12 Immunologic and Plasma Protein Disorders
Serum Total Protein and Albumin
Analysis
Total protein can be estimated in fluid, serum, or plasma (ethylenediaminetetraacetic acid [EDTA] or heparinized) by refractometry. Temperature-controlled refractometers calibrated for total protein are preferable to those that read only total solids (see comments under Artifacts).6 Total protein and albumin can be measured in serum, urine, or fluid by spectrophotometric or dry reagent methods. Serum globulin concentration is calculated by subtracting the serum albumin from the serum total protein.
Normal Values
TABLE 12-1 NORMAL SERUM TOTAL PROTEIN AND ALBUMIN VALUES (g/dl)
| DOGS | CATS | |
|---|---|---|
| Plasma total protein | 6.0–7.8 | 6.0–7.5 |
| Serum total protein | 5.5–7.5 | 5.5–7.8 |
| Serum albumin* | 2.5–4.0 | 2.5–4.0 |
* Serum globulin concentration should mirror serum albumin concentration.
Danger Values
Albumin less than or equal to 1.0 g/dl can be associated with major fluid shifts, but animals with concurrent increased portal hypertension can be at risk for abdominal effusion formation at higher values (i.e., >1.5 g/dl). Patients with severe hypoalbuminemia may also have decreased antithrombin III (AT III) activity (see under Causes of Hypoalbuminemia), thus also putting them at risk for thromboembolism of pulmonic, mesenteric, or portal vasculature.
Artifacts
Falsely increased values (refractometer) can result from lipemia, severe hyperglycemia, azotemia, and significant hypernatremia and hyperchloremia. Hyperbilirubinemia is historically listed as a cause for falsely increased serum total protein, but this likely occurs only with marked elevations.15 Likewise, hemolysis interferes with visual interpretation of total protein reading but does not directly interfere with measurement.6
Drug Therapy That May Alter Protein Values
Hormonal changes generally have a slight effect on serum proteins, even though physical changes (e.g., body weight, muscle mass) may be marked. Hyperproteinemia may be caused by anabolic steroids, progesterone, insulin, and thyroid hormones in people, but a similar effect is not expected in dogs and cats. Prolonged, high-dose corticosteroid therapy can cause hyperproteinemia and hyperalbuminemia in normal dogs, but values return to normal within weeks after cessation of therapy.15 Hypoproteinemia may be due to estrogens; hypoalbuminemia may be due to anticonvulsants, acetaminophen, estrogens, and various antineoplastic agents in people. Anticonvulsants and antineoplastic drug administration in dogs and cats are not expected to cause similar changes by themselves without associated underlying causes (e.g., hepatic cirrhosis).
Causes of Hypoalbuminemia
The first consideration is typically to concurrently determine the serum globulin concentration and determine if it is also similarly decreased (nonselective), or only albumin is decreased (selective). If both are decreased (i.e., panhypoproteinemia), nonselective causes for hypoproteinemia such as hemorrhage, exudation from severe skin lesions, protein-losing enteropathy (PLE), and hemodilution are usually more likely (Box 12-1). Overt bleeding should be apparent, but gastrointestinal hemorrhage can be difficult to determine if it is relatively mild and chronic. GI hemorrhage can be due to many types of gastrointestinal diseases (see Chapter 9), including PLE, but other nonspecific disorders such as hypoadrenocorticism are also of consideration. Causes of PLE are numerous and are discussed in Chapter 9. Although both serum albumin and globulin are often decreased in PLE, globulin concentrations may be normal to increased in some cases (especially those with other concurrent diseases such as heartworm infection, ehrlichiosis, or chronic skin disorders). Hemodilution rarely causes hypoalbuminemia but can occur due to intravenous fluid overload or plasma expanders (e.g., hetastarch), diseases causing edema (e.g., congestive heart failure), and rarely from excess antidiuretic hormone (ADH) secretion. Hemodilution usually causes mild decreases (albumin 2.1 to 2.4 g/dl), whereas PLE can cause moderate (1.5 to 2.0 g/dl) to severe (<1.5 g/dl) hypoalbuminemia.
Box 12-1 Causes of Hypoalbuminemia in Dogs and Cats
Chronic hepatic insufficiency*,1
Protein-losing nephropathy (PLN) because of glomerular disease*,1
Gastrointestinal: protein-losing enteropathy (PLE)*,2
Exudates from cutaneous lesions2
* Most common and important causes of serum albumin ≤2.0 g/dl. Other causes rarely, if ever, cause serum albumin ≤2.0 g/dl.
† Doubtful importance as a sole cause of serum albumin ≤2.0 g/dl. Probably more important as a contributing factor when there is another problem that results in hypoalbuminemia.
‡ Can be important in very young animals or animals fed diets that are extremely restricted in protein for prolonged periods.
Decreased albumin plus normal to increased globulins can be referred to as selective hypoalbuminemia. The most common and clinically significant causes are decreased albumin production from chronic hepatic insufficiency, increased loss from protein-losing nephropathy (PLN), or sequestration in a body cavity due to major effusion (see Box 12-1). Chronic hepatic insufficiency can produce marked hypoalbuminemia (<1.0 g/dl) if the liver is severely affected. Causes for hepatic insufficiency are numerous and can be congenital (e.g., congenital portosystemic shunt) or acquired (e.g., cirrhosis, neoplasia) (see Chapter 9). Hypoalbuminemia from PLN can be substantial (e.g., <2.0 g/dl, even <1.5 g/dl). If renal protein loss is detected, urinalysis ± urine protein : creatinine ratio (see Chapter 7) are typically indicated to confirm presence and severity of albuminuria. Causes for PLN are discussed in Chapter 7.
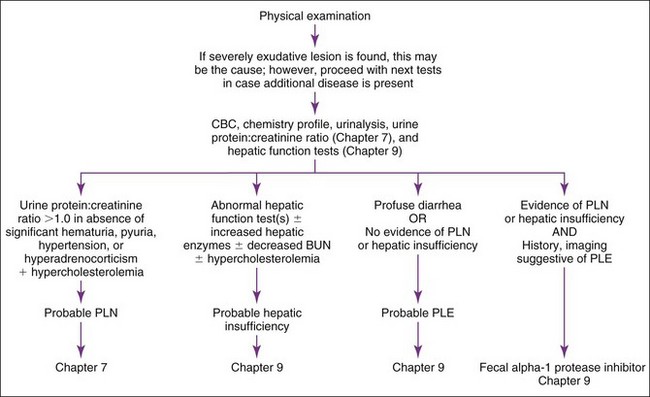
Basic diagnostic approach to hypoalbuminemic patients is outlined in Figure 12-1. Clinical pathology testing should include a complete blood count (CBC), clinical chemistry, and urinalysis on animals with physical exam findings suggestive of hypoalbuminemia. Serum bile acids and blood ammonia are indicated if hepatic insufficiency is suspected (see Chapter 9). If proteinuria is found without pyuria or hematuria and hypoalbuminemia is present, a urine protein : creatinine ratio (see Chapter 7) should be performed. Pyuria and hematuria can cause proteinuria, making it impossible to determine if there is glomerular loss; therefore follow-up urinalysis following resolution of pyuria or hematuria is indicated. An attempt should be made to categorize the degree of hypoalbuminemia (2.1 to 2.4 g/dl, mild; 1.5 to 2.0 g/dl, moderate; <1.5 g/dl, marked) in order to establish initial differential diagnoses. However, definitive exclusion of potential causes by this categorization should not occur until additional testing has been performed, because mild hypoalbuminemia can be observed in some cases of PLE and PLN and early hepatic insufficiency.
Next, recognizing certain patterns on diagnostic samples can be suggestive of different disease processes. Hypercholesterolemia plus hypoalbuminemia suggests PLN. Significant proteinuria without pyuria and hematuria indicates a diagnostic workup for PLN (see Chapter 7). Hypocholesterolemia plus hypoalbuminemia is suggestive of hepatic insufficiency or PLE. Hypoalbuminemia associated with hepatomegaly; microhepatia; neurologic signs; icterus; decreased blood urea nitrogen (BUN) with or without increased alanine aminotransferase (ALT), serum alkaline phosphatase (SAP), or both; or abnormal hepatic function test results (e.g., serum bile acids) indicates a diagnostic workup for hepatic insufficiency (see Chapter 9).
Note
ALT and SAP may be normal in many patients with severe hepatic disease, and decreased mean corpuscular volume (MCV) (see Chapter 2) is sometimes present in dogs with congenital portosystemic shunts.
A congenital portosystemic shunt is more likely in young animals; however, congenital shunts can be diagnosed in animals more than 10 years old. Acquired hepatic disease is more common in adults and requires hepatic biopsy for diagnosis; however, some dogs less than 1 year old have severe, acquired hepatic disease with acquired shunting. Hypoalbuminemia with normal hepatic function tests and absence of proteinuria or cutaneous lesions allows one to diagnose PLE by exclusion (see Chapter 9), even if feces are normal. If the patient has renal or hepatic disease and PLE is still a concern, then measurement of fecal alpha1-protease inhibitor concentrations (Chapter 9) may allow diagnosis of PLE by inclusion. Intestinal biopsy may then provide a definitive diagnosis of which intestinal disease is causing PLE. Endoscopic biopsies are safer than surgical biopsy, but it is critical that excellent-quality tissue samples be obtained; many endoscopically obtained samples are poor quality and nondiagnostic. If exploratory laparotomy is performed, hepatic biopsy should generally be performed along with intestinal biopsies. It is important to obtain biopsy specimens at several sites along the small intestine, even when no apparent gross lesions are found.
Causes of Altered Globulins
Changes in globulin levels are most often attributed to alterations in immunoglobulin values. Nonselective causes for hypoglobulinemia are similar to nonselective causes for hypoalbuminemia (see previous discussion of Causes of Hypoalbuminemia). True selective hypoglobulinemia (i.e., normal or increased albumin) occasionally occurs in dogs and cats from congenital or acquired immunodeficiencies. However, neonatal immunodeficiency patients are likely to succumb to this disorder early in life, and definitive diagnosis is often not established. Acquired immunodeficiencies are often secondary to chemotherapy or radiation therapy or directly from neoplastic transformation of lymphocytes (i.e., lymphoproliferative disorder) where antibody production is impaired or deficient. Hyperglobulinemia can be either nonselective (i.e., albumin elevated concurrently) from dehydration or selective due to three main processes, (1) acute phase protein increase (usually only induces mild elevation in globulins), (2) increased immunoglobulins from generalized antigenic stimulation with chronic inflammation, or (3) paraproteinemia (abnormal immunoglobulin production in blood) from a lymphoproliferative disorder (see following discussions).
Acute Phase Proteins
Occasionally Indicated
Acute phase protein analysis is performed in certain clinical situations where more specific information regarding inflammation or coagulation is needed. Collectively, acute phase proteins are part of the α and β globulins measured in protein electrophoresis (see Protein Electrophoresis later) and, in conjunction with gamma globulins, compose the globulin fraction of the total protein analysis. Acute phase proteins typically include fibrinogen, haptoglobin, C-reactive protein (see Chapter 9), complement (C3a), serum amyloid A, α1-acid glycoprotein, α1-antiprotease, transferrin, α2-macroglobulin, and ceruloplasmin.
Analysis
Normal Values
Normal reference intervals for fibrinogen in dogs and cats are generally accepted as approximately 100 to 300 mg/dl but are slightly variable depending on the methodology used to measure fibrinogen. Normal values on other acute phase proteins are variable depending on published data, so it is important to use reference intervals established at each laboratory performing the analysis.3
Causes of Increased Acute Phase Proteins
Some acute phase proteins increase with associated inflammation and are appropriately termed positive acute phase proteins. Hyperfibrinogenemia is one of the best indicators of acute inflammation in large animal species, but has traditionally not been utilized in small animal medicine. This concept may change because of more accurate methods for fibrinogen analysis being employed by reference laboratories. Regardless, elevated levels of fibrinogen are frequently seen in infectious disease (e.g., bacterial, viral, protozoal, fungal), trauma, neoplasia, and necrosis. C-reactive protein is increased in pregnant dogs, glucocorticoid therapy increases haptoglobin in dogs, and phenobarbital treatment in dogs can cause elevated α1-acid glycoprotein.8–10,15
Causes of Decreased Acute Phase Proteins
Acute phase proteins can decrease in patients as well and are often referred to as negative acute phase proteins when the decrease is associated with inflammation. Albumin (see previous discussion on Causes of Hypoalbuminemia) and transferrin are considered the classic negative acute phase proteins. However, other mechanisms such as lack of production or consumption can be associated with decreases in acute phase proteins that are not a result of the protein representing a negative acute phase protein. Hepatic insufficiency, if severe enough, can lead to decreased production levels of most acute phase proteins, similar to albumin. Fibrinogen, while most frequently considered a positive acute phase protein related to inflammation (see previous discussion on Causes of Increased Acute Phase Proteins), is equally clinically relevant in patients with decreased values. Primary consideration with hypofibrinogenemia is consumptive coagulopathy, such as disseminated intravascular coagulation (DIC). While not present in every case (in some instances fibrinogen is normal or increased), hypofibrinogenemia in conjunction with significant thrombocytopenia, decreased AT III, schistocytes, high D-dimer, and prolongation of activated partial thromboplastin time (aPTT) or prothrombin time (PT) is highly suggestive of DIC (see Chapter 5). Rare reports of inherited or congenital hypofibrinogenemia in dogs are documented.15
Protein Electrophoresis
Electrophoresis
Analysis
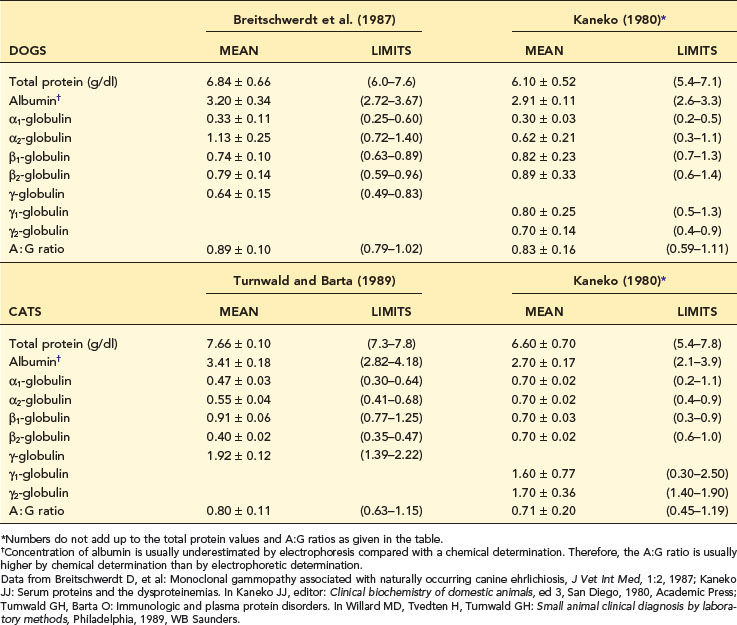
The cellulose acetate technique is the method of choice. Interpretation of electrophoretograms is based on densitometric measurements of intensity of staining of protein bands on cellulose acetate strips. Serum separates into four fractions: (1) albumin, (2) alpha (α) globulins, (3) beta (β) globulins, and (4) gamma (γ) globulins (Table 12-2). Canine and feline α- and β- globulins are usually divided into two subfractions each: α1, α2; β1, β2. Gamma globulins are usually listed as having one fraction composed primarily of immunoglobulin G (IgG), although older references do list subfractions γ1 and γ2. Important: Immunoglobulin M (IgM) and immunoglobulin A (IgA) antibodies will often appear in the β2 region or “bridge” the β2-to-γ region on electrophoresis. Normal-appearing electrophoretograms from dogs and cats are presented in Figures 12-2 and 12-3.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree