intestinal epithelium that facilitate transport of IgG into the systemic circulation. The process is maximal within 24 hours of birth but also occurs to a limited degree throughout the remainder of the nursing period. Once absorbed, MDA concentrations gradually decrease as the neonate becomes older. The time period for their elimination is dependent on the initial level received by the neonate. Until MDA levels decline to a certain level, they create a feedback loop that inhibits endogenous IgG production. As MDAs decline in the circulation, the animal becomes more vulnerable to systemic disease-causing organisms in the environment and begins to synthesize its own immunoglobulins. The level of antibody in colostrum-deprived animals gradually increases until it eclipses that of neonates receiving MDA, which have a steady decline in their serum antibody titer after birth. In one study, colostrum-deprived kittens had unmeasurable IgG at birth that gradually increased with time; however, IgG did not reach the level of colostrum-receiving kittens until 4 to 5 weeks of age.144 Antibody production in the neonate can be stimulated only when the circulating MDA levels decrease below a specific threshold. This level is not absolute but is determined by the ratio of MDA to the dose level of pathogenic or vaccine antigen. Usually, pathogenic organisms can break through a higher level of this blocking antibody than can attenuated organisms in live vaccines. Increasing the dose (titer) of vaccine antigen allows for earlier immunization in the face of MDA blockade. (See Maternal Immunity and Immunization, later.) Although absorption of IgG and other antibodies is very important in the first few days of neonatal life, secretory IgA (S-IgA) is also present in breast milk throughout lactation and becomes the primary defense against enteric and upper respiratory mucosal surface infections in the neonate.870 S-IgA, which is highly effective in inhibiting the adherence of pathogens at the mucosal surface, causes their immediate neutralization. Artificial (passive) transfer of specific antibodies or other immunoreactive substances from one individual to another has historically been used to treat a variety of infectious diseases in humans and animals. Use of passive immunotherapy has been decreasing; however, it is still beneficial for diseases in which serum antibody is protective, when the host does not have the time or ability to mount an immune response, or when antimicrobial chemotherapy is not available or effective for the incriminated infectious agent. When given to animals prophylactically or therapeutically, antibodies that neutralize specific infectious agents can provide protection against those organisms, or at least reduce the organisms’ numbers. Various aspects of passive immunization are summarized in Table 100-1. Currently, passive immunization is used in small animal veterinary practice in a few special instances. Developing passive immunization protocols for opportunistic and nosocomial infections is also being considered in human medicine.880 TABLE 100-1 Comparison of Passive and Active Immunoprophylaxis Furthermore, immune sera can prophylactically or therapeutically benefit litters of puppies that are clinically infected with neonatal herpesvirus (see Therapy, Chapter 5). Serum for treating CHV infection should be prepared from recovered bitches that previously have had affected litters. Hyperimmune serum has been beneficial in treating parvovirus-infected cats and dogs within 4 days of experimental postinoculation (PI), which corresponded to the first day or two of clinical illness.389,570 In a placebo-controlled study involving dogs admitted to a teaching hospital with parvoviral infection; the efficacy of a preparation of specific canine lyophilized (freeze-dried) IgG was investigated.521 Dogs receiving lyophilized IgG as adjunctive treatment had significantly decreased hospitalization time, reduced severity of disease, and reduced cost of treatment compared with dogs treated with conventional therapy alone. Administration of immunoglobulin against endotoxin has also been used in dog and cats. Increased levels of endotoxins and tumor necrosis factor have been observed in dogs with naturally occurring parvoviral enteritis.637 Heterologous antiserum produced in horses for Salmonella typhimurium endotoxin is commercially available (SEPTI-serum, Immvac, Columbia, MO.) and has shown benefit in treating the endotoxemia in dogs experimentally infected with parvovirus. (See Chapters 8 and 36 and the Drug Formulary in the Appendix.185) Passive immunization with heterologous (equine- or human-serum-origin) antitoxin is used in the initial treatment of dogs and cats with tetanus (see Chapter 41). The effect and efficacy of passive immunization depends on many factors, including the animal’s antibody titer to the specific agent, as well as the volume administered, the relative importance of serum antibody in controlling the particular infection, and the timing of administration of antibody compared with exposure. Allergic reactions are more likely with passive than with active immunization because of the large amounts of foreign protein that are administered. An additional concern is that transfer of infectious agents is more likely with administration of serum when noncommercially prepared products are used. Unfortunately, the administration of immunoglobulins also delays the ability to stimulate active immunity in the host by vaccination. Large amounts of exogenously administered antibody may negate endogenous antibody production by tying up vaccine antigens or by direct feedback mechanisms that are not clearly understood. The duration of protection provided from passively administered antisera is short-lived (often 2 to 3 weeks). The amount of antisera administered is finite and, as with all exogenous proteins, undergoes accelerated elimination from the body, especially if it originates from a different species. Although not readily available commercially in many countries, canine and feline immune sera can be prepared in veterinary practices by sterilely harvesting serum or plasma. Immune serum (or plasma) is derived either from clinically healthy, specific pathogen-free individuals or from groups of animals that have recovered from, or have been vaccinated against, the disease in question. Companies producing commercial high-titer antisera obtain their sera from laboratory-infected dogs. Immune serum prepared in practice generally comes from animals that have been currently vaccinated against specified infectious agents; however, very high levels of antibody are difficult to achieve with attenuated vaccines because their effect is neutralized by preexisting protective immune responses. Inactivated adjuvanted vaccines would likely be required if hyperimmune serum is needed, and these are not available for many diseases. The infections in which passive antisera might be of benefit in providing neonatal protection would be CPV infection, canine distemper, infectious canine hepatitis, CHV infection, and feline panleukopenia. Veterinarians who prepare their own serum must carefully screen donors for insidious bloodborne infectious diseases such as bartonellosis, hemotropic mycoplasmosis, feline leukemia virus (FeLV), or feline immunodeficiency virus (FIV) infections, canine brucellosis, leishmaniasis, babesiosis, or ehrlichiosis (see Precautions with Blood Product Transfusions, Chapter 93). See Web Table 93-5 for a listing of infectious agents of concern and the type of screening needed to control them. If noncommercial sera harvested from donor cats are used, the duration of protection in the recipient is unknown and depends on the amount of antibodies in the sera. In cats, because of naturally occurring blood-group antibodies, ideally, the blood type of both donor and recipient should match. If blood typing or cross-matching is not performed, only type A cats should be used as donors. Careful attention must be paid to sterility during collection, storage, and administration of blood-derived products. Jugular venipuncture is preferred for collection, and the area over the jugular vein should be shaved and prepared aseptically. For plasma, blood is collected in anticoagulant contained in commercially available bags or bottles. The erythrocytes can be removed by centrifugation and sedimentation. Other resources should be consulted for collection guidelines. For a specific amount of serum, at least double the amount of blood should be collected into sterile clotting tubes without additives. After centrifugation, serum can be harvested and stored frozen in single-dose aliquots since IgG is a highly stable molecule. The collected serum can be stored up to 1 year if frozen (−20° C [−4° F]) promptly after collection.499 The minimum amount of immune sera required to confer protection is unknown and will depend on the donor’s antibody concentration. Doses of adult dog serum given to colostrum-deprived pups have ranged from 22 to 40 mL/kg; however, the serum IgG concentrations in the recipient pups at this dose have been lower than that derived from naturally acquired colostrum.76,673 Therefore, higher range doses might be needed for pups unless they will be given an earlier initial vaccination series. The dose shown effective for increasing total IgG concentration in recipient kittens, equal to that provided by nursing colostrum, is 5 mL of adult cat donor serum given subcutaneously or intraperitoneally to each recipient kitten at birth. This application is repeated again at 12 and 24 hours of age.499 The total dose of 15 mL/kitten is equivalent to 150 mL/kg based on the body weight of neonatal kittens. Immune plasma can be given intravenously; however, this is not desirable for serum because it can precipitate thrombotic complications. Furthermore, intravenous administration to small pups or kittens is often avoided because of the difficulty of cannulating a vein and the risk of volume overload. Usually, serum is given subcutaneously; although intraperitoneally or intramedullary routes are possible and sometimes more accessible for puppies and kittens that have small veins and that are hypotensive. Oral administration of IgG on the first day of life has poor bioavailability compared to parenteral routes.143 Any mucosal protection afforded by orally administered serum is only temporary because unstable serum IgG, IgM, and IgA are destroyed by proteolytic enzymes once the neonate develops improved digestive function and bacterial colonization. In contrast, because of its stability, natural S-IgA in the dam’s milk offers continued protection against mucosal pathogens during the entire period that the animal is nursing. Newborn pups and kittens have the inherent capacity to respond immunologically to numerous antigens at birth, but this response is slower and inferior compared with that of older animals.158,587,587 As described earlier, protection against infection during these early weeks of life is provided primarily by passive transfer of immunoglobulins and small amounts of cellular material from the dam’s colostrum. However, only up to 18% of the serum antibody received by the neonate from its dam is transferred in utero. This small amount of antibody protects colostrum-deprived puppies or kittens but makes them resistant to immunization for a variable time period. Table 100-2 lists the effects of MDA on vaccination for selected diseases of dogs. The immunoglobulin absorbed systemically from colostrum can give the neonate a titer that can be equal or exceed that of the dam in some instances (Table 100-3). Over time, the MDA declines at a characteristic half-life that is specific for each disease-producing agent (Table 100-4).387 Antibody class is also important with respect to titer loss. In neonates, serum contains maternally derived IgA, IgM, and IgG that are usually lost in the order given. The absolute titer of MDA in the serum of a neonate depends on the quantity of immunoglobulin received during nursing and the absolute titer of the dam. The amount is also inversely proportional to the size of the litter. TABLE 100-2 Effect of Maternal Immunity on Vaccination for Selected Canine Infectious Diseases HI, Hemagglutination inhibition; NR, not reported; SN, serum neutralization (viral neutralization). aAbsolute titers will vary between laboratories. These values were obtained from studies at the Baker Institute, Cornell University, Ithaca, NY. bOne product is licensed for 4 weeks of age; see text. cRecommendation cited here is for high-titer–lower passage (“potentiated”) parvoviral vaccines.655 There are a few remaining older (“conventional”) parvoviral vaccines that may require up to 20 weeks until the primary vaccination series is completed (see text). TABLE 100-3 Comparison of Maternal Immunity for Selected Canine and Feline Infectious Diseases NR, Not reported. TABLE 100-4 Half-Life of Maternally Derived Immunoglobulins in Neonatal Dogs and Cats aThe duration of maternal antibody protection against disease usually corresponds to the interval over which vaccines are ineffective. In some cases, titers may be increased by vaccination of either the canine or feline dam just before conception. Although not advised, vaccination with inactivated products during pregnancy has sometimes been shown to help protect feline neonates694 (see later discussion, Feline Vaccination Recommendations, Feline Viral Respiratory Disease); however, caution should be exercised when vaccinating any pregnant dam by avoiding use of attenuated-live products. Titer values are so variable among individual animals that quantitative predictions cannot be made short of direct measurement of serum immunoglobulins of the dam or neonate. Commercially available enzyme-linked immunosorbent assay (ELISA) kits are available for semiquantitative measurement of antibodies to CPV, with or without CDV (Immunocomb, Biogal, Kibbutz Gal’ed, Israel; TiterCHEK CDV/CPV, Synbiotics, Kansas City, MO, division Pfizer Animal Health; see Web Appendix 6).883 Repeated measurements (nomogram determinations) in neonates are usually impractical and expensive. Veterinarians usually use multiple vaccines, given at 2- to 4-week intervals, in an attempt to “break through” maternal immunity before exposure to virulent organisms (Figs. 100-1 and 100-2). Although not considered deleterious, frequently administered vaccines can also accelerate the depletion of the MDA present in the neonate’s circulation through formation and clearance of immune complexes. Attempts to overcome the interfering effects of MDA on vaccination have included antigenically related vaccines, such as measles or genetically engineered vector vaccines for CDV; alternate routes, such as intranasal administration for canine or feline respiratory viruses or Bordetella; and different vaccine strains or types that are able to overcome maternal immunity at a younger age as with higher-titer, lower-passage CPV vaccine. For further information, see the specific disease recommendations later and respective chapters for each disease. Conventional vaccines have generally been derived from whole organisms and are either live attenuated (modified live) or noninfectious or inactivated (killed). The advantages and disadvantages of these whole-agent products are summarized in Web Table 100-1. To increase potency and remove nonessential, potentially allergenic proteins, newer types of vaccines are being developed.112,182,182 For example, live agents are being modified by genetic deletion or recombination or use of naked nucleic acids. On the other hand, newer noninfectious vaccines are using subunit fractions of purified agents or synthetic peptides, produced by genetic recombination or formation of anti-idiotypes (see later discussion). Web Table 100-2 compares these newer vaccines. WEB TABLE 100-1 Comparison of Two Main Types of Conventional Whole-Agent Vaccines CMI, Cell-mediated immunity; FeLV, feline leukemia virus; FIV, feline immunodeficiency virus; IN, intranasal; MDA, maternally derived antibody; PO, by mouth. WEB TABLE 100-2 Comparison of Newer Types of Vaccines A, Advantage; CDV, canine distemper virus; CPV, canine parvovirus D, disadvantage; FeLV, feline leukemia virus; FHV, feline herpesvirus-1; FIPV, feline infectious peritonitis virus; FPV, feline panleukopenia virus. aSee Web Appendix 3 for more information on particular commercial products. The agents in live vaccines must be modified (attenuated) so that they retain immunogenicity and the ability to replicate in the intended host without producing illness. Attenuation of agents is usually achieved by adapting them to unusual hosts, by subjecting them to prolonged storage, or by serial passage in tissue culture. Attenuation can also be done by intentional genetic manipulations as described under genetically engineered vaccines, later. The various routes of administration also affect attenuation of the vaccine antigens. For instance, vaccines have been developed that require administrating only partially attenuated strains of organisms at sites other than those for which they have an affinity. For example, rather than inoculating a cat intranasally with a viral respiratory pathogen such as FHV-1 or FCV, the vaccine is administered at an alternate subcutaneous site. The agents undergo limited replication at the alternative sites, but, unfortunately, subcutaneous administration can stimulate a greater systemic immune response as compared to the local mucosal S-IgA antibody response. Furthermore, inadvertent contact of these partially attenuated agents with respiratory tissues during vaccination can cause disease (see later discussion, Postvaccinal Complications). Quality control is an essential factor in the production of biologics. Cell lines used in attenuating vaccine agents must not contain latent pathogens. This is especially true for veterinary vaccines, because production usually involves putting the target antigen in primary and secondary cell cultures (see Adventitious Agents under Postvaccinal Complications, later).231 Primary cell cultures are those derived from tissues harvested directly from an animal. Secondary cultures originate from further cultivation of the primary cell lines. Both of these lines are at risk of containing pathogens. Unlike human vaccines, the primary or secondary cell lines for animals are usually produced from cells of the species for which the vaccine is intended. This practice increases the risk of contamination with potentially pathogenic, latent, or passenger organisms. Live genetically engineered vaccines involve manipulation of virulent genetic sequences while maintaining the organism’s replicative function. Gene deletion is one method allowing for selective attenuation of infectious agents when existing modified live-agent vaccines are ineffective. For example, a gene-deleted FHV-1 vaccine has been developed experimentally; however, it is not commercially available.305 Insertion of a genetic marker may also be desired when it is important to determine whether an animal has been vaccinated or has actually had the disease in question. When antibody titers are being used to distinguish between natural and vaccine exposure, such as with FIV infection, a unique vaccine marker would be beneficial but would not distinguish an animal that is both vaccinated and infected. Potential expression vectors include poxviruses, simian virus 40, bovine papillomavirus, adenovirus, and herpesvirus.44,917 Bacterial vectors such as Escherichia coli, Salmonella typhimurium, and Mycobacterium bovis bacille Calmette-Guérin (BCG) can also be used. Various plant sources such as Agrobacterium tumefaciens TL plasmid, cow pea mosaic virus, and potato virus C have been used as expression vectors for CPV-2 vaccination of dogs.316,567,567 All potentially pathogenic vectors must be attenuated for the host. Poxviruses such as vaccinia work well because of their large genome and wide host range. One potential problem with genetically engineered vector vaccines is that the host can mount an immune response to the vector. For example, a recombinant vaccinia virus expressing FeLV gp70 envelope protein was not successful in producing antiviral antibodies in cats.278 Instead, inoculated cats developed neutralizing antibody against the vaccinia virus. Experimental recombinant vaccinia virus products have induced immune responses in dogs to human influenza virus, rabies virus, herpesvirus, and hepatitis virus.36 Commercially available oral rabies vaccines with poxviruses as vectors have been used to protect wildlife. Canarypox virus has been an effective vector in vaccines for canine distemper, rabies, and feline leukemia.307,835,838,839 For instance, a canine canarypox virus–vectored distemper vaccine is commercially available worldwide for administration by injection (Recombitek CDV). Experimentally, pups vaccinated with canarypox-rabies vaccine are protected at a young age despite the negating influence of MDAs.838 Parenteral canarypox-FeLV recombinant vaccine (Eurifel), expressing the envelope and gag genes, demonstrated protection against oronasal challenge with virulent FeLV.307,838 A needle-free injection system (VET JET, Bioject Medical Technologies, Tualatin, OR) administers a similar product (PureVax, Merial) transdermally.307,645 A recombinant baculovirus was used to express the VP2 capsid component of CPV, and the respective vaccine produced titers in dogs that were higher than those from a no longer available inactivated vaccine.181,185 A CAV-2-based CDV vaccine expressing the H and F antigens was effective, in the face of MDAs, in protecting pups against a challenge with virulent CDV.238 Nucleic acids (RNA or DNA) can stimulate production of immunogenic proteins by the host without being permanently incorporated into the host cell genome. This happens when the RNA or DNA is inoculated into the host as naked or plasmid-containing molecules with complete gene coding. Thus, unlike attenuated vaccines, nucleic acids are noninfectious and have no risk of causing disease. Nucleic acid vaccines are stable, resisting extremes in temperature, making their storage and preservation more practical and less expensive. They can be used to develop broader antigenic subtypes and to induce both humoral and CMI responses. DNA vaccines appear to be most effective in stimulating long-lasting cytotoxic T cells and variable antibody responses against expressed proteins. Direct intramuscular or intradermal inoculation using DNA from pathogenic organisms is a promising method for vaccination against some diseases.182,708 Despite the potential benefits, there are practical limitations to the use of the nucleic acid vaccines. One important consideration is that they can alter the genome of inoculated hosts. In addition, there are numerous patent considerations on the technology used to create the final product because the nucleic acids are injected by inoculation devices, with or without adjuvants such as liposomes or gold particles, which facilitate uptake by host cells. Second-generation DNA vaccines have been designed to contain genes encoding for cytokines or immunostimulatory molecules that act as biologic facilitators of the immune response. Nevertheless, without being able to deliver the genes and express them at sufficient levels at the correct site, the technology may not be commercially viable.41 Classical DNA vaccines are injected intramuscularly and have little ability to stimulate S-IgA responses. Formulations with microparticles, cationic lipids, protein polymers, or biodegradable microspheres allow for oral or intranasal delivery and result in significant S-IgA production at the mucosal sites.804 Such DNA vaccines have been used in clinical trials in humans; unfortunately, they stimulate weaker responses as compared with conventional vaccines. Giving a combination of a DNA vaccine first, followed by a booster using a different type of vaccine for the same disease, can provide an accentuated immune response. A CDV nucleic acid vaccine has been produced that causes host cell expression of the nucleocapsid, fusion, and hemagglutinin proteins.117 Three intramuscular injections of 12-week-old puppies, at 4-week intervals, induced a humoral IgG response and protected dogs against a challenge with virulent CDV. In subsequent studies, this vaccine was found to break through MDA in neonates because it primed the immune system to subsequent vaccination. This was demonstrated after administration of this nucleic acid vaccine at 2 weeks of age followed by a conventional modified live virus (MLV) CDV vaccine to pups at 9 weeks of age or earlier.306 In another study, a DNA rabies vaccine, given intradermally in the ear pinna, protected dogs against virulent rabies virus challenge 1 year later.511 Noninfectious whole agent (inactivated or killed) vaccines are produced in a similar fashion to live vaccines. Because noninfectious vaccines fail to replicate in the host, the level of antigenic mass is a critical determinant in the efficacy of a particular product. Inactivated whole-agent products contain agents subjected to various forms of denaturation without destroying their immunogenicity. Heat and light treatments have been relatively ineffective because, in many cases, they destroy immunogenicity without complete inactivation. Chemical inactivation with formalin produces slight modification in antigenic composition of the product, reduction in immunogenicity, and severe irritation to the animal at the site of injection. Ethylenediamine and β-propiolactone are inactivating agents that overcome many of these disadvantages. Having greater antigenic mass and added adjuvants (see Adjuvants, later), inactivated vaccines have an increased tendency to produce local and systemic allergic reactions. Noninfectious vaccines are stable and have no risk of producing vaccine-induced illness. They are sometimes considered safer for this reason. However, these vaccines do not mimic natural infection and therefore may not produce adequate mucosal immunity or CMI. The immune response to inactivated vaccines is generally of shorter duration and narrower spectrum than that of corresponding attenuated live vaccines. With the exception of rabies vaccines, noninfectious vaccines must generally be given at least twice to produce an anamnestic response that equals one attenuated vaccination (Fig. 100-3). Full protection may not develop until 2 to 3 weeks after the last immunization. Despite this shortcoming, immunity that is stimulated by noninfectious products is commonly sufficient for protection against clinical disease and routine use. After vaccination with noninfectious vaccines, many partially protected animals probably become infected when exposed to virulent agents. They develop a mild or subclinical infection that further boosts their immunity to the disease. Genetically engineered subunit protein vaccines allow for in vitro production of large quantities of immunogenic proteins by introducing genetic coding for specific antigens into bacteria, yeasts, or continuous cell lines. The altered cells replicate in the laboratory, are lysed and then put through filtration to eliminate all but the immunogenic target antigen. The FeLV vaccine (Leucogen, Virbac, Carros Cedex, France; Nobivac, Intervet, Boxmeer, The Netherlands) consists of synthetic glycoprotein for FeLV (gp70) subgroup A expressed in E. coli.540 An outer surface protein (Osp) A only vaccine is also available for dogs to protect against infection due to Lyme borreliosis (Recombitek Lyme, Merial, Iselin, NJ). A synthetic peptide VP2 vaccine has been effective against experimental CPV-2 challenge infection in dogs.465 Synthetic antigens can be produced by identifying the antigenic or genetic determinants using monoclonal antibodies (MABs). Once the nucleotide and amino acid sequences have been determined, the protein can be synthesized. Unfortunately, many antigenically active peptides by themselves are weak antigens that require adjuvants. Genetically engineered subunit protein vaccines have a duration of immunity (DOI) similar to that of inactivated whole-agent vaccines, whose DOI can be shorter compared with live attenuated products. Adjuvants have been added to noninfectious biologics in an attempt to increase the amount of immunostimulation and to extend the DOI of inactivated-agent vaccines to a level comparable to that of attenuated-agent vaccines. Adjuvants are helpful in accentuating the immune response to killed whole organism or purified antigens. They can prolong antigen exposure, enhance antigen presentation, and increase cytokine and immune responses.508 By definition and in principle, adjuvants produce a heightened inflammatory reaction, which can sometimes be deleterious. Fever, anorexia, swelling, hyperesthesia, uveitis, arthritis, meningitis, and glomerulitis are potential complications. Examples and properties of adjuvants used clinically and experimentally are listed in Web Table 100-3. WEB TABLE 100-3 Comparison of Vaccine Adjuvantsa
Immunoprophylaxis
Maternally Derived Immunity
Passive Immunization
Variable
Passive
Active
Advantages
Disadvantages
Indications

Indications for Immunoglobulin Administration
Preparing Homologous Immune Sera or Plasma
Administering Immune Sera or Plasma
Maternal Immunity and Immunization
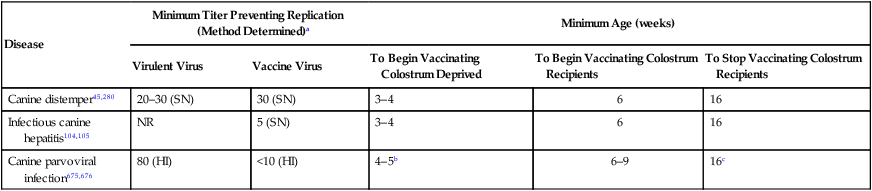
Disease
Minimum Titer Preventing Replication (Method Determined)a
Minimum Age (weeks)
Virulent Virus
Vaccine Virus
To Begin Vaccinating Colostrum Deprived
To Begin Vaccinating Colostrum Recipients
To Stop Vaccinating Colostrum Recipients
Canine distemper45,280
20–30 (SN)
30 (SN)
3–4
6
16
Infectious canine hepatitis104,105
NR
5 (SN)
3–4
6
16
Canine parvoviral infection675,676
80 (HI)
<10 (HI)
4–5b
6–9
16c
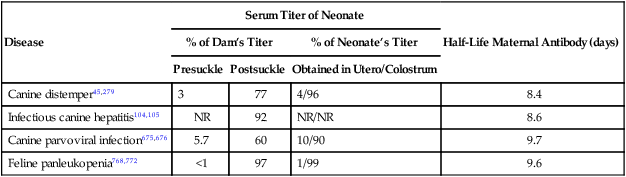
Disease
Serum Titer of Neonate
Half-Life Maternal Antibody (days)
% of Dam’s Titer
% of Neonate’s Titer
Presuckle
Postsuckle
Obtained in Utero/Colostrum
Canine distemper45,279
3
77
4/96
8.4
Infectious canine hepatitis104,105
NR
92
NR/NR
8.6
Canine parvoviral infection675,676
5.7
60
10/90
9.7
Feline panleukopenia768,772
<1
97
1/99
9.6
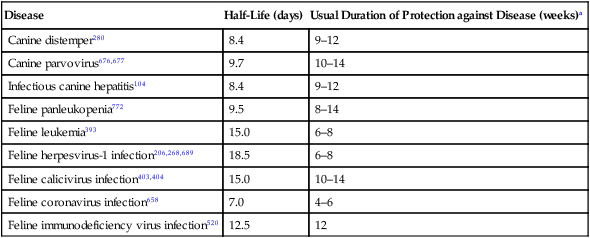
Disease
Half-Life (days)
Usual Duration of Protection against Disease (weeks)a
Canine distemper280
8.4
9–12
Canine parvovirus676,677
9.7
10–14
Infectious canine hepatitis104
8.4
9–12
Feline panleukopenia772
9.5
8–14
Feline leukemia393
15.0
6–8
Feline herpesvirus-1 infection206,268,689
18.5
6–8
Feline calicivirus infection403,404
15.0
10–14
Feline coronavirus infection658
7.0
4–6
Feline immunodeficiency virus infection520
12.5
12
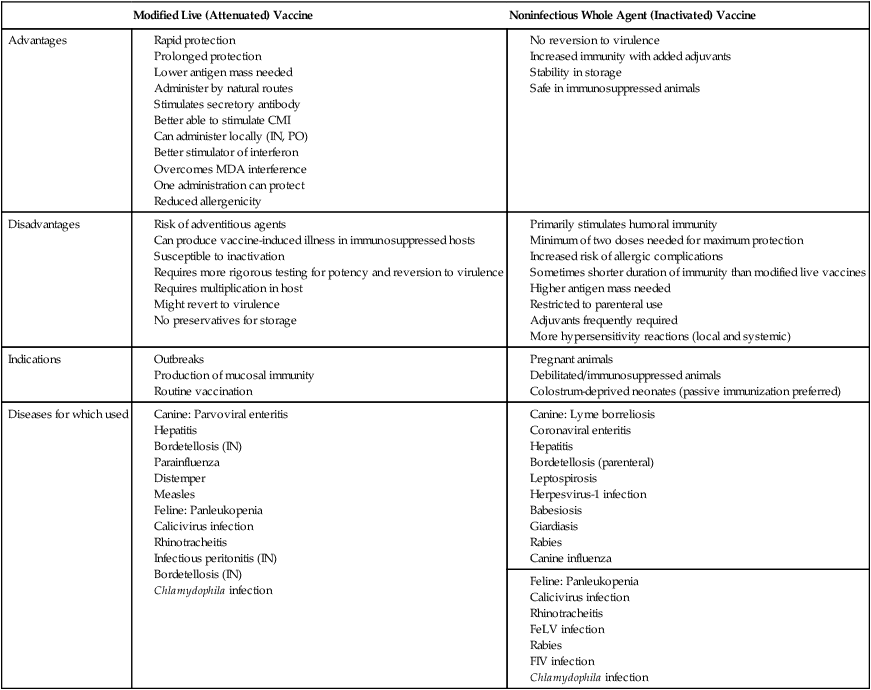
Active Immunization
Types of Vaccines
Modified Live (Attenuated) Vaccine
Noninfectious Whole Agent (Inactivated) Vaccine
Advantages
Disadvantages
Indications
Diseases for which used
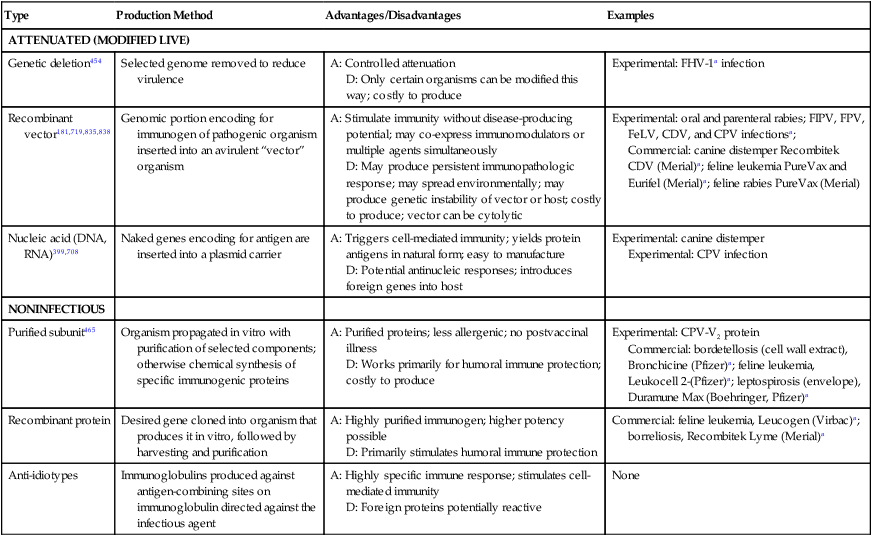
Type
Production Method
Advantages/Disadvantages
Examples
ATTENUATED (MODIFIED LIVE)
Genetic deletion454
Selected genome removed to reduce virulence
A: Controlled attenuation
D: Only certain organisms can be modified this way; costly to produce
Experimental: FHV-1a infection
Recombinant vector181,719,835,838
Genomic portion encoding for immunogen of pathogenic organism inserted into an avirulent “vector” organism
A: Stimulate immunity without disease-producing potential; may co-express immunomodulators or multiple agents simultaneously
D: May produce persistent immunopathologic response; may spread environmentally; may produce genetic instability of vector or host; costly to produce; vector can be cytolytic
Experimental: oral and parenteral rabies; FIPV, FPV, FeLV, CDV, and CPV infectionsa;
Commercial: canine distemper Recombitek CDV (Merial)a; feline leukemia PureVax and Eurifel (Merial)a; feline rabies PureVax (Merial)
Nucleic acid (DNA, RNA)399,708
Naked genes encoding for antigen are inserted into a plasmid carrier
A: Triggers cell-mediated immunity; yields protein antigens in natural form; easy to manufacture
D: Potential antinucleic responses; introduces foreign genes into host
Experimental: canine distemper
Experimental: CPV infection
NONINFECTIOUS
Purified subunit465
Organism propagated in vitro with purification of selected components; otherwise chemical synthesis of specific immunogenic proteins
A: Purified proteins; less allergenic; no postvaccinal illness
D: Works primarily for humoral immune protection; costly to produce
Experimental: CPV-V2 protein
Commercial: bordetellosis (cell wall extract), Bronchicine (Pfizer)a; feline leukemia, Leukocell 2-(Pfizer)a; leptospirosis (envelope), Duramune Max (Boehringer, Pfizer)a
Recombinant protein
Desired gene cloned into organism that produces it in vitro, followed by harvesting and purification
A: Highly purified immunogen; higher potency possible
D: Primarily stimulates humoral immune protection
Commercial: feline leukemia, Leucogen (Virbac)a; borreliosis, Recombitek Lyme (Merial)a
Anti-idiotypes
Immunoglobulins produced against antigen-combining sites on immunoglobulin directed against the infectious agent
A: Highly specific immune response; stimulates cell-mediated immunity
D: Foreign proteins potentially reactive
None
Live
Noninfectious
Adjuvants
Adjuvant
Composition and Examples
Advantages/Effects
Immune Effect
Disadvantages and Complications
Metal salts
Aluminum hydroxide, gold salts
Safety
Stimulate Th2
Granulomas and injection-site inflammation
Oil emulsions
Oil-in-water and water-in-oil emulsions with surfactant
Strong response, can prolong antigen exposure
Humoral response
More granuloma and injection-site inflammation
Liposomes
Vesicles of cholesterol and phospholipids
Safe
Humoral and CTL response
Weaker response without co-administered immunomodulators
Microparticles
Particles of biodegradable polymers such as cyanoacrylates
Long-term antigen release with dissolution
Humoral and CMI response, can use for topical administration
Microencapsulation may alter antigen properties
Saponins
Chemical extracts of the tree Quillaia saponaria (e.g., Quil A)
Relatively safe, currently used in many veterinary vaccines
Strong Th1, Th2, and CTL responses
Hemolysis with inadvertent IV administration
ISCOMs
Lipid moieties containing saponins, cholesterol, and phospholipids
Relatively safe, used in a few veterinary vaccines, many preclinical trials
Strong Th1, Th2, and CTL responses
Toxic only at high doses
Copolymers
Nonionic hydrophobic petroleum-based polymers
Relatively safe
Humoral and some CTL responses
Local reactions because not biodegradable
Polysaccharides
High-molecular-weight dextrans
Nonirritating locally
Block phagocytosis and slow antigen degradation
Systemic hypersensitivity reaction with repeated use
Proteins
Diphtheria or tetanus toxoid, bovine serum albumin, KLH
Relatively safe
Improve antigen recognition
Systemic hypersensitivity reaction with repeated use
Bacterial products
Propionibacterium spp., muramyl dipeptide, cholera toxins, LPS components, Bacillus anthracis toxin
Potent immunostimulants
Strong humoral and CTL responses
Can induce more severe sensitivity reactions
Cytokines
IFN-γ, IL-1, IL-2, GM-CSF, IL-12
Can direct specific immune response
Response depends on cytokine used
Often species specific in composition and effects; may promote autoimmune or hypersensitivity reactions
Complement derivatives
C3d fragment
Can direct specific immune response
Response depends on fragment used
Theoretical but unproven humoral hypersensitivity or autoimmunity ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Immunoprophylaxis