Fig. 8.1
Light micrographs of HE staining (HE; a, b), immunostaining for IgA (c), Igκ (d), IgG1 (e), and IgM (f), confocal laser scanning micrographs of double-fluorescence immunostaining for IgA (g), IgG1 (h), and merge (i) in paraffin sections of the mouse small intestines prepared with IVCT. (a) and (b) Flowing erythrocytes are well preserved in open blood vessel s (arrowheads in a, b). (b) Higher magnified view of the rectangular part in (a). The epithelial morphology is well preserved with few ice crystal s at the light microscopic level (arrows in b). (c) Many IgA-immunopositive plasma cell s are mainly observed in intestinal villi (white arrowheads). Epithelial cells are also IgA-immunopositive along intestinal crypts (small inset) and villi (large inset). (d) Igκ-immunopositive plasma cells (white arrowheads) are abundant in the villi and crypt areas. (e) IgG1 immunoreactivity is detected in interstitial matrices of intestinal villi and crypts, as well as inside blood vessels (arrowheads), whereas it is hardly detected in the cytoplasm of epithelial cell s . (f) IgM immunoreactivity is detected only in blood vessels (arrowheads). (g–i) IgA immunoreactivity (green in a, c) is detected in the cytoplasm of epithelial cells (asterisks), as well as plasma cells in the lamina propria mucosa. However, IgG1 immunoreactivity (red in h, i) is detected in some plasma cells (arrowheads) and blood vessels (arrows). (i) From a color-merged image of (g) and (h), IgA- and IgG1-double immunopositive plasma cells are identified in the intestinal mucosa (arrowheads; yellow). Cr crypt, ML muscle layer, Vi villi. Bars: (a–f) 100 μm, (g–i) 50 μm (From Shimo S et al., J Immunol Methods 361(1–2):64–74, 2010)
Furthermore, to examine what class of Ig was produced in identical plasma cell s and immunolocalized in epithelial cell s of the intestinal mucosa, double-fluorescence immunostaining for IgA and IgG1 was performed for tissue samples, as prepared with IVCT-FS which were examined with confocal laser scanning microscopy (Fig. 8.1g–i). The IgA immunoreactivity was clearly detected in almost all plasma cells in the lamina propria mucosa, as well as in the cytoplasm of some epithelial cells (Fig. 8.1g, i). In addition, small granular immunoreaction products were more clearly observed in the apical cytoplasm of the epithelial cells at some optical section levels under the confocal laser scanning microscope (asterisks in Fig. 8.1g). However, the IgG 1 immunoreactivity was mainly detected in blood vessel s of intestinal villi and a few plasma cells at these intestinal areas (Fig. 8.1h, i). IVCT is reasonable to prevent translocation of soluble protein s that easily occurs during the conventional chemical fixation of aqueous solution s [12, 13] because it can immediately capture all dynamic biological movements within milliseconds at the time of freezing, as already shown by our time-dependent studies of signal molecules with IVCT [14, 15]. In addition, artificial antigen masking of various target molecules, which was sometimes problematic on immunohistochemistry [16–18], was also improved with IVCT followed by FS, as reported before [19]. The well-frozen tissue areas with few visible ice crystal s were within 300–400 μm from the frozen tissue surface at the light microscopic level. At the electron microscopic level, however, meshwork-like structures damaged by the tiny ice crystal formation were usually observed in the deep frozen tissues of living animal organ s , and these ice crystal sizes always depended on the distance from the frozen tissue surface [20]. These fine meshwork-like structures probably aid more effective penetration of antibodies during immunohistochemical steps, resulting in formation of antigen-antibody complexes [19].
8.3 Electron Micrographs of Epithelial Cells in Intestinal Tissues Prepared by IVCT
At the electron microscopic level, the uppermost part of the cytoplasm or the cuticular border of epithelial cell s consists of numerous microvilli , containing filaments that extend into the deep cytoplasm and intermingle with the terminal web, as shown in Fig. 8.2. No cytoplasmic organelles of the terminal web region were seen, but the actin microfilament bundles were organized along their entire length, as well as microfilament networks in the terminal web [21, 22]. On the other hand, many cytoplasmic organelles including numerous mitochondria, endoplasmic reticulum, and ribosome were localized under the terminal web region, as shown in Fig. 8.2c, d. Morphological alteration was probably due to rapidly changing solute permeability of cell membranes during perfusion -fixation with artificial perfusion pressure [23–25]. Such relatively high pressure in the interstitium during perfusion-fixation may also cause other dilations in intercellular spaces between epithelial cells, such as lateral interdigitation and alterations in the total cell sizes. Therefore, careful attention must be paid to preserve the native ultrastructure of cells and tissues and not lose them during the tissue preparation steps, which may be compatible with functional analyses by in situ imaging to visualize the intestinal mucosal immunity [26–28].
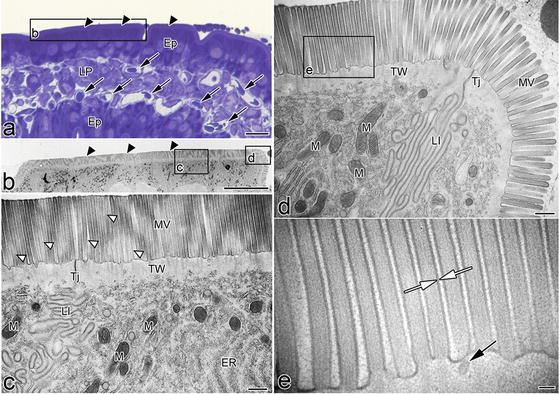

Fig. 8.2
Electron micrographs of the apical cytoplasm of living mouse intestinal epithelial cell s prepared by IVCT. (a) Epon thick section stained with toluidine blue . The cuticular border and open blood capillaries are seen (arrowheads and arrows). (b) At lower magnification, microvilli show a conspicuous surface structure. This layer is the band that shows low-intensity staining with toluidine blue. In regions under the microvilli, the electron-lucent band appears to be separated from the deeper cytoplasm. (c) The microvilli of epithelial cells are frequently seen to have different electron density from the slicing plane because they are waving back and forth (white arrowheads). Furthermore, many cytoplasmic organelles are abundantly localized under the terminal web, such as electron-dense mitochondria, obliquely cut endoplasmic reticulum, and ribosome. (e) At a higher magnification in (d), the separating inner or outer leaflets of the apposed cell membranes are visible along the microvilli (white arrows). Ep epithelial cell, ER endoplasmic reticulum, LI lateral interdigitation, LP lamina propria, M mitochondria, MV microvilli, TJ tight junction , TW terminal web. Bars: (a, b) 10 μm, (c, d) 500 nm, (e) 100 nm (From Shimo S et al., Microscopy 64(3):189–203, 2015)
8.4 Immunolocalizations of IgA, J-Chain, and Igκ in Epon Thick Sections
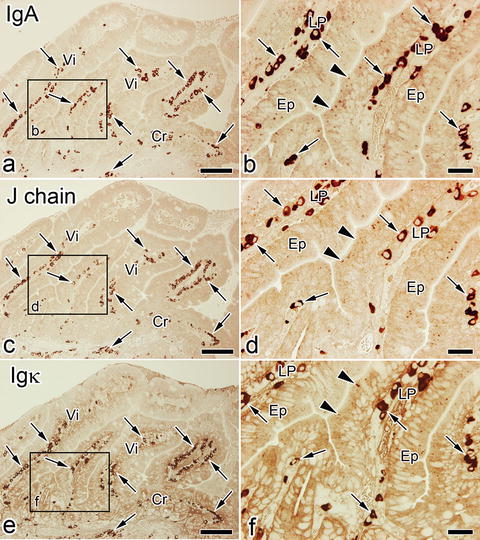
Immunolocalizations of soluble IgA, J-chain , and Igκ were compared with each other in the small intestinal tissues of living mice, which were prepared by IVCT. By IVCT, IgA-immunopositive plasma cell s were detected from the middle to the basal areas of the lamina propria, and also small granular immunoreaction products were clearly detected in the apical cytoplasm of epithelial cell s in intestinal villi, as shown in Fig. 8.3a, b. J-chain- and Igκ-immunopositive plasma cells were also observed in the same areas of the lamina propria. However, the correspondence of Epon-embedded thick sections to electron microscopic images has been difficult to achieve because of much less localization of IgA in some apical areas of epithelial cells. Immunostaining for IgA, small granular immunoreaction products were detected in the epithelial cells, corresponding to vesicular structures, which were revealed in an ultrathin section by electron microscopy , as shown in Fig. 8.2, by using the in vivo cryoapparatu s (Eiko Engineering, Hitachinaka, Ibaraki, Japan) in combination with the cryoknife with cryogen [29].