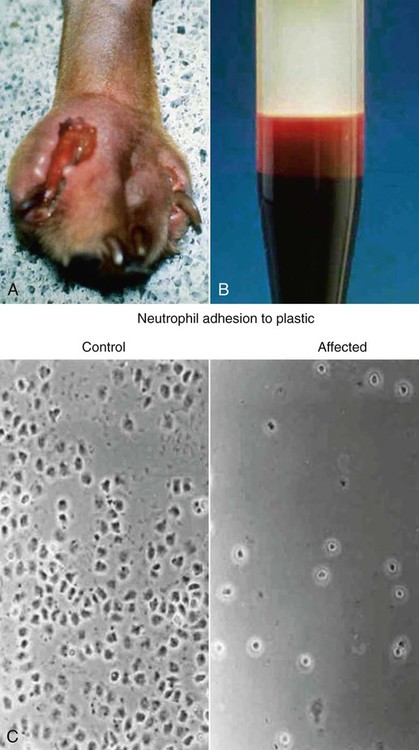
Immunodeficiencies are a large heterogeneous group of hereditary and acquired disorders of host immunity that can be associated with an increased risk of infection (Table 94-1).57,97,115,126 They can arise through disturbances in antigen-specific defense mechanisms mediated by lymphocytes, the nonspecific defense system (which includes phagocytes, plasma proteins, and physical barriers), or both. Although the exact pathogenesis of many canine and feline immunodeficiencies remains unknown, the molecular defects for several forms have been elucidated. Because many effective preventive and therapeutic measures are now available to control infectious diseases in individuals with intact host defense mechanisms, other animals with persistent, antimicrobial-unresponsive infections likely suffer from an immunodeficiency disorder. A few hereditary immunodeficiency disorders are prevalent within certain breeds of dogs and cats, whereas others have been described once and may exist only in research animal colonies. TABLE 94-1 Features of Immunodeficiency Diseases Based on the Underlying Defect Certain major clinical features suggest that a patient has an impaired immune system (Box 94-1). Specific organisms and medical conditions often implicated in immunocompromised hosts are listed in Box 94-2. A definitive diagnosis often requires specific immune testing in addition to routine laboratory tests, and therapeutic interventions are limited. Immunodeficiencies can be divided into primary or secondary forms depending on whether they are inherited or acquired. Many genetically determined immune defects have been described in the dog, whereas only a few are known in cats. They occur rarely and are summarized in Table 94-2; some are discussed next in more detail in the Specific Primary or Inherited Immunodeficiencies section of this chapter. They can be broadly classified according to defects of the specific or nonspecific immune system as well as combinations thereof.92 The nonspecific immune system, also known as innate or natural immunity, should be functional at birth and available on short notice to protect the host from invasion by all sorts of organisms. It includes physicochemical barriers, phagocytes, complement and other plasma proteins, and natural killer cells. Congenital barrier defects particularly involve the skin and mucous membrane surfaces and are associated with infections of particular organs. A variety of hereditary skin diseases are being further defined and other immunodeficiencies are being recognized. The Ehlers-Danlos syndrome, causing fragile, hyperextendable skin in many dogs and cats as well as the myxedematous skin and immunodeficiencies of shar-peis, predisposes the animals to pyoderma, whereas ciliary dyskinesia in dogs increases the susceptibility to rhinosinusitis and pneumonia. Similarly, X-chromosomal ectodermal dysplasia in German shepherd dogs is associated with skin problems as well as other immunodeficiencies. TABLE 94-2 Primary or Hereditary Immunodeficiencies of Dogs and Catsa AD, Autosomal dominant; AR, autosomal recessive; G-CSF, granulocyte colony-stimulating factor; IL, interleukin; U, unknown; XR, X-linked recessive. aFor additional references, see Reference 102. In addition to the listed syndromes, bone marrow dyscrasias have been described in miniature and toy poodles and transient hypogammaglobulinemia in Samoyeds, but less is known of these disorders. bFor example, springer spaniel, old English sheepdog, English setter, West Highland white terrier, pointer. cReferences 75, 117, 243, 283–285. dReferences 80, 97, 98, 140, 150, 264–267. The specific immune system can be divided into humoral and cell-mediated immune systems and includes B and T lymphocytes, immunoglobulins, and cytokines.92 Deficiencies of B lymphocytes or humoral immunity affect the production of immunoglobulins and lead to increased susceptibility to pyogenic bacterial infections. Deficiencies of T lymphocytes or cell-mediated immunity (CMI) are associated with viral and fungal infections, but intracellular bacterial infections may also occur. Animals with cellular immunodeficiencies may have smaller thymic and tonsillar tissues as well as intestinal and peripheral lymph nodes and decreased numbers of circulating lymphocytes. The degree of immunodeficiency varies greatly between defects. Infections may be systemic or restricted to a particular organ system such as the skin or respiratory tract. Some immunodeficiencies in neonates lead to overwhelming infections and death within the first few days to weeks of life, whereas others, such as morphologic leukocyte changes, are not consistently associated with any noticeable predisposition to infection. Chédiak-Higashi syndrome in smoke-colored Persian cats is characterized by abnormally large eosinophilic granules in polymorphonuclear leukocytes. It causes no immunodeficiencies but does cause a bleeding tendency resulting from a platelet storage pool disease.206 Similarly, birman cats with acidophilic granulation of neutrophils and dogs and cats with various lysosomal storage diseases (e.g., mucopolysaccharidosis, gangliosidosis, mannosidosis) have granulation or vacuolation of leukocytes without being immunocompromised; frequently they also exhibit a lymphocytosis. The Pelger-Huët anomaly, which is characterized by hyposegmentation of granulocytes, causes no immunodeficiency in animals, despite the fact that the leukograms of affected dogs and cats reveal the most severe left shift with a normal leukocyte count. In foxhounds, in vitro chemotactic response of these neutrophils was mildly diminished, whereas in basenjis no functional abnormalities have been found.22,170,170 Although an increased susceptibility to opportunistic infections develops, the type of infection varies depending on the type of defect within the immune system. A few immunodeficiency disorders predispose animals to a restricted group of unusual infectious agents. Some male dachshunds appear to be predisposed to Pneumocystis pneumonia, and German shepherd dogs may be prone to systemic aspergillosis, other fungal infections or rickettsioses.166 Doberman pinschers and Rottweiler dogs are more likely to develop parvoviral disease. Golden and Labrador retrievers and Bernese mountain dogs that had high serum antibody titers to Borrelia burgdorferi were more likely to have glomerulonephritis.64 Basset hounds and miniature schnauzers have an increased susceptibility to systemic avian mycobacteriosis, toxoplasmosis, and neosporosis. American and English foxhounds appear to be predisposed to developing leishmaniasis. Great Danes and Dobermans may be more susceptible to cryptococcal infections.190 A genetic predisposition to demodicosis has been proposed in various canine breeds and families within breeds. The predisposition to develop feline infectious peritonitis (FIP) has also been suggested to have a genetic basis, more commonly in some purebred cats (see Chapter 10). The mechanisms predisposing particular animals to specific infections remain unknown in many breeds. The previously mentioned key signs of infection (see Box 94-1) develop in animals with a primary immunodeficiency generally early in life. Despite receiving colostrum, clinically affected animals can have illness during the neonatal to juvenile period and can develop recurrent and overwhelming infections that lead to severe debilitation and death before 1 year of age. Several animals, but typically not all, in a litter may be affected, whereas the parents are usually clinically healthy. A genetic predisposition to infection is rarely noted after 1 year of age (e.g., avian tuberculosis in basset hounds). Furthermore, animals with primary immunodeficiencies may have other special clinical manifestations. Hypersensitivity reactions can occur and reflect an overall dysregulation of the immune system caused by a lack of one or more components or a chronic antigen stimulation from inadequate clearance of infections. Chronic systemic infections may also hamper the animal’s growth rate (Fig. 94-1). Characteristic coat color dilutions and increased tendency for surface bleeding are seen for example in collies with cyclic hematopoiesis, Persian cats with Chédiak-Higashi syndrome, and Weimaraners with an incompletely defined immunodeficiency. Nude birman kittens and ectodermal dysplasia are associated with a complete lack or loss of hair. All components of the immune system of animals with secondary immunodeficiencies are initially intact and functional but become transiently or permanently impaired during or after an underlying disease condition or exposure to certain agents. Thus, secondary immunodeficiencies occur much more commonly than primary forms and are associated with organ impairment. Depending of the type and severity of the immunosuppression, resulting opportunistic infections may be manifested by a localized or systemic spread. Various barrier disturbances lead to surface infections, such as respiratory tract, urogenital, dermatologic, and gastrointestinal (GI) infections. Furthermore, certain infections, particularly viral diseases in cats, directly impair the immune system, predisposing the animals to secondary bacterial, fungal, or protozoal infections. For example, cats infected with the feline immunodeficiency virus (FIV) harbor more diverse fungal microflora on the hair and mucosal surfaces than noninfected cats,262 which may predispose the FIV-infected cats to a greater prevalence of secondary fungal infections. Feline leukemia virus (FeLV)-infected cats are also more likely than uninfected cats to have clinical illness with hemotropic Mycoplasma infections. Puppies and kittens are particularly vulnerable to infections because of their incompletely developed immune systems. Colostrum intake only during the first day of life transfers maternal-derived antibodies (MDAs) and provides protection during the first few weeks of life in small animals. Although colostrum deprivation has been shown in large animals to result in major neonatal losses, neonatal kittens and puppies kept in high-quality catteries or kennels do not seem to be extremely predisposed to infections. However, a transient hypoglobulinemia after the decline of MDAs in the plasma and before the immunocompetency of 2- to 4-month-old animals can increase the susceptibility to various infections.261 Similarly, older animals can again become immunocompromised and susceptible to infection. Various drugs and chemicals as well as nutritional deficiencies can drastically impair the production and function of leukocytes. Known secondary immunodeficiencies in companion animals are listed in Box 94-3, and some are discussed in detail at the end of this chapter. Although an immunodeficiency may be suspected on the basis of clinical evidence, specific laboratory tests are generally required to reach a definitive diagnosis.113 A minimum database of information, including results of a complete blood count, serum chemistry screen, and urinalysis, should always be obtained and can suggest a specific disorder. Results of differential leukocyte count and microscopic evaluation of a blood smear are most important. Neutropenia in the presence of an active bacterial infection is by far the most feared condition. It should be noted that generally, some breeds, such as greyhounds, have low reference values for blood leukocyte counts. Neutropenia can be transient, as it occurs with cyclic hematopoiesis every 12 to 14 days or with parvovirus infection, or can be persistent, as it is seen in animals with cobalamin malabsorption defects or overwhelming infections (sepsis). Lymphopenia may be observed in dogs with a T-cell disorder or SCID. Although leukocytosis is expected during periods of infection, defects in leukocyte adhesion and egress from blood circulation at sites of infection can be associated with disproportionately high leukocytosis for the degree of infection as seen with hereditary LAD or with concurrent glucocorticoid usage. Dachshunds with Pneumocystis pneumonia also have very marked leukocytosis. Nonregenerative anemia with normocytosis or microcytosis, often observed in infected animals, is caused by several factors related to the persistent inflammatory disease state. The erythrocyte count can be within reference limits even if the animals have active infections and during periods of treatment and remission. Careful review of a blood smear can reveal leukocyte abnormalities such as granulation and vacuolation resulting from lysosomal storage diseases or Chédiak-Higashi syndrome,165 acidophilic granulation of leukocytes in birmans,142 phagocytized microorganisms, or toxic leukocyte changes that suggest overwhelming bacterial infections. T-cell or combined immunodeficiencies cause defective CMI responses. The animal may have prolonged allograft rejection times and decreased delayed-type hypersensitivity to skin testing with viral vaccines, tuberculin, or dinitrochlorobenzene.223 Reduced in vitro lymphocyte stimulation results can also be caused by a primary lymphocyte defect or the infection. No practical treatments for primary immunodeficiencies exist, except for parenteral cobalamin administration to animals with hereditary cobalamin malabsorption. Immunocompromised animals with infection generally have a guarded to poor prognosis. Despite aggressive antimicrobial therapy, their infections are difficult to control, leading to overwhelming infections, protracted courses, and recurrences. Some leukocyte defects cause death before 1 year of age, whereas others may not lead to a markedly increased predisposition to infection. In experimental studies, bone marrow transplantation and gene therapy corrected several canine leukocyte defects.137,184 Dogs with hereditary immunodeficiencies and other genetic defects have served as intermediate test subjects between experiments in mice with genetic alterations and treatment of humans to test safety and efficacy of novel therapies. Granulocyte colony-stimulating factor (G-CSF) has been used to increase neutrophil numbers and treat chemotherapy-induced and cyclic neutropenia (see Chapter 2 and the Drug Formulary in the Appendix). Owners must consider the potential zoonotic risks involved with keeping an immunodeficient animal with infections that can be contagious to humans, particularly immunosuppressed humans exposed to foxhounds with leishmaniasis and miniature schnauzers and basset hounds with avian mycobacteriosis (see Chapter 99). Primary ciliary dyskinesia, also known as immotile cilia syndrome, is caused by various functional and ultrastructural ciliary abnormalities, including lack of outer or inner dynein arms, abnormal microtubular pattern, random ciliary orientation, and electron-dense cores in the basal body. Because of impaired mucociliary clearance, affected animals have recurrent bacterial rhinitis, sinusitis, and bronchopneumonia with bronchiectasis. Poorly motile or immotile live sperms lead to male infertility, and although evidence is lacking, dysfunction of ependymal cilia can cause hydrocephalus. Otitis media can also occur. The lack of coordinated cilia motility during embryogenesis is responsible for the 50% prevalence of concurrent situs inversus in some forms of primary ciliary dyskinesia. The clinical triad of rhinosinusitis, bronchiectasis, and situs inversus is known as Kartagener’s syndrome.88,214,214 Primary ciliary dyskinesia is inherited by an autosomal recessive trait. It represents a heterogeneous group of defects that have been described in more than a dozen breeds.297 Clinical signs typically begin at a young age, and recurrent respiratory infections lead to death or euthanasia before 1 year of age. However, a few dogs appear clinically healthy for months to years.102 A diagnosis is reached by documenting an absence of ciliary clearance of a microaggregated albumin that is labeled with technetium-99m and placed through a catheter into the nasal cavity or the tracheal bifurcation and by tissue biopsy of ciliated mucosae with ultrastructural analysis and in vitro motility studies.50,65 Induction of ciliogenesis can also be determined by use of in vitro cell culture.50A mutation in the CCDC39 gene has been found in Old English sheepdogs with axonemal disorganization and abnormal ciliary beating.205a Chronic recurrent respiratory infections have been observed in related Irish wolfhounds.51,173 In puppies the disease begins with a serous rhinitis that later becomes catarrhal to hemorrhagic with turbinate ulceration and destruction. Pneumonia and generalized lymphadenomegaly are apparent in later stages. Although the disease is responsive to antimicrobial treatment, it can progress in intervening periods. Although a familial relationship exists, the exact mode of inheritance and the underlying defect are uncertain. A primary ciliary dyskinesia has been identified in some wolfhounds35 but has not been a consistent feature of the disease. Serum IgA levels were reduced in some dogs, but bronchoalveolar lavage fluid had high IgA concentrations in tested dogs, which can relate to their inflammatory response to infection.51 Neutrophil dysfunction was described in a family of Doberman pinschers in which a young animal had chronic recurrent respiratory tract infections.24 Oxygen radical formation and bactericidal activity of neutrophils were reduced despite normal phagocytosis of bacteria. The specific defect has not yet been identified, and primary ciliary dyskinesia has not been completely ruled out. The ciliary morphology appeared normal, but no functional studies have been performed. Affected Doberman pinschers develop clinical signs of upper respiratory tract infections at a few weeks of age. They respond to antibacterials, but the prognosis is guarded. Cyclic hematopoiesis is characterized by a periodic production and maturation defect of hematopoietic cells in the bone marrow.215 In human patients the cycle is 21 days, whereas in dogs it is every 12 to 14 days. Because of the short half-life of granulocytes, severe neutropenia (fewer than 1000/µL) is seen every 12 to 14 days and lasts for 3 to 4 days; thus the synonym cyclic neutropenia syndrome. Serial blood counts are used to reach a definitive diagnosis. During periods of severe neutropenia, dogs are highly susceptible to bacterial infections. Clinical illness appears at 6 to 8 weeks of age with regularly recurring bacterial infections (Fig. 94-2). The chronic exposure leads to systemic amyloidosis, and death can be result from organ failure (of the kidney and other organs) and sepsis before 1 year of age. Furthermore, gingival bleeding can occur from an associated platelet storage pool disease. Affected collies have a silver gray or light beige to tan coat color, and secondary hormonal cycling has been observed.154,183 Cyclic hematopoiesis was the first reported canine immunodeficiency observed and has only been clearly described in collies that are hypopigmented, hence the term gray collie syndrome. It is inherited as an autosomal recessive trait, but no clinical case has been reported since the 1970s outside of the research colony in this breed. A basset hound with an apparent cyclic hematopoiesis has been observed but not further characterized. Experimentally, bone marrow transplantation completely corrects this syndrome, including the coat color dilution. Furthermore, administration of lithium carbonate at extremely high and otherwise toxic doses is known to induce leukocytosis and can ameliorate the cyclic neutropenia in affected animals. Similarly, injections of G-CSFs, alone or in combination with steel factor, abolish the cycling. Mutations in a leukocyte elastase have been identified in humans with a similar syndrome.215 However, the defect in collie dogs is associated with a homozygous mutation of the gene encoding the dog adaptor protein complex 3 β-subunit that directs trans-Golgi export of transmembrane cargo proteins to lysosomes.17,158 This leukocyte defect, originally described as canine granulocytopathy syndrome in Irish setters and cattle, is referred to as canine or bovine LAD (or CLAD or BLAD).243 It is known to be caused by the absence of a family of three leukocyte integrins.75,117,284,286 These proteins are heterodimers (CD11a-d/CD18) with a specific α-chain (CD11a-d) and a common β-chain (CD18). In LAD, the β2-subunit is missing (CD18 deficiency), resulting in a lack of surface expression and function of all leukocyte integrins. The CD11b/18 is the most critical integrin because is the CR3 receptor that binds C3bi and ICAM-1 of activated endothelium, thereby mediating tight adhesion leukocytes. Because of this deficiency, granulocytes are unable to marginate, migrate randomly or by chemotaxis, and kill microorganisms, resulting in an impaired inflammatory response despite marked leukocytosis (Fig. 94-3, A). Furthermore, the in vitro lymphocyte stimulation response is reduced. A single missense mutation substituting a cystine with a serine has been identified in affected Irish setters, and the same mutation has been found in red and white setters.75,105,152,160–162 Affected dogs have a severely increased susceptibility to bacterial and fungal infections. Signs of pyogenic infections develop during the first weeks to months of life, are often recurring, and are poorly responsive to antibacterials. Omphalophlebitis and gingivitis are often the first infections and might be followed by pyoderma, pododermatitis, thrombophlebitis, pneumonia, pyometra, osteomyelitis (especially craniomandibular and metaphyseal), and fatal sepsis. Pups are often stunted or have poor body condition.60,283 The sites of infections exhibit only minimal inflammation and pus formation despite a most severe persistent leukocytosis of 25,000 to 500,000/µL (see Fig. 94-3, B). Regional lymphadenomegaly and poor wound healing are commonly noted. Long-term therapy with bactericidal antibacterials is required to keep affected animals alive. LAD has been reported in Irish setters worldwide and is an autosomal recessive disease. LAD due to other CD18 mutations also has been documented in Holstein cattle and in humans.160 Moreover, an adult domestic medium-haired cat with severe leukocytosis and with signs of infection and inflammation has been found to have LAD.116 The diagnosis in dogs is currently made by mutation-specific DNA testing,105,161,162,290 whereas a different mutation, β2-integrin (CD18), was detected in the cat. In the past, demonstration of a lack of neutrophil adherence to glass, plastic, or wool and deficiency of leukocyte glycoproteins CD11/CD18 by flow cytometry analysis were used (see Fig. 94-3, C) (see Web Appendix 5 for commercial laboratories). Carrier dogs can be detected by genetic testing.75 If the mutation is not known, leukocyte flow cytometry for CD18 is the most broad and simple screening test in dogs and cats. Hematopoietic stem cell transplantation of CD18+ donor cells and gene therapy can reverse the defect.12 Mixed chimeric hematopoietic stem cell transplantation from a histocompatible littermate has been shown to reverse the clinical signs of illness in an affected dog.61 At times, donor recipients were immunosuppressed with ciclosporin and mycophenolic acid mofetil during the 2 months after transplantation. A C3 deficiency was found in a colony of Brittany spaniels with spinal muscular atrophy.20,306,306 C3 is a key factor of the complement system required in opsonizing bacteria. Affected dogs have defective chemotaxis and bacterial phagocytosis from a complete deficiency of C3 (0.1%) and less than 1% plasma complement activity. The immunodeficiency is generally mild and animals live until adulthood, but serious infections can occur, including pneumonia, pyoderma, sepsis, pyometra, and septic arthritis. Affected dogs can develop renal failure with amyloidosis. This is an inherited autosomal recessive disorder, and a diagnosis can be reached by documenting a lack of serum complement activity or mutation test. Chronic neutropenia and megaloblastic anemia have been described in giant schnauzers, beagles, Australian shepherds, Komondors, and border collie pups.107,109,109 Affected animals fail to thrive and often show signs of lethargy, inappetence, and cachexia but are only rarely predisposed to infection accompanied by fever, lameness, or diarrhea. Blood smears show neutropenia with hypersegmented neutrophils. Affected animals have very low serum cobalamin concentrations and a marked methylmalonic aciduria. A cobalamin ileal receptor defect was documented in affected giant schnauzers and Australian shepherds.109 Treatment with parenteral administration of cobalamin once or twice monthly is highly effective.107 Chronic neutropenia and recurrent bacterial infections have been found in certain lines of border collies in Australia and New Zealand.3 This autosomal recessive defect is referred to as Trapped Neutrophil Syndrome and is characterized by stunted growth in pups by 2 weeks of age and fever, anorexia, diarrhea, and lameness by 2 months of age. Metaphyseal osteomyelitis is seen radiographically, and neutropenia (fewer than 3 × 103 cells/µL) is consistently found. Bone marrow cytologic abnormalities include granulocytic hyperplasia with a shift to the right, suggesting that the developing cells cannot enter the systemic circulation. Fasting hypercholesterolemia is also observed. A linkage test has been offered at www.bordercolliehealth.com/TNSdatabase.html.259a Pups respond poorly to antimicrobial therapy and eventually succumb to systemic infections. Certain lines of miniature dachshunds are predisposed to developing Pneumocystis pneumonia by 1 year of age (see Chapter 66). This predisposition also occurs in Cavalier King Charles spaniels that seem to have lower serum IgG concentrations;298 however, additional genetic and immunologic studies in those dogs are not available. Affected miniature dachshunds have hypogammaglobulinemia, with decreased IgA, IgG, and IgM; decreased lymphocyte transformation to phytohemagglutinin and pokeweed mitogens; and absence of B cells and presence of T cells in lymphoid tissues (stained by CD3 and CD79a cell markers).178 Increased susceptibility to avian mycobacteriosis has been recognized in basset hounds and miniature schnauzers (see Chapter 48). The underlying defect is unknown, but it may represent a defect in the natural resistance against mycobacterial protein. Similarly, English and American foxhounds in the United States are commonly infected with leishmaniasis (see Chapter 73)111,225 and pit bulls and greyhounds with babesiosis (see Chapter 76); thus a genetic predisposition is suspected. Although the Pelger-Huët anomaly has been observed in dogs, it has not been associated with clinical illness.170 German shepherds are more severely infected with Ehrlichia canis (see Chapter 26), Rickettsia rickettsii (see Chapter 27), Pythium spp. (see Chapter 65), and Aspergillus spp. or Neosartorya spp. (see Chapter 62).166 Some German shepherd dogs also are predisposed to pyoderma.43,67,67 These predispositions can involve separate defects that have yet to be defined. Affected dogs have pruritus with a deep pyoderma over the lumbosacral region, which may spread to other regions.252 Coagulase-positive Staphylococcus pseudintermedius is most commonly cultured. Proposed mechanisms for this susceptibility include hypothyroidism, cell-mediated immunodeficiency associated with serum inhibitors or defective helper T cells, and bacterial hypersensitivity reactions to staphylococci.43,67,70,308 Although the inciting causes may be multivariate, the infection responds favorably to long-term administration of systemic antimicrobials.
Immunodeficiencies and Infectious Diseases
Defect
Susceptibility to Specific Infections
Diagnostic Tests to Confirm Defect
Physical barrier
Ciliary dyskinesia
Respiratory infections
Electron microscopy of cilia
Ehlers-Danlos syndrome
Skin injury and infections
Histopathology and electron microscopy of skin
Ectodermal dysplasia
Histopathology of skin, DNA
B cell
Pyogenic bacteria, Giardia spp.
Globulin, serum electrophoresis, tetanus antibody response
T cell
Bacterial sepsis, mycobacteria, Candida and Pneumocystis spp.
Thymic radiographs, skin testing delayed hypersensitivity, CD4 and CD8 lymphocytes
Granulocyte
Bacteria; streptococci; Pseudomonas, Candida, Nocardia, and Aspergillus spp.
Neutrophil count, phagocytosis assays, DNA
Complement
Pyogenic bacteria
CH50 assay, DNA
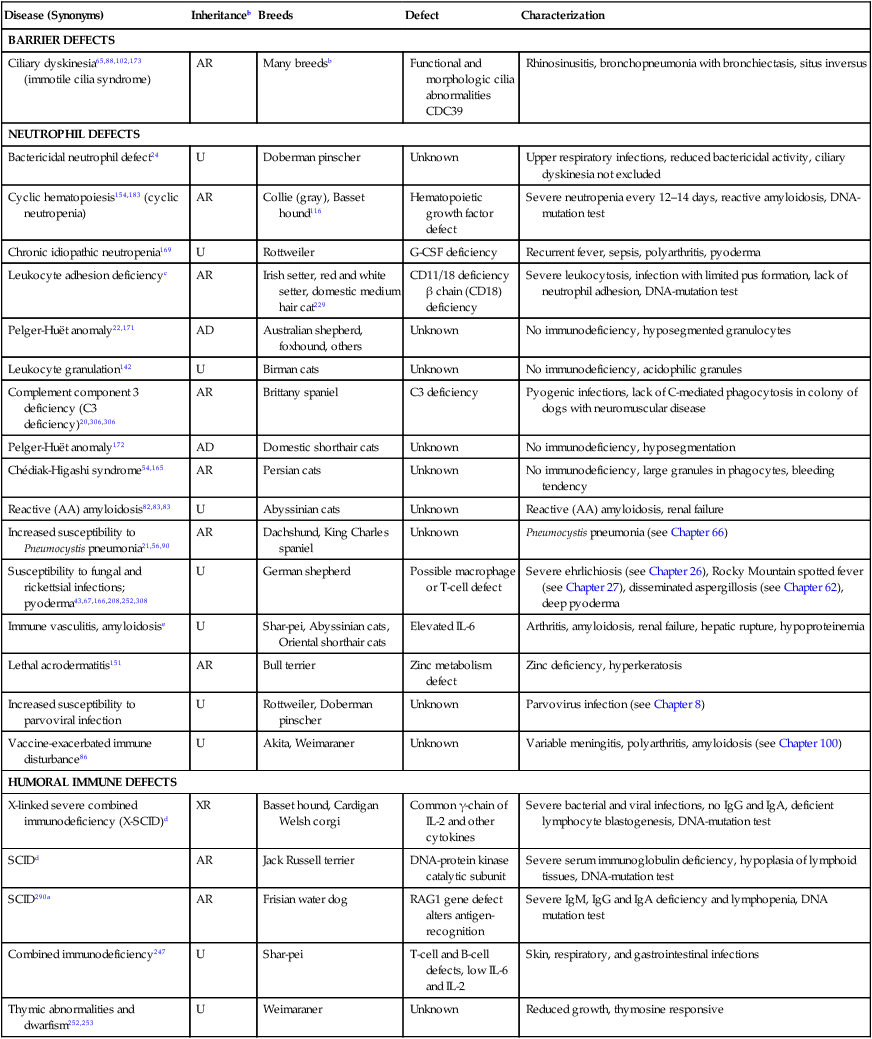
Primary or Hereditary Immunodeficiencies
Disease (Synonyms)
Inheritanceb
Breeds
Defect
Characterization
BARRIER DEFECTS
Ciliary dyskinesia65,88,102,173 (immotile cilia syndrome)
AR
Many breedsb
Functional and morphologic cilia abnormalities
CDC39
Rhinosinusitis, bronchopneumonia with bronchiectasis, situs inversus
NEUTROPHIL DEFECTS
Bactericidal neutrophil defect24
U
Doberman pinscher
Unknown
Upper respiratory infections, reduced bactericidal activity, ciliary dyskinesia not excluded
Cyclic hematopoiesis154,183 (cyclic neutropenia)
AR
Collie (gray), Basset hound116
Hematopoietic growth factor defect
Severe neutropenia every 12–14 days, reactive amyloidosis, DNA-mutation test
Chronic idiopathic neutropenia169
U
Rottweiler
G-CSF deficiency
Recurrent fever, sepsis, polyarthritis, pyoderma
Leukocyte adhesion deficiencyc
AR
Irish setter, red and white setter, domestic medium hair cat229
CD11/18 deficiency β chain (CD18) deficiency
Severe leukocytosis, infection with limited pus formation, lack of neutrophil adhesion, DNA-mutation test
Pelger-Huët anomaly22,171
AD
Australian shepherd, foxhound, others
Unknown
No immunodeficiency, hyposegmented granulocytes
Leukocyte granulation142
U
Birman cats
Unknown
No immunodeficiency, acidophilic granules
Complement component 3 deficiency (C3 deficiency)20,306,306
AR
Brittany spaniel
C3 deficiency
Pyogenic infections, lack of C-mediated phagocytosis in colony of dogs with neuromuscular disease
Pelger-Huët anomaly172
AD
Domestic shorthair cats
Unknown
No immunodeficiency, hyposegmentation
Chédiak-Higashi syndrome54,165
AR
Persian cats
Unknown
No immunodeficiency, large granules in phagocytes, bleeding tendency
Reactive (AA) amyloidosis82,83,83
U
Abyssinian cats
Unknown
Reactive (AA) amyloidosis, renal failure
Increased susceptibility to Pneumocystis pneumonia21,56,90
AR
Dachshund, King Charles spaniel
Unknown
Pneumocystis pneumonia (see Chapter 66)
Susceptibility to fungal and rickettsial infections; pyoderma43,67,166,208,252,308
U
German shepherd
Possible macrophage or T-cell defect
Severe ehrlichiosis (see Chapter 26), Rocky Mountain spotted fever (see Chapter 27), disseminated aspergillosis (see Chapter 62), deep pyoderma
Immune vasculitis, amyloidosise
U
Shar-pei, Abyssinian cats, Oriental shorthair cats
Elevated IL-6
Arthritis, amyloidosis, renal failure, hepatic rupture, hypoproteinemia
Lethal acrodermatitis151
AR
Bull terrier
Zinc metabolism defect
Zinc deficiency, hyperkeratosis
Increased susceptibility to parvoviral infection
U
Rottweiler, Doberman pinscher
Unknown
Parvovirus infection (see Chapter 8)
Vaccine-exacerbated immune disturbance86
U
Akita, Weimaraner
Unknown
Variable meningitis, polyarthritis, amyloidosis (see Chapter 100)
HUMORAL IMMUNE DEFECTS
X-linked severe combined immunodeficiency (X-SCID)d
XR
Basset hound, Cardigan Welsh corgi
Common γ-chain of IL-2 and other cytokines
Severe bacterial and viral infections, no IgG and IgA, deficient lymphocyte blastogenesis, DNA-mutation test
SCIDd
AR
Jack Russell terrier
DNA-protein kinase catalytic subunit
Severe serum immunoglobulin deficiency, hypoplasia of lymphoid tissues, DNA-mutation test
SCID290a
AR
Frisian water dog
RAG1 gene defect alters antigen-recognition
Severe IgM, IgG and IgA deficiency and lymphopenia, DNA mutation test
Combined immunodeficiency247
U
Shar-pei
T-cell and B-cell defects, low IL-6 and IL-2
Skin, respiratory, and gastrointestinal infections
Thymic abnormalities and dwarfism252,253
U
Weimaraner
Unknown
Reduced growth, thymosine responsive
Secondary or Acquired Immunodeficiencies
Diagnostic Studies
Treatment and Prevention
Specific Primary or Inherited Immunodeficiencies
Dogs
Primary Ciliary Dyskinesia
Rhinitis and Bronchopneumonia in Irish Wolfhounds
Neutrophil Bactericidal Defect with Respiratory Infection in Doberman Pinschers
Cyclic Hematopoiesis
Leukocyte Adhesion Deficiency
Complement C3 Deficiency
Selective Cobalamin Malabsorption
Trapped Neutrophil Syndrome—Hereditary Neutropenia in Border Collies
Common Variable Immunodeficiency in Miniature Dachshunds with Pneumocystosis
Miscellaneous Defects in Dogs
German Shepherd Infections
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine