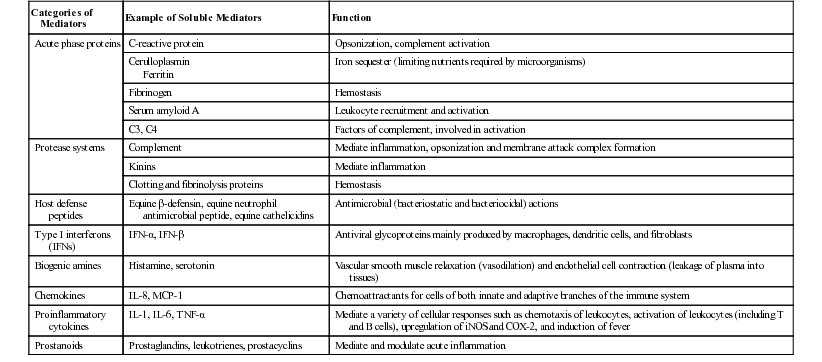
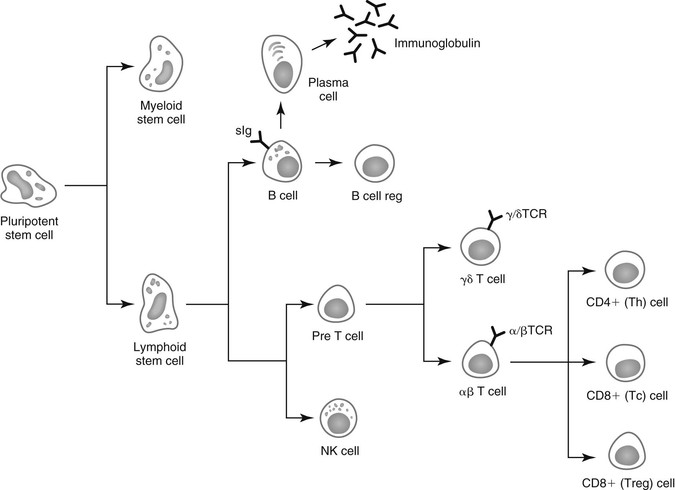
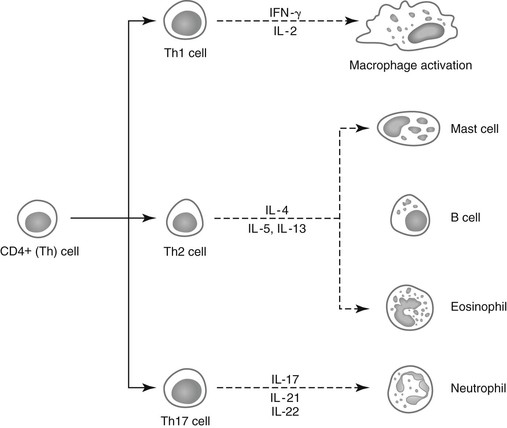
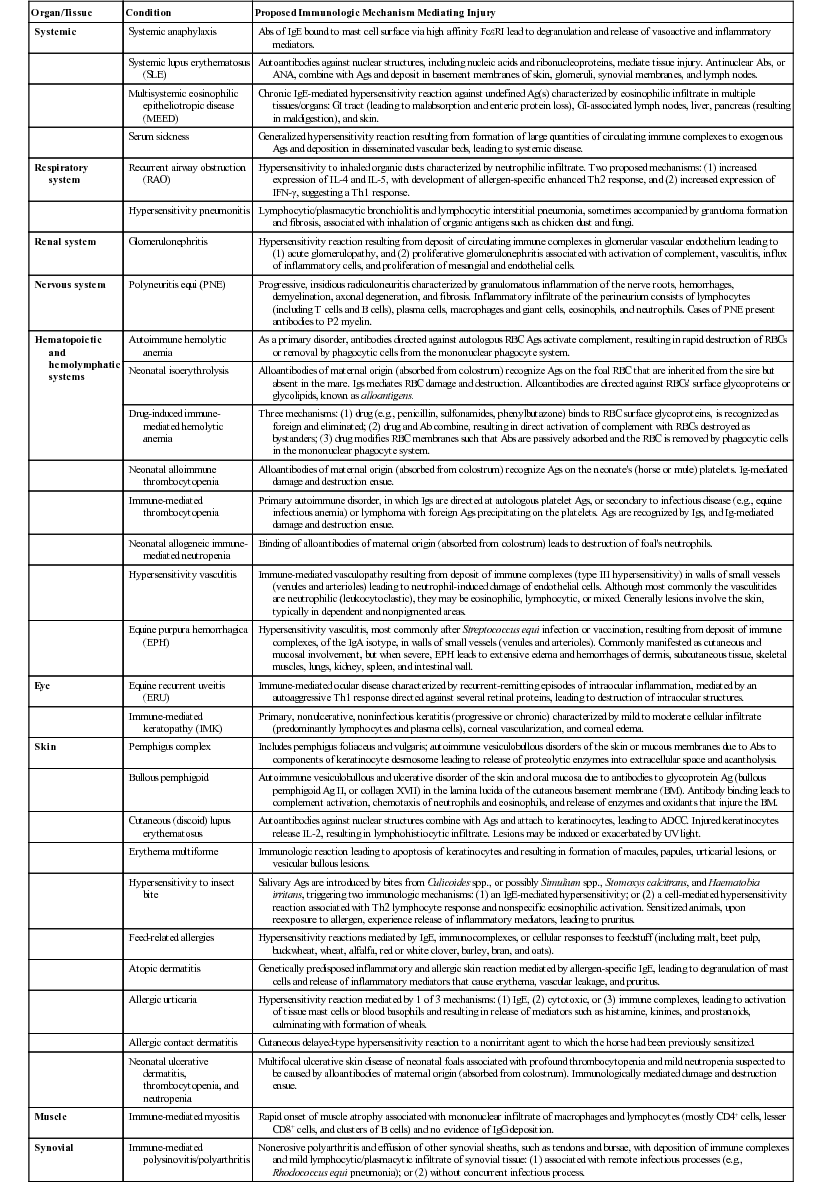
Lais R. Costa, George M. Barrington, Consulting Editors Lais R. Costa* Complex defense mechanisms have developed to protect the host from outside challenges, particularly against the pathogenic effects of microorganisms. Diseases, especially infectious diseases, can result from failure of the host’s normal defense mechanisms, as well as from overwhelming challenge from the outside. When animals are plagued by repeated or chronic infections, the clinician should always determine whether host factors are involved. On the other hand, the defense mechanisms might lead to exaggerated responses and result in immune-mediated injury of tissues and organs. The analysis of immunologic disorders, including immunodeficiency diseases, immunosuppression, and immune-mediated injury, depends on an understanding of the normal immune response. Lais R. Costa* The immune response results from an intricate network of innate mechanisms of resistance and complex adaptive immune mechanisms. Cells and soluble factors of the innate branch of the immune system interact with cells of the adaptive (clonal) branch of the immune system by promoting chemotactic stimuli for leukocyte trafficking, providing co-stimulatory signals and antigen presentation. The adaptive immune mechanisms confer specific, anamnestic response following the initial encounter with an antigen or pathogen. The adaptive immune response is largely mediated by cells of the lymphocytic lineage that are capable of distinction of antigenic determinants or epitopes that are self from those that are nonself, providing constant surveillance to identify and eliminate cells and organisms recognized as foreign. The cellular and molecular mechanisms involved in the immune response of horses have been recently reviewed in detail.1 The innate immune mechanisms are the first line of defense and do not require previous exposure to the pathogen, allowing time for development of antigen-specific acquired immune response. Innate defense mechanisms are ever ready or induced immediately. They include soluble mediators, cells, and cell-associated molecules that lead to inflammatory reactions and effector mechanisms to eliminate the offending organism. Inflammatory reaction leads to increased blood flow, increased vascular permeability, and leukocyte migration and activation. Table 53-1 depicts some of the important soluble mediators of the innate immune mechanisms. Complement activation following association of factor C3b to microbial molecules (alternative pathway), to mannose-binding lectin (lectin pathway), or following specific immunologic recognition by antibodies (classical pathway) leads to a cascade of sequential proteolytic cleavages capable of remarkable amplification. Complement activation results in opsonization by factor C3b; release of soluble factors C3a and C5a, which increase vascular permeability and exert chemoattraction of leukocytes; and finally the formation of lytic multiprotein complex, the membrane attack complex (MAC) that lyses the cell or microorganism. The diverse cellular components of the innate immune system include granulocytes (neutrophils, eosinophils, and basophils); macrophages; dendritic cells (DCs); mast cells; and natural killer (NK) cells. All these cells play critical roles in both innate and adaptive immune responses. Macrophages and neutrophils are capable of phagocytosis and destruction of the offending pathogen by oxidative burst. Neutrophils are quick responders specialized in killing invading organisms, particularly extracellular pathogens. They are the first effector cells to act on an invader, whereas macrophages compose the second line of defense and are particularly effective at dealing with intracellular pathogens. Macrophages originate from monocytes and become specialized phagocytic cells resident in various tissues; for instance, microglial cells in the brain, histiocytes in connective tissue, Kupffer cells in the liver, alveolar macrophages in alveoli of lungs, pulmonary intravascular macrophages in capillaries of lungs, and osteoclasts in bones. Macrophages have diverse functions in addition to phagocytic and antimicrobial functions, including secretion of cytokines that lead to amplification of the inflammatory response, stimulation of lymphocytes, modulation and even suppression of the immune response, and antigen processing and presentation. Subpopulations of macrophages exert specific immunologic functions. Macrophages along with DCs are the principal antigen-processing cells. Antigen processing and presentation in the context of major histocompatibility complex (MHC)-class II is a critical function linking the cells of the innate immune system and those of the adaptive immune system. Dendritic cells encompass a heterogeneous population of immune cells, originating either from myeloid or lymphoid precursors. Myeloid DCs, follicular DCs, and Langerhans cells comprise the population called DC1 cells, which originate from monocytes, and therefore are derived from myeloid precursors. They are the main antigen presenters, whereas plasmacytoid DCs, referred to as DC2 cells, are specialized in secreting type I interferon (IFN; i.e., IFN-α and IFN-β) during viral infections. Type I interferons activate lymphocytes and NK cells. NK cells recognize cells under stress, including infected or tumor cells expressing antigenic epitopes that are recognized as foreign. NK cells are important in mediating antibody-dependent cell-mediated cytotoxicity (ADCC). γδ T cells comprise a heterogeneous population of T cells expressing γδ T-cell receptors (TCRs), and exert a wide range of immune functions from response to heat-shock proteins, cytokine production, and antigen processing and presentation to promoting delayed hypersensitivity. NK cells and γδ T cells are lymphoid cells considered as the interface between innate and adaptive immunity. Lymphokine-activated killer (LAK) cells comprise a subset of cytotoxic effector cells with a wide target cell spectrum. LAK cells are distinct from T cells and NK cells.2 Cells of the innate immune system, including neutrophils, cells of the mononuclear phagocytic system, DCs, mast cells, NK cells, and a subset of γδ T cells, are capable of recognizing pathogen incursions by pattern recognition receptors (PRRs). The ligands for PRRs are collectively called pathogen-associated molecular patterns (PAMPs) and include toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD), and nucleotide-binding domain leucine-rich repeat containing protein (NLR).3 In addition, the innate immune system detects markers of endogenous cell-damage, called danger-associated molecular patterns (DAMPs).3,4 The antigen-specific or adaptive immune response involves two major populations of lymphocytes, T cells (expressing αβ-TCR) and B cells (Fig. 53-1). Classically, T cells are associated with cell-mediated immune responses that confer protection against fungal, protozoal, intracellular bacterial, and viral infections. B cells are associated with humoral immunity, which provides defense against toxins, extracellular bacterial infections, parasites, and certain viral infections. T cells originate from stem cells, which probably develop in the fetal liver. These cells must undergo a maturation process in the thymus before becoming fully functional. T cells comprise about 70% to 80% of peripheral blood lymphocytes of horses and populate the periarteriolar regions of the spleen and the paracortical regions of lymph nodes. As with T cells, B cells originate from stem cells in the fetal liver. The site of B-cell maturation varies with species and includes several different organs, such as the bursa of Fabricius in birds and the bone marrow and certain Peyer patches in mammals. Of peripheral blood lymphocytes of horses, 15% to 30% are B cells. B cells populate germinal centers of the spleen and lymph nodes.5,6 T lymphocytes are important in regulating the adaptive immune response, both the humoral and cellular immune responses. T lymphocytes are characterized as helper T (Th) cells, cytotoxic T (Tc) cells, and regulatory T (Treg) cells based on their primary function. T cells might be differentiated on the basis of cell surface antigens, with most Th cells expressing the CD4 surface receptor and most Tc cells expressing CD8 surface receptor.7,8 CD4+ T lymphocytes recognize antigens in the context of MHC-class II expressed on the surface of antigen-presenting cells, and CD8+ T lymphocytes recognize antigens in the context of MHC-class I. Further work has shown that the function of these cells is complex and that the pattern of cytokine expression is important in regulation of the immune response. On the basis of their profile of cytokine expression, CD4+ cells have been further subdivided into distinct subsets (e.g., Th1, Th2, and recently identified Th17 cells) (Fig. 53-2). Although there is some species variation, Th1 cells generally produce IFN-γ and interleukin-2 (IL-2) and are involved primarily in the generation of cell-mediated immune responses, whereas Th2 cells produce IL-4, IL-5, and IL-13 and are involved in humoral responses. Th17 cells produce IL-17, IL-21, and IL-22 and are important in clearing extracellular pathogens during host defense reactions that are not efficiently cleared by Th1- and Th2-type immunity.9,10 Protection from disease or development of lesions can be associated with the particular type of Th-cell response, and the clinical relevance of this is currently being investigated in a number of equine diseases. The second major class of lymphocytes is B lymphocytes, which produce immunoglobulins (Igs) and are the precursors of plasma cells. There are several classes or isotypes of Igs. They are classified according to their heavy chain: IgG (γ heavy chain), IgM (µ heavy chain), IgA (α heavy chain), IgE (ε heavy chain), and IgD (δ heavy chain). Originally, four subisotypes of IgG were identified in the horse on the basis of their immune functions and properties, IgGa, IgGb, IgGc, and IgG(T). Recently, seven IgG heavy chain constant region genes were identified, and the IgG subisotypes were reassigned.11,12 The original subisotype IgGa includes new subisotype IgG1, the original subisotype IgGb includes IgG4, and the original subisotype IgGc includes new subisotype IgG6. The formerly called IgG(T) includes two subisotypes, IgG3 and IgG5. The characteristics and immunologic functions of the equine Ig isotypes and subisotypes are summarized in Table 53-2.11–15 Furthermore, B cells function as important antigen-presenting cells by processing and presenting antigens in the context of MHC-class II. In addition to antigen recognition by surface Igs, B cells also recognize TLR ligands.3 A less appreciated function of subsets of B cells is the production of cytokines.13 Subsets of B cells have recently been recognized as regulators of the immune responses during inflammation and autoimmunity.16,17 TABLE 53-2 Characteristics and Immunologic Functions of Equine Immunoglobulin (Ig) Isotypes and Subisotypes11–15 ADCC, Antibody-dependent cellular cytotoxicity; NA, not applicable. Numerous factors influence adequacy of the immune response of an individual animal to potential environmental pathogens. Important factors to consider in the horse are age, genetic predisposition, general fitness, training, the level of stress associated with exercise, state of health, including nutritional status, and the presence of concurrent diseases. Moreover, increased concentrations of corticosteroids can result in varying degrees of immunosuppression. The increase in endogenous corticosteroids may be associated with stress or disease. The most common condition in horses associated with increased endogenous corticosteroids is pituitary pars intermedia dysfunction (PPID). In addition, corticosteroid treatment is the most common iatrogenic cause of immunosuppression. Regardless if endogenously produced or exogenous in origin, increased concentrations of corticosteroids may exacerbate preexisting infectious diseases or decrease resistance to environmental pathogens.18,19 Corticosteroids have a number of effects on the immune system, many of which are dose dependent. The effects of corticosteroids on the immune system are discussed later in “Drug-Induced Immunosuppression”. It is well recognized that nutritional status has a major impact on immune function, and nutritional deprivation produces impairment of immune response. Collectively called nutritionally acquired immunodeficiency syndrome (NAIDS), these immunologic dysfunctions include protein-calorie malnutrition and deficiencies of single nutrients. Protein-calorie malnutrition and deficiencies of single nutrients that assist in nucleic acid metabolism lead to dysfunction of the adaptive immune response, especially the T-cell functions, and result in atrophy of lymphoid tissues.20 In these individuals, the cell-mediated immunity and humoral response to T cell–dependent antigens are subnormal. Deficiency of single nutrients can impair production of key proteins by lymphocytes and phagocytic cells.20 Inadequate nutritional status may result from (1) increased and unmet nutritional requirement, including during growth, pregnancy, lactation, aging, and intense work, (2) disease state associated with altered nutrient intake such as conditions leading to hypophagia or anorexia, and (3) disease state associated with altered absorption of nutrients.21 Young animals are more likely to develop NAIDS, followed by geriatric patients, contributing to the greater risk of infections in both age groups. Age has been shown to affect immune function in multiple species. Altered immune function has been identified in both ends of the age spectrum, in foals and older horses. Foals are immunocompetent at birth, but their immune system is naive, therefore they rely on the transfer of maternal immunity through the colostrum for immunologic protection. Newborn foals have practically no circulating Igs of their own; however, they normally receive maternal transfer of colostral antibodies, especially IgG.22–25 As the foals age, the maternal antibody levels decline and no longer provide comprehensive protection, but at the same time they might interfere with the foals’ ability to mount an immune response. The immune response of foals differs from those of adults.22–25 Phagocytocytic ability of neutrophils (when autologous serum is used as opsonin) is impaired until 3 weeks of age.26 Killing capacity of neutrophil is reduced in neonates but gradually improves, reaching levels similar to adults by 3 months of age.26 Oxidative burst activity and phagocytosis by peripheral blood leukocytes (only if adult serum is used as opsonin) and LAK cell activity do not differ significantly between foals from birth to 6 months of age and adult horses.27 Total circulating lymphocyte counts at birth are similar to those of adult horses and increase linearly 2.5-fold from birth to 18 months of age.25,27,28 This increase in circulating lymphocyte counts results principally from an increase in B cells and CD4+ T cells.25,27–29 Age-dependent changes in humoral immune response from birth to 6 months of age include the gradual increase in the numbers of peripheral blood B cells and in circulating IgM above those found in adult horses, concomitant with decline of maternal Ig levels.25 Proliferative response of peripheral lymphocytes to mitogenic stimulation is slightly reduced at birth but rapidly increases.27 The expression of MHC-class II on the surface of peripheral blood mononuclear cells is low in neonatal foals and gradually increases.27 The vulnerability of foals between 2 and 4 months of age to respiratory tract infections, particularly the pathogens Rhodococcus equi and Pneumocystic jiroveci (formerly Pneumocystis carinii), was thought to reflect a general cellular immune-deficient state that is uncovered by the decline in protective maternal antibodies.22,28,30 Studies evaluating cytokine production by lymphocytes of foals from birth to 6 months of age have yielded conflicting results. Mitogen stimulation of lymphocytes from newborn foals was shown to evoke a limited production of IFN-γ due to a diminished ability to express the IFN-γ gene, associated with hypermethylation of the promoter of the IFN-γ gene.24,31 This ability was shown to increase steadily, reaching adult levels within the first year of life, suggesting that foals have an inherent inability to mount a Th1 immune response, which may contribute to their susceptibility to intracellular pathogens. Further studies showed that the number of IFN-γ and IL-4 producing cells vary with the type of mitogen used.31 Regardless of the mitogen, the number of IFN-γ producing cells was equally low in foals younger than a week of age and at 3 to 4 months of age, compared with adult horses.32 The number of IL-4 producing cells was low in foals younger than a week of age and even lower at 3 to 4 months of age.32 Expression of IFN-γ, IL-4, and IL-10 by mitogen-stimulated lymphocytes from foals at 36 hours and 5 days was minimal and gradually increased by 6 and 12 weeks of age, although it remained inferior to that of adult horses.33 Antigen-specific stimulation with R. equi of peripheral lymphocytes from foals during the first 3 weeks of life (i.e., days 1, 3, 7, 10, 15, and 20) induced expression of IFN-γ and IL-4 that was lowest at day 1 and gradually increased.34 Immunization of neonates with a killed adjuvanted vaccine invoked a modest induction of vaccine-specific humoral and lymphoproliferative immune responses.35 Both humoral and lymphoproliferative responses improved by 3 months of age, but their magnitude was far less than that of adult horses.35 On the other side of the age spectrum, aging has been associated with an increased susceptibility to infection.36,37 Immunosenescence is discussed in detail later in this chapter. Exercise represents not only an increased nutritional requirement but also a physiologic stress that can significantly alter the immune response.38,39 However, defining the precise effects of exercise on the equine immune system and susceptibility to disease has been difficult because of the complexity of the immune system; host factors (e.g., age, level of fitness); and the variable nature of exercise.36,38,39 In general, it appears that exercise may have both positive and negative effects on the immune response.36,38–44 Suppressive effects, such as a decline in the ratio of CD4+:CD8+ T cells, decreased lymphoproliferative responses, and suppression of the innate immune system, have been associated with strenuous high-intensity exercise, prolonged exhaustive exercise, or overtraining. In contrast, moderate exercise tends to have beneficial effects on the host defense mechanisms. Data in horses directly linking exercise-induced immunosuppression and increased susceptibility to infectious disease are limited. However, the potential immunosuppressive effects of exercise need to be recognized. In one study, unconditioned ponies vaccinated with a killed influenza vaccine and subjected to 5 days of strenuous exercise had an increased susceptibility to clinical influenza after challenge exposure compared with rested ponies.45 However, ponies with exercise-induced immunosuppression responded to the administration of an intranasal modified-live equine influenza vaccine and were protected from challenge.46 In a study of influenza infection in trained horses, moderate exercise led to increased signs of clinical disease, but the duration of disease was unaffected.47 Increased susceptibility to infections, either because of recurrence, chronicity, or severity of infectious illness beyond what is considered “normal,” raises the suspicion of inadequate immune response. Inadequacy of the immune response due to deficiency of the innate, humoral, or cell-mediated immunity, or any combination of these, predisposes animals to infections that might culminate with their death. Pathogens most often associated with immunodeficiencies in horses include infections by P. jiroveci, Candida spp., Aspergillus spp., adenovirus, Cryptosporidium, and R. equi in adult horses. General clinical features associated with immunodeficiencies include the following5,21,48,49: (1) onset of infections during the first 6 weeks of life, (2) repeated infections that respond poorly to standard therapy, (3) increased susceptibility to organisms with low pathogenicity, (4) infection with organisms rarely observed in immunocompetent individuals, (5) systemic illness after administration of attenuated live vaccines, (6) failure to respond to vaccination, and (7) persistent marked abnormalities in leukocyte numbers. Immunodeficiencies of the adaptive immune response can be classified according to (1) the site of defect in the host defense system and (2) whether the mechanism is primary or secondary.5,21,48,49 In primary disorders there is an inherent abnormality in the immune system that has a proven or suspected genetic basis, whereas in secondary disorders the host’s initially normal ability to respond immunologically is altered. Both primary and secondary immunodeficiencies can affect various components of the immune system, and by careful dissection of the immune response, the site of the defect can often be identified. Some drugs, toxicities, neoplasia, and microbial infections lead to immunosuppression.5,21,49 Immunosuppression associated with drugs, leukoproliferative and autoimmune conditions, and infectious processes are discussed later in this chapter. Table 53-3 depicts salient characteristics of immunodeficiency disorders of the horse. Table 53-4 lists some case reports of impaired or inadequate immune response in foals that do not fit any of the well-described immunodeficiency disorders. TABLE 53-3 Features of Immunodeficiency Disorders of Horses * Onset of signs varies with the degree of transfer of maternal antibodies. Onset of signs listed in this table is in face of adequate colostral transfer of maternal immunity. † Report of Caspian foal with clinical and postmortem findings of SCID but negative for SCID genetic testing. Abs, Antibodies; ConA, concavalin A; NR, not reported; PHA, phytohemagglutinin; PWM, pokeweed mitogen; QH, Quarter Horse; SCID, severe combined immunodeficiency; STB, Standardbred; THB, Thoroughbred; TIA, turbidimetric immunoassay; WMB, warmblood. TABLE 53-4 Reports of Impaired or Inadequate Immune Response in Foals ASB, American Saddlebred; ConA, concavalin A; LPS, lipopolysaccharide; NR, not reported; PHA, phytohemagglutinin; PWM, pokeweed mitogen; QH, Quarter Horse. Inherited disorders of the innate immune system such as lethal trait A46, Chédiak-Higashi syndrome, and leukocyte adhesion deficiency have not been identified in horses. Pelger-Huët anomaly, an inherited disorder of neutrophils, occurs in horses. Heritability of Pelger-Huët anomaly has been recently described in Arabian horses.50,51 Pelger-Huët neutrophils are characterized by reduced numbers of nuclear segmentation into lobes. They often resemble band forms, appearing mostly as bilobed dumbbell-shaped nuclei. Immunophenotyping of Pelger-Huët neutrophils of horses revealed normal expression of myeloid-specific markers and adhesion molecules, and evaluation of phagocytosis and oxidative burst via flow cytometry revealed normal neutrophil function.51 Similar to the condition in other species, the functions of Pelger-Huët neutrophils are virtually normal, and the neutrophil survival is not affected. However, their inflexible nuclei restrict their ability to emigrate from blood vessels. Nonetheless, no clinical signs of immunodeficiency are observed and this morphologic anomaly represents an incidental finding during the evaluation of blood smears. Inherited forms of complement dysfunction or factor deficiency have not been reported in the horse. However, depletion of complement C3 component associated with chronic infections, such as equine infectious anemia, has been reported. Moreover, the foal is born with low serum complement activity, and colostrum is not a significant source of complement. Therefore foals are transiently deficient in serum opsonic capacity and rely on their own production of complement after birth. In sepsis, complement C3 components are rapidly consumed, which delays the physiologic age-dependent increase otherwise observed in healthy foals.52 Inappropriate or exaggerated immunologic responses can directly injure bodily tissues. The clinical manifestation of immune-mediated injury depends on the nature and site(s) of tissue injury. Immune-mediated tissue injury can be from humoral or cell-mediated immune responses, or a combination of both. Immune-mediated tissue/organ damage, often referred to as hypersensitivity, has been classified according to the nature of the immunologic pathway involved. Comprehensive reviews of these disorders in the horse may be found elsewhere.1,53–56 Table 53-5 summarizes disorders caused by immune-mediated injury in the horse. IgE-mediated hypersensitivity reactions, often referred to as immediate or type I hypersensitivity, consist of responses to antigens mediated by antibodies of the IgE isotype.1,53–55 These reactions typically occur within 15 minutes from the time of reexposure to the triggering factor, unless the triggering antigen requires metabolic processing. Binding of the antigen (often referred to as allergen) to the IgE molecules bound to the surface of mast cells via high-affinity IgE receptors (FcεRI) leads to degranulation of mast cells and release of mediators such as histamine, serotonin, leukotrienes, prostaglandins, various chemokines, and cytokines. These mediators, broadly classified as to their ability to cause vasodilation, edema, smooth muscle contraction, and chemotaxis, are responsible for inflammation, which may be localized or systemic. Of clinical significance is that localized reactions may propagate to systemic reactions. The classic example of systemic IgE-mediated hypersensitivity is anaphylaxis. In horses anaphylaxis may be manifested as dyspnea, tachypnea, coughing, sweating, hypotension, and abdominal pain because the respiratory and gastrointestinal tracts are the shock organs in horses. Immune complex–mediated hypersensitivity reactions are also referred to as cytotoxic hypersensitivity. They include reactions to cell-associated antigenic epitopes, or type II hypersensitivity, and reactions to soluble antigens, or type III hypersensitivity.1,53–55 Immune complex–mediated hypersensitivity reactions to cell-associated antigenic epitopes or soluble antigens are mediated mostly by antibodies of the IgM or IgG isotypes. Some conditions are mediated by IgA isotypes. Immune complex formation activates complement and results in the deposition of C3b and iC3b. Complement deposition enhances destruction of antigen-bearing cells by formation of the MAC and by opsonization, which enhances phagocyte-mediated destruction. In addition, Igs also mark the cell target for ADCC by NK cells. Beyond cell destruction that is mediated by complement and ADCC, activation of C5a potently recruits neutrophils that release proteolytic enzymes (including acid phosphatases, acid hydrolytic enzymes, cathepsins, elastase, collagenase) and oxidative agents. Enzymatic and oxidative damage mediated by neutrophils and other chemoattracted cells leads to further tissue destruction. The binding of Ig molecules to antigenic epitopes on cells that are fixed in a tissue or on tissue structural elements, such as basement membrane, results in damage to the respective tissue. For instance, antibodies to pemphigus antigens of the keratinocyte desmosome are responsible for the immune complex–mediated damage that results in acantholysis, blistering, and desquamation in pemphigus complex.56 If the antigen-bearing cell is circulating, as is the case in neonatal isoerythrolysis, the cell may be destroyed in circulation but more typically is removed by the mononuclear phagocytic system. Examples of hypersensitivity to antigenic epitopes on circulating cells include alloimmune reactions (e.g., neonatal isoerythrolysis, neonatal alloimmune thrombocytopenia, neonatal allogeneic neutropenia) and drug hypersensitivities (e.g., penicillin-induced hemolytic anemia). In the case of soluble antigens that are not cell associated, Ig binding results in Ag-Ab complexes that deposit in a variety of vascular beds, including lymph nodes, kidneys, joints, and skin. Tissue in the vicinity of the precipitated Ag-Ab complexes is damaged by the ensuing immune reaction. An important condition characterized by hypersensitivity reaction to soluble antigens in horses is purpura hemorrhagica. Delayed hypersensitivity reactions, also referred to as type IV hypersensitivity, consist of exaggerated cell-mediated immune responses. Once sensitization has occurred, the reaction becomes evident within 24 to 48 hours from the time of reexposure to the triggering agent. Initial exposure leads to development of antigen-specific memory T cells that remain dormant until reexposure to the antigen. Following reexposure, APCs capture and process the antigen to present it to T cells in the context of MHC. This interaction results in IL-1 production by APC, as well as production of IL-2 and clonal expansion of the antigen-specific T cell of the Th1 phenotype, culminating with the production of large amounts of Th1 cytokines (IFN-γ, macrophage inhibitory factor, TNF-α, and TNF-β). This cascade of cytokines recruits nonspecific circulating leukocytes, including monocytes, segmented neutrophils, and lymphocytes to infiltrate the site. Following infiltration, which is responsible for the hallmark sign of induration in type IV hypersensitivity reactions, these chemoattracted cells also produce inflammatory mediators and cytokines that amplify the reaction. Examples of delayed-type hypersensitivity in horses include insect-bite hypersensitivity, contact dermatitis often to heavy metals and aniline dyes found in tack, bedding, soaps, and blankets.1,53–55 Lais R. Costa† Assessment of immune response encompasses in vivo, in vitro, and ex vivo measurements of immune function and can be divided into three categories: (1) assays for characterizing the adequacy of the immune response when an immunodeficiency or immunosuppression is suspected (e.g., assays to assess humoral, cellular, and innate immune responses); (2) assays for identifying inappropriate or exaggerated immunologic responses when immune-mediated injury is suspected (e.g., antinuclear antibody titer, assay for platelet surface–associated IgG, isotype-specific antibodies coating erythrocytes); and (3) assays of immunologic recognition of pathogens (e.g., antibody titer to specific antigens or pathogens) and protection against infectious agents.1–4 The aspects of immune response evaluation discussed in this section include only assays for characterizing the adequacy of the immune response when immunodeficiency or immunosuppression is suspected. Assays for identifying inappropriate immunologic responses when immune-mediated condition is suspected are discussed elsewhere.2–4 Assays of immunologic recognition of pathogens and protection against infectious agents are discussed in this book under body systems or found elsewhere.3,4 Assessment of the adequacy of a patient’s immune response is warranted when there is an increased recurrence, severity, or chronicity of infectious illness above and beyond what is considered normal, especially if the possibility of enhanced virulence of the pathogen involved has been ruled out. Because immunodeficiency disorders are uncommon, they should only be considered after more common causes of recurrent infection have been excluded. It is important to remember that inadequate immune response may occur in association with malnutrition, neoplasia (especially leukoproliferative disorders), autoimmune diseases, immunosuppressive therapy, and the presence of underlying diseases such as PPID. In addition, immunosuppression and immune evasion mediated by the infectious agent itself should be considered. Therefore the diagnostic plan should include evaluation of the patient’s immune response, as well as ruling out conditions that may account for the inadequate immune response of the patient. In foals, adequate transfer of maternal immunity can transiently mask the onset of signs resulting from immunodeficiency. Therefore immunodeficiency in foals with adequate passive transfer of maternal immunity may not be recognized early in life. Moreover, increased susceptibility to infections is often accompanied by failure to thrive and poor growth. Weight and height should be compared with breed-appropriate age standards, in order to recognize abnormal growth patterns. A list of assays to assess and characterize the adequacy of adaptive (humoral and cell-mediated) and innate immune responses is depicted in Table 53-6. In horses, only a limited number of assays to evaluate immune response are available from a practical standpoint, and most are crude indicators of immune response and therefore detect only severe deviations from normal. Nevertheless, a number of immunodeficiency syndromes have been characterized in horses. Moreover, the clinician may be able to characterize the patient’s immunosuppressive state. Reagent development for characterization of the equine immune response is evolving, and the large animal clinician is encouraged to search for up-to-date information on assays for equine immunology. Information can be found at the following websites: (1) available tests—http://www.vet.k-state.edu/depts/dmp/service/immunology/index.htm and http://www.vet.cornell.edu/labs/EquineImmuno/testOverview.cfm and (2) equine immunology reagents, including monoclonal antibodies to cell surface molecules, equine cytokine reagents and receptors, and cloned equine cytokines and chemokines—http://www.umass.edu/vetimm/equine/index.html and http://www.ca.uky.edu/gluck/HorohovDW_EIR.asp. TABLE 53-6 List of Assays to Assess and Characterize the Adequacy of Immune Response in Horses
Immunologic Disorders

Equine Immunologic Disorders
Overview of Immunologic Disorders in Horses
Normal Immune Response in Horses
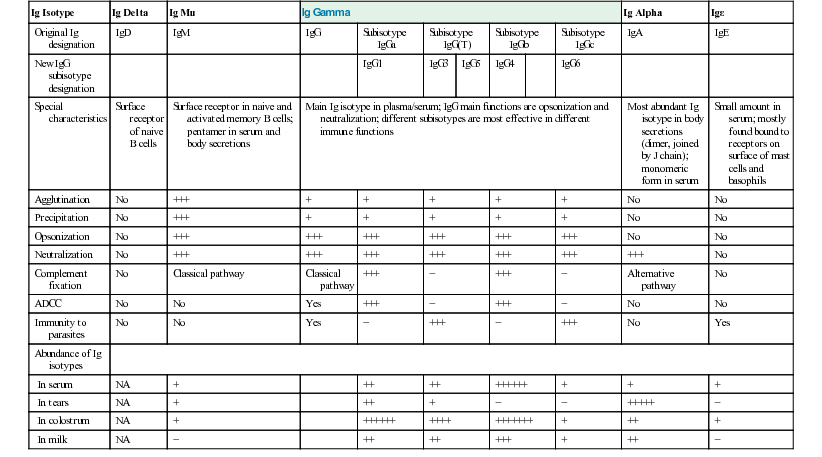
Ig Isotype
Ig Delta
Ig Mu
Ig Gamma
Ig Alpha
Igε
Original Ig designation
IgD
IgM
IgG
Subisotype IgGa
Subisotype IgG(T)
Subisotype IgGb
Subisotype IgGc
IgA
IgE
New IgG subisotype designation
IgG1
IgG3
IgG5
IgG4
IgG6
Special characteristics
Surface receptor of naive B cells
Surface receptor in naive and activated memory B cells; pentamer in serum and body secretions
Main Ig isotype in plasma/serum; IgG main functions are opsonization and neutralization; different subisotypes are most effective in different immune functions
Most abundant Ig isotype in body secretions (dimer, joined by J chain); monomeric form in serum
Small amount in serum; mostly found bound to receptors on surface of mast cells and basophils
Agglutination
No
+++
+
+
+
+
+
No
No
Precipitation
No
+++
+
+
+
+
+
No
No
Opsonization
No
+++
+++
+++
+++
+++
+++
No
No
Neutralization
No
+++
+++
+++
+++
+++
+++
+++
No
Complement fixation
No
Classical pathway
Classical pathway
+++
−
+++
−
Alternative pathway
No
ADCC
No
No
Yes
+++
−
+++
−
No
No
Immunity to parasites
No
No
Yes
−
+++
−
+++
No
Yes
Abundance of Ig isotypes
In serum
NA
+
++
++
++++++
+
+
+
In tears
NA
+
++
+
−
−
+++++
−
In colostrum
NA
+
++++++
++++
+++++++
+
++
+
In milk
NA
−
++
++
+++
+
++
−
Factors Associated with Inadequate Immune Response
Immunologic Disorders Resulting from Compromised or Deficient Immune Responses
Immunodeficiency Disorders Characterized by Defects of Components of the Adaptive Immune Response
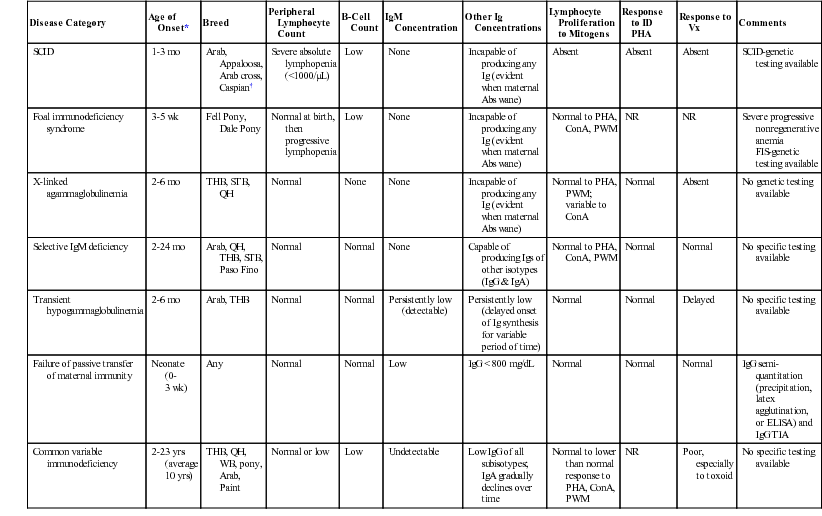
Disease Category
Age of Onset*
Breed
Peripheral Lymphocyte Count
B-Cell Count
IgM Concentration
Other Ig Concentrations
Lymphocyte Proliferation to Mitogens
Response to ID PHA
Response to Vx
Comments
SCID
1-3 mo
Arab, Appaloosa, Arab cross,
Caspian†
Severe absolute lymphopenia (<1000/µL)
Low
None
Incapable of producing any Ig (evident when maternal Abs wane)
Absent
Absent
Absent
SCID-genetic testing available
Foal immunodeficiency syndrome
3-5 wk
Fell Pony, Dale Pony
Normal at birth, then progressive lymphopenia
Low
None
Incapable of producing any Ig (evident when maternal Abs wane)
Normal to PHA, ConA, PWM
NR
NR
Severe progressive nonregenerative anemia
FIS-genetic testing available
X-linked agammaglobulinemia
2-6 mo
THB, STB, QH
Normal
None
None
Incapable of producing any Ig (evident when maternal Abs wane)
Normal to PHA, PWM; variable to ConA
Normal
Absent
No genetic testing available
Selective IgM deficiency
2-24 mo
Arab, QH, THB, STB, Paso Fino
Normal
Normal
None
Capable of producing Igs of other isotypes (IgG & IgA)
Normal to PHA, ConA, PWM
Normal
Normal
No specific testing available
Transient hypogammaglobulinemia
2-6 mo
Arab, THB
Normal
Normal
Persistently low (detectable)
Persistently low (delayed onset of Ig synthesis for variable period of time)
Normal
Normal
Delayed
No specific testing available
Failure of passive transfer of maternal immunity
Neonate (0-3 wk)
Any
Normal
Normal
Low
IgG < 800 mg/dL
Normal
Normal
Normal
IgG semi-quantitation (precipitation, latex agglutination, or ELISA) and IgG TIA
Common variable immunodeficiency
2-23 yrs (average 10 yrs)
THB, QH, WB, pony, Arab, Paint
Normal or low
Low
Undetectable
Low IgG of all subisotypes; IgA gradually declines over time
Normal to lower than normal response to PHA, ConA, PWM
NR
Poor, especially to toxoid
No specific testing available
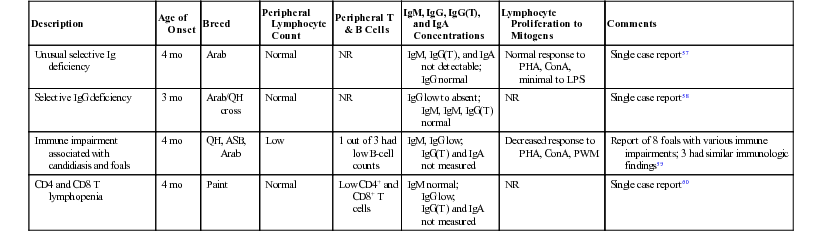
Description
Age of Onset
Breed
Peripheral Lymphocyte Count
Peripheral T & B Cells
IgM, IgG, IgG(T), and IgA Concentrations
Lymphocyte Proliferation to Mitogens
Comments
Unusual selective Ig deficiency
4 mo
Arab
Normal
NR
IgM, IgG(T), and IgA not detectable;
IgG normal
Normal response to PHA, ConA, minimal to LPS
Single case report57
Selective IgG deficiency
3 mo
Arab/QH cross
Normal
NR
IgG low to absent;
IgM, IgM, IgG(T) normal
NR
Single case report58
Immune impairment associated with candidiasis and foals
4 mo
QH, ASB, Arab
Low
1 out of 3 had low B-cell counts
IgM, IgG low;
IgG(T) and IgA not measured
Decreased response to PHA, ConA, PWM
Report of 8 foals with various immune impairments; 3 had similar immunologic findings59
CD4 and CD8 T lymphopenia
4 mo
Paint
Normal
Low CD4+ and CD8+ T cells
IgM normal;
IgG low;
IgG(T) and IgA not measured
NR
Single case report60
Immunodeficiencies Characterized by Defects of Components of the Innate Immune Response
Immunologic Disorders Leading to Immune-Mediated Injury
Evaluation of Immunologic Response in Horses
Characterizing the Adequacy of the Immune Response
Test
Description
Purpose
Comments
Complete blood count (CBC)3,5
Standard hemogram including evaluation of cell morphology.
Identify abnormalities in number and morphologic changes of lymphocytes, monocytes, neutrophils, and eosinophils.
Part of the initial assessment as the minimum database of any patient. Equine CBC can be performed by most clinical pathology laboratories.
Immunophenotyping of peripheral lymphocytes3,4,6–14
Enumeration of specific subsets of lymphocytes using fluorescent antibodies to cell surface markers and flow cytometry (FCM).
Determination of circulating lymphocyte types: B cells (CD19+ like, CD20+, CD21+, sIgM+), T cells (CD2+, CD3+, CD5+), CD4+ T cells, and CD8+ T cells, and determination of CD4:CD8 ratio.
In normal horses, about 20% of circulating lymphocytes are B cells, about 62% are CD4+ T cells, and 18% CD8+ T cells. Normal CD4:CD8 ratio is 3.5 ± 1.0. Peripheral blood lymphocyte phenotyping is offered at the Animal Heath Diagnostic Center—Cornell University, and at the Clinical Immunology Laboratory—Kansas State University.
Immunophenotyping of lymphocyte types and subsets in tissues10,11
Immunohistochemistry using antibodies to specific cell markers in order to sort out different lymphocyte types and subsets.
Determination of B cells (sIgM+), T cells (CD3+), CD4+ T cells (CD4+), and CD8+ T cells (CD8+) in lymphoid tissue specimens (such as lymph node, spleen, and bone marrow).
Enumeration of cell types in tissue samples may be done in formalin-fixed (with limitations), fresh, or frozen samples; the proper preparation of sample must be confirmed with the pathology laboratory performing the procedure.
Immunophenotyping of lymphocyte types and subsets in body fluids10,11
Immunohistochemistry or FCM using labeled antibodies to specific cell markers in order to sort out different lymphocyte types and subsets.
Determination of B cells (sIgM+), T cells (CD3+), CD4+ T cells (CD4+), and CD8+ T cells (CD8+) in samples from CSF, pleural fluid, peritoneal fluid, bone marrow, and lymph node aspirates.
Enumeration of cell types in body fluids and aspirates using immunocytochemistry is offered at the Animal Heath Diagnostic Center—Cornell University; FCM is offered at the Clinical Immunology Laboratory—Kansas State University.
Histologic evaluation of lymph node10,11,14
Histochemistry and immunohistochemistry with specific antibodies to B-cell markers.
Lack of germinal centers and decrease or absence of expression of B-cell markers are indicative of B-cell deficiency.
Offered at most diagnostic laboratories and at the Animal Health Diagnostic Center—Cornell University.
Serum protein electrophoresis3,4
Separation of serum proteins by gel electrophoresis.
To quantitate γ-globulins, which are primarily Igs, and determine if they are monoclonal or polyclonal.
In horses with repeated infections, serum Ig levels are likely to be high and polyclonal in nature. Monoclonal hypergammaglobulinemia is evidence of lymphocyte malignancy. Low levels of γ-globulins are supportive of Ig deficiency. Assay is offered at most clinical pathology laboratories.
Semiquantitation of immunoglobulins (Igs)3,15–18
(1) Precipitation of Igs using serial concentrations of specific salts (e.g., zinc sulfate or sodium sulfite precipitation, glutaraldehyde coagulation).
(2) Species-specific tests based on antigen-antibody reactions, either latex bead agglutination or enzyme-linked immunosorbent assay (ELISA).
Semiquantitative tests are quick and easy to perform and therefore useful for some conditions, such as failure of transfer of passive immunity after colostrum ingestion and plasma transfusion.
Commercial tests based on these principles are available: (1) zinc sulfate precipitation: Equi-Z test kit, VMRD, Inc.; (2) glutaraldehyde coagulation: GammaCheck E, Plasvacc USA; (3) latex bead agglutination: Foalchek, Centaur, Inc.; (3) ELISA: equine IgG ELISA, GenWay Biotech, Inc.; Horse IgG ELISA kit, Immunology Consultants Laboratory, Inc.; SNAP-Foal IgG Test, IDEXX Laboratories, Inc.
Precipitation of Igs can be used in several species, although the test may work better in some species than others. ELISAs are species specific. Specificity and sensitivity vary among tests, and scientific literature should be consulted.
Radial immunodiffusion (RID) for quantitation of Igs 3,15–18
RID quantitates Igs of specific isotypes using a precipitation reaction to species-specific, isotype-specific immunoglobulin (where the precipitation area is proportional to the concentration of Ig).
Accurate method for quantitating specific classes of Igs (e.g., IgG, IgM, IgA). RID requires incubation for 18-24 hr. Not recommended for evaluation of foals for failure of transfer of passive immunity after colostrum ingestion because of long turnaround time.
RID kits for many Ig isotypes of domestic animals are available commercially. Although test reagents are for a single species, some cross-reactivity exists among species but must be standardized and calibrated accordingly. RID kits for equine Ig isotypes are commercially available: RID for Equine IgG (Plasvacc USA); RID Test Kit (Kent Laboratories USA); Horse IgG Test (IDBiotech France). RID for IgG and IgM is performed at several veterinary clinical immunology laboratories. Specificity and sensitivity varies among tests and scientific literature should be consulted. RID kit for equine IgA is not currently available.
IgG turbidimetric immunoassay (TIA)19
TIA is based on the principles of immunologic agglutination and light scattering of the agglutination products. The automated TIA is performed on a chemistry analyzer.
Accurate method for quantitating equine IgG, with good correlation with RID but shorter turnaround time. TIA can be used to evaluate foals for failure of transfer of passive immunity after colostrum ingestion.
TIA is a quantitative test with high sensitivity and specificity. Kits for equine IgG TIA are commercially available: Midland Bioproducts Corporation, for use on Roche Cobas Mira chemistry analyzers and Hitachi 704 automatic analyzers; Equine IgG serum/plasma test kit from HAR-VET for use with DVM Rapid Test Instruments. TIA for equine IgG is offered in veterinary diagnostic laboratories (e.g., Animal Health Diagnostic Center—Cornell University).
Measurement of specific antibody responses pre vaccination and post vaccination10,11
Paired serum samples pre vaccination and 15 to 20 days post vaccination are tested to measure Ag-specific Ab response.
To determine B-cell function by evoking an anamnestic response to a protein Ag.
Serum tetanus toxoid Ab titers pre vaccination and post vaccination are offered at the Equine Clinical Immunology Laboratory—Cornell University.
Peripheral blood phagocytic functions of opsonization capacity, phagocytosis, and oxidative burst20–26
Peripheral blood leukocytes are incubated simultaneously with oxidative sensitive dye dihydrorhodamine (DHR), and inactivated Staphylococcus aureus labeled with red fluorescent dye propidium iodide (PI), opsonized with pooled or autologous serum (heat-inactivated serum or no serum used as controls). Oxidation of DHR generates green-fluorescent rhodamine 123 (rho). Two-color FCM analysis is performed for rho and PI, for oxidative burst and phagocytosis, respectively.
To evaluate functions of the innate immune response: opsonization by serum complement components and Igs, phagocytosis by neutrophils and monocytes, and oxidative burst by neutrophils.
The opsonization capacity and phagocytosis are generally performed for research purposes.
The oxidative burst activity in peripheral blood neutrophils is offered upon special request at the Equine Clinical Immunology Laboratory—Cornell University.
Intradermal (ID) skin testing with the plant lectin phytohemagglutinin (PHA)27
Measure the thickness of a skin fold before injection, then inject ID 50-µg of PHA dissolved in 0.5 mL of phosphate-buffered saline (PBS), and at a different site 10 cm (4 inches) away, 0.5 mL of PBS is injected as control. After 24 hr the skin thickness of both injection sites is measured.
PHA, unlike other compounds such as dinitrochlorobenzene, is capable of eliciting a delayed-type hypersensitivity response without requirement of prior sensitization. Increase in skin thickness should be greater than 0.6 mm; otherwise there is a defect in cell-mediated immunity.
The ID testing with PHA might yield false results; therefore confirmation with in vitro assay of lymphocyte blastogenesis assay is recommended.
Lymphocyte proliferation (blastogenesis) assay11–14
Lymphocytes are harvested from peripheral blood sample, incubated with serial concentrations of various mitogens, and labeled nucleotide. Incorporation of radiolabeled isotope into newly synthesized DNA is measured. Alternatively, proliferation analysis is evaluated using fluorescent-label and measured via FCM.
In vitro lymphocyte blastogenesis with pokeweed mitogen (PWM) requires both B- and T-cell function for normal responses, whereas in blastogenesis with PHA, concavalin A indicates primarily T-cell function.
No longer routinely offered. Generally performed for research purpose or academic interest by immunology laboratories (e.g., the Equine Clinical Immunology Laboratory—Cornell University).
Equine cytokine 5-plex assay28
Detects soluble equine IL-4, IL-10, IL-17, IFN-γ, and IFN-α in biological samples using paired monoclonal Abs specific for each cytokine and fluorescent bead/microspheres that are color coded.
Detects the concentration of each cytokine as pg/mL of biological sample in order to identify typical T-cell and antiviral cytokines in biological samples from horses.
Offered at the Animal Heath Diagnostic Center—Cornell University (see http://ahdc.vet.cornell.edu/docs/Equine_Cytokine_5plex_assay.pdf)
SCID genetic testing29
Collect a sample from a convenient source of DNA: (1) swab of the inside of the mouth with a mucosal brush and submit the brush containing mucosal cells, (2) blood sample in EDTA or ACD anticoagulant, or (3) pulled mane or tail hair, place in a clean bag, and submit to DNA testing.
A DNA-based carrier test for the genetic mutation affecting the DNA-dependent protein kinase (DNA-PK) located in chromosome 9.
Offered by VetGen, Ann Arbor, MI (see www.vetgen.com; http://www.vetgen.com/equine-scid-service.html)
Foal immunodeficiency syndrome genetic testing30
Collect a sample (pulled mane or tail hair), place in a clean bag, and submit to DNA testing.
A DNA-based carrier test for genetic defect affecting the sodium/myo-inositol cotransporter gene (SLC5A3) located in chromosome 26.
Offered at the Animal Health Trust, Newmarket, UK (see http://www.aht.org.uk/cms-display/genetics_fis.html) ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Immunologic Disorders
Chapter 53