Fig. 18.1
Light micrographs of hematoxylin-eosin staining of paraffin sections of living mouse thymic tissues prepared by IVCT (a–f). (a) A low-magnification view of the thymic tissues shows widely panoramic tissue areas, extending from the superficial cortex (Co) under the capsule (Cp) to the deep medulla (M). (b–f) Each micrograph is a highly magnified view of the area enclosed with each rectangle in a and b. (b) Many small thymocytes, large epithelial reticulum cells, and open blood vessel s (asterisks) with flowing erythrocyte s are detected in the areas of the cortex (Co) and medulla (M). (c) Cortical blood capillaries with erythrocytes (asterisks) are seen in the subcapsular region in addition to capsular blood capillaries (asterisks). (d) The medulla (M) is composed of fewer thymocytes (arrows) and epithelial reticulum cells (arrowheads), and a Hassall’s corpuscle (HC) is also seen. (e) Corticomedullary boundary areas contain thick blood vessels (asterisks), extending into both cortical (Co) and medullary (M) areas. Their opening lumen is the well-delineated endothelial cell s and the basal lamina . (f) Note flowing erythrocytes in anastomosing blood capillaries of cortical tissues prepared with IVCT (asterisks). Arrows thymocytes, Arrowheads epithelial reticulum cells. Bars (a) 100 μm, (b, d–f) 50 μm, (c) 20 μm
18.3 Enzyme Histochemistry of Injected HRP in Cryosections of Thymic Tissues
Enzyme-reactive intensities of HRP with methyl green (MG) staining were detected at various time intervals in cryosections of mouse thymic tissues, which were injected with HRP (100 mg/ml) via left ventricle s (Fig. 18.2). The DAB reactivity of HRP with high sensitivity was not found in the interstitium of the cortex without HRP injection (Fig. 18.2e, inset). However, flowing erythrocyte s in the blood vessel s were usually positive for the DAB reaction in cryosections. Enzyme reaction products of injected HRP protein were serially localized not only in the blood vessels but also in the interstitial matrices of the thymic cortex of living mice (Fig. 18.2a–d). At 30 s after the HRP injection, enzyme reaction products were slightly detected in the interstitium around some thick blood vessels (Fig. 18.2a). Fine cortical vessels have diameters of 4–8 μm in largest dimension, as already shown in Fig. 18.1f. At 1 and 3 min, such enzyme reaction products were more widely found in the similar interstitium around thick blood vessels in the corticomedullary boundary areas (Fig. 18.2b, c). They appeared to be limited at variable distances in the interstitium from the permeable blood vessels. At 10 min, they were more diffusely detected in the interstitium of cortical areas and also detected in the interstitium of medullary areas (Fig. 18.2d). At 15 min, they were finally found to be distributed throughout all the interstitium of thymic tissues including both the cortex and medulla (Fig. 18.2e). In addition, at 30 min, HRP phagocytosis by many macrophages was seen throughout all the interstitium of the thymic tissues (Fig. 18.2f), which was accompanied by the rapid decrease of DAB reaction products in the interstitium of the cortex (Fig. 18.2f).
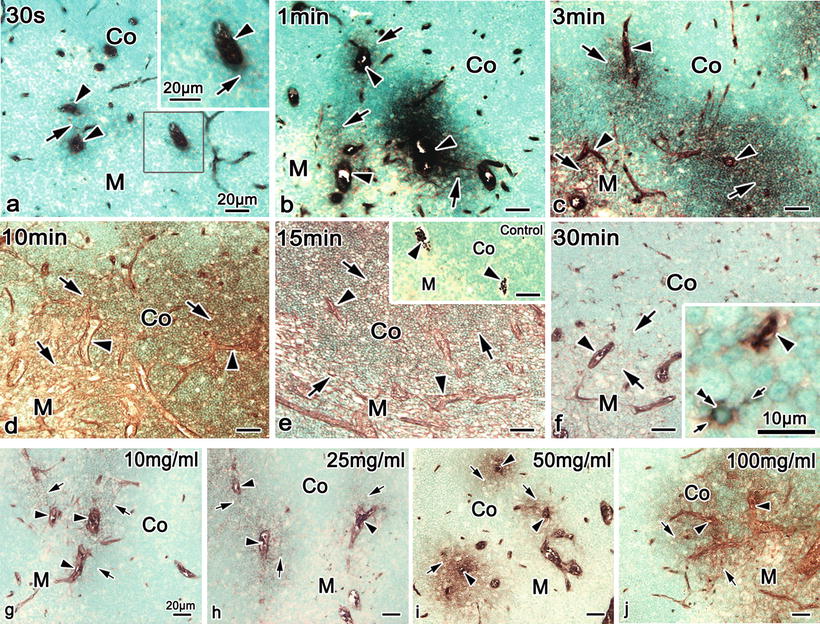

Fig. 18.2
Enzyme-histochemical images of DAB reaction of HRP in cryosections of mouse thymic tissues, which were injected with HRP (100 mg/ml) via left ventricle s and prepared after various time intervals by IVCT. (a) At 30 s, HRP reaction products are slightly detected in the interstitium around some thick blood vessel s in corticomedullary boundary areas (arrows) and also within other blood vessels (arrowheads). (b, c) At 1 and 3 min, they are more widely detected in the interstitium around thick blood vessels in corticomedullary boundary areas (arrows) and within some thick blood vessels in both cortical and medullary areas (arrowheads). (d) At 10 min, they are diffusely detected in all the interstitium of cortical and medullary areas (arrows). (e) At 15 min, they are uniformly distributed throughout all the interstitium of thymic tissues (arrows) in addition to perivascular spaces of blood vessels (arrowheads). Immunocontrol (inset). (f) At 30 min, HRP phagocytosis by macrophages (double arrowheads) is seen to be scattered, which is accompanied by the decrease of DAB reaction intensity in all interstitial matrices. The images (g–j), which were injected with various concentrations of HRP (10, 25, 50, and 100 mg/ml) via left ventricles and prepared at 3 min after HRP injection by IVCT. (g–j) HRP enzyme reactivities are variously detected in the interstitium (arrows) around some blood vessels in corticomedullary boundary areas and other thick blood vessels of the medulla, depending on the HRP concentration, but they are also found within blood vessels (arrowheads) in addition to tiny blood capillaries . Arrowhead blood vessel, Small arrow process, Co cortex, M medulla. Bars 20 μm, insets 10 μm
It was previously unknown that the leakage of HRP protein through the blood vessel s depends on not only molecular sizes or charges but also on molecular concentrations in the blood vessels. At 3 min after their injection via left ventricle s with lower concentrations of HRP, such as 10 and 25 mg/ml (Fig. 18.2g, h), a slight gradient of transudation into the interstitium through thick blood vessels was seen in the thymus of living mice, depending on the HRP concentration. The HRP enzyme reactivity was similarly detected in the interstitium around thick blood vessels in corticomedullary boundary areas and within some blood capillaries in the cortex (Fig. 18.2g, h). However, HRP enzyme reaction products were more heavily detected in the interstitium of inner cortical and medullary areas at higher concentrations of HRP, such as 50 and 100 mg/ml (Fig. 18.2i, j). A series of HRP injection s with different concentrations revealed that the distribution of exogenous HRP was also dependent on the concentration of injected HRP. Therefore, no HRP was detected in the interstitium around blood capillaries in outer cortical areas at any concentration of injected HRP.
18.4 Immunolocalizations of HRP in Paraffin Sections and HRP Phagocytosis by Macrophages
To reexamine the thymic tissues at a higher resolution, both HE staining and HRP immunostaining were performed in serial paraffin sections of living mouse thymic tissues after short time intervals in the order of seconds to minutes and were injected with a lower concentration of HRP (25 mg/ml) via the left ventricle . At 30 s and 1 min (Fig. 18.3a–d), HRP immunoreactivity was detected only within the blood vessel s . At 3 and 10 min, however, it was weakly detected in the interstitium around the blood vessels in corticomedullary boundary areas and within some blood capillaries in cortical areas (Fig. 18.3e–h). A striking feature was that immunostaining changes involved the inner cortex and especially the corticomedullary boundary of thymic tissues (Fig. 18.3f, h). Perivascular spaces of thick blood vessels were also immunopositive for HRP (Fig. 18.3f, h, insets). At 15 min, HRP immunoreactivity was more widely seen in the interstitium of the thymic cortex (Fig. 18.3i, j, arrows), but less than in the blood vessels (Fig. 18.3i, j, arrowheads). At 30 min, it was a little more heavily detected in the interstitium of the medulla (Fig. 18.3l, m) as compared with the cortex. The permeability of HRP across the blood capillary walls was hardly seen in the outer cortical areas , as shown in Fig. 18.3f, h. In addition, HRP phagocytosis by macrophages was clearly seen at 30 min after the HRP injection (Fig. 18.3m, double arrowheads), which was similar to that revealed by enzyme histochemistry of HRP (Fig. 18.2f).
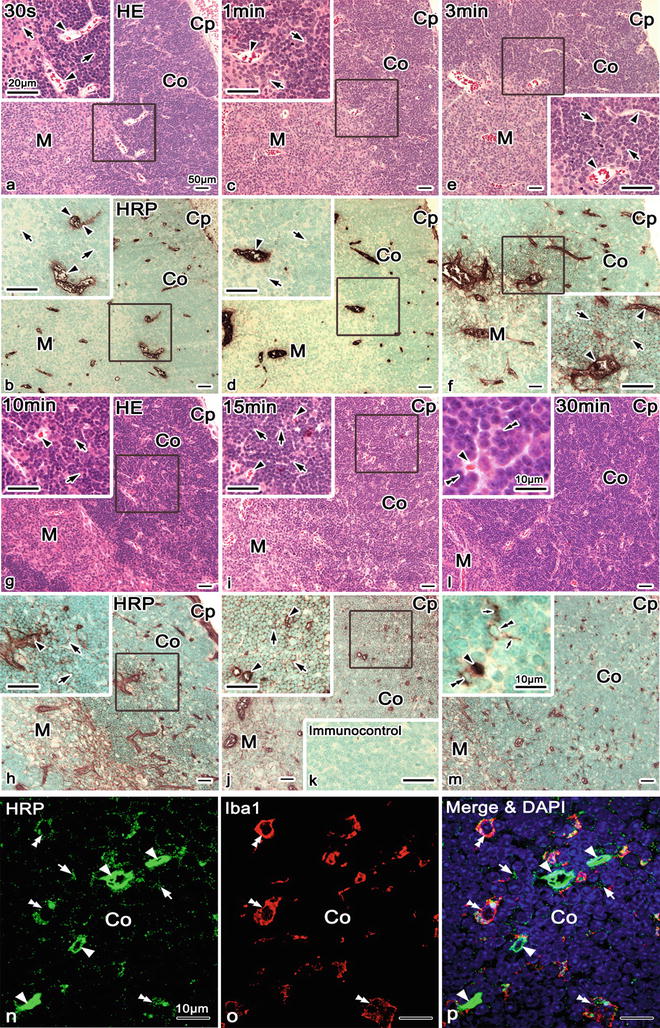

Fig. 18.3




Light microscopic images of hematoxylin-eosin (HE) staining (a, c, e, g, i, l) and HRP immunostaining (b, d, f, h, j, m) in serial paraffin sections of mouse thymic tissues, which were injected with a lower concentration of HRP (25 mg/ml) via left ventricle s and prepared after various time intervals by IVCT. (a–d) At 30 s and 1 min, HRP immunoreactivity is detected only within blood vessel s (arrowheads), not in the interstitium around them. (e, f) However, at 3 min, it is weakly detected in the interstitium (arrows) around blood vessels in corticomedullary boundary areas and within some thick blood vessels (arrowheads). (g, h) At 10 min, HRP immunoreactivity is also detected in the interstitium (arrows) around blood vessels in corticomedullary boundary areas and within thick blood vessels (arrowhead). (i, j) At 15 min, it is more widely seen in the interstitium (arrows), as compared with blood vessels (arrowheads). (k) Immunocontrol. (l, m) In addition, HRP phagocytosis by macrophages (double arrowheads) is seen at 30 min after HRP injection . The immunofluorescence micrographs (n–p) of cryosections of thymic cortical areas at 30 min after HRP (100 mg/ml) injection via left ventricles of living mice, prepared by IVCT. Double-immunofluorescence staining for HRP (n, p, green) and ionized calcium-binding adapter molecule 1 (Iba1) (o, p, red) with nuclear DAPI staining (blue) show their colocalization in almost all macrophages. Iba1 immunofluorescence shows the macrophages of thymic tissues (o, p, double arrowheads), which contain HRP in their cytoplasm (n, p, double arrowheads). HRP immunostaining is still detected within the blood vessels (n, p, arrowheads) and some areas of the cortical interstitium among compact thymocytes (n, p, arrows). Arrowheads blood vessels, Small arrows processes, Co cortex, Bars 10 μm, Cp capsule, Co cortex, M medulla. Bars 10, 50 μm; insets 10, 20 μm
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


