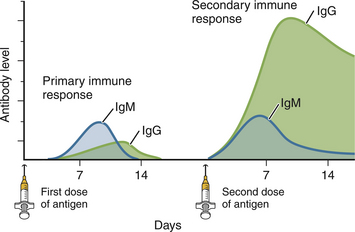
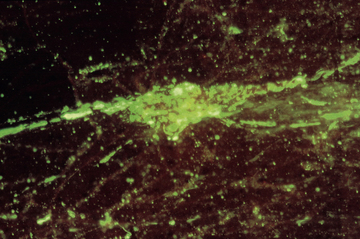
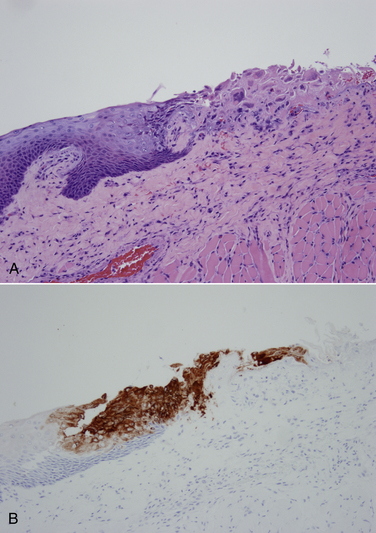
Chapter 2 • Immunoassays are commonly used in practice to detect antigen or antibody in body fluids. • The term serology is most often used to refer to assays that detect antibody in serum. • Immunoassays that detect antigen or antibody in dogs and cats suspected to have infectious diseases include direct and indirect immunofluorescent antibody (IFA), immunohistochemistry (IHC), ELISA, Western immunoblotting, agglutination tests, gel immunodiffusion assays, and serum neutralization. • Positive immunoassay results do not always imply infection, and negative results do not rule out infection. • When interpreting the results of immunoassays, veterinarians should consider what is being measured (antigen or antibody), the likelihood of antigen or antibody being present in relation to the onset of illness, whether the positive test result is consistent with the animal’s clinical signs, and the animal’s vaccination history and immune status. The possibility of false positives due to immunologic cross-reactivity, as well as the analytical sensitivity and specificity of the test used (i.e., the test’s performance), must also be considered. • Additional assays may be required to support immunoassay test results. Some immunoassays, such as ELISA and IFA, provide quantitative results, expressed as optical density units (ODs) or titers (e.g., 1:800, or reciprocal titer = 800). Exposure to an infectious agent results in a rise in antibody titer over time (Figure 2-1). Usually, the first antibody class to be produced by plasma cells is IgM, after which IgG synthesis by plasma cells occurs. When exposure to a pathogen occurs for the first time, there is a lag phase of 5 to 7 days before IgM antibodies can be detected in the blood (primary antibody response). The antibody titer increases, and if the infectious agent is cleared, there is a progressive decline in the titer over weeks to months. The speed and magnitude by which the antibody titer rises depends on host factors and the infectious agent involved. For some diseases, such as leptospirosis, a significant rise occurs within 3 to 4 days of the onset of clinical signs. In secondary, or anamnestic, immune responses, the lag phase is shorter, the antibody titer increases more rapidly and to a greater magnitude, and antibodies may persist for longer periods of time (months to years). FIGURE 2-1 Time course of primary and secondary immune responses, showing relative amounts of IgM and IgG produced at different times after antigen exposure. (Modified from Tizard IR. Veterinary Immunology, 8 ed. St Louis: Saunders; 2008) Blood collected for antibody testing should be allowed to clot, and the serum separated immediately and then refrigerated or frozen. Specimens can be refrigerated for up to a week before they are shipped to the laboratory. If testing is to be delayed for longer than a week, specimens should be frozen at −20°C or −80°C. Specimens can be stored frozen for years without loss of antibody titer. For specimens collected for antigen testing, assay results may be more susceptible to variation with specimen storage. The laboratory should be contacted in order to determine specimen storage and handling requirements. Immunofluorescent and Immunoperoxidase Antibody Assays Direct IFA (also known as direct FA) detects antigen in clinical specimens. IFA involves the use of an antibody that has been tagged (conjugated) with a fluorescent label (such as fluorescein isothiocyanate) to detect the presence a specific antigen. IFA is performed directly on a glass slide, and fluorescence is detected by microscopy. The sensitivity of direct IFA is too low to detect individual virus particles or soluble antigen, so usually IFA detects antigen present in association with eukaryotic or bacterial cells. If the antigen is intracellular, pretreatment of the slide to permeabilize the cells may be necessary. The glass slide is then incubated with a solution that contains the fluorescent antibody, then washed to remove unbound antibody, and the cells are examined using a fluorescence microscope (Figure 2-2). Examples of the use of direct IFA in veterinary medicine include detection of Giardia oocysts, FeLV within monocytes in peripheral blood or bone marrow, or canine distemper virus within epithelial cells from a conjunctival scraping. Nonspecific fluorescence can result in false positives using these methods, but this can be overcome with the inclusion of proper controls and technical expertise. An alternative to the use of a fluorescein-conjugated antibody is the use of an antibody that has been conjugated to an enzyme such as horseradish peroxidase (immunoperoxidase). A substrate, most commonly 3,3′-diaminobenzidine (DAB), is then added to the slides. In the presence of hydrogen peroxide, DAB is converted to an insoluble brown precipitate, which can be visualized using conventional light microscopy. This technique is known as immunocytochemistry. The same method, when applied to tissue sections, is known as immunohistochemistry (IHC) (Figure 2-3). FIGURE 2-2 Direct fluorescent antibody stain of brain tissue for rabies virus.Frozen tissue sections were stained with a commercial reagent that contains three monoclonal antibodies specific for the rabies virus nucleocapsid. Antibodies were labeled with fluorescein. Positive staining is present in a Purkinje cell. (Courtesy of Galligan J. New York State Department of Health. In Maclachlan NJ, Dubovi EJ. Fenner’s Veterinary Virology, 4 ed. New York: Elsevier; 2011.) FIGURE 2-3 Immunohistochemistry. A, Hematoxylin and eosin stained section of a lingual ulcer from a cat infected with feline herpesvirus-1. B, The brown stained cells lining the ulcer are positive using an immunohistochemical stain for feline herpesvirus-1. (Courtesy of Dr. Patricia Pesavento, School of Veterinary Medicine, University of California, Davis.) Indirect IFA (also known as IFA testing, or IFAT) is generally used to establish a serum antibody titer to an infectious agent, but it can also be used to detect antigen. When used to detect antigen, it is more sensitive than direct IFA. For measurement of antibody titers, patient serum is serially diluted in the laboratory and reacted with a known antigen that has been fixed to glass slides. IFA slides are commercially manufactured for this purpose. Slides are then washed to remove unbound antibody. A secondary antibody, which is designed to react with the bound patient antibodies (e.g., dog IgG or dog IgM), is applied to the slides, which are then washed again. This secondary antibody has been conjugated to a fluorescent label. The slides are then examined using a fluorescence microscope. The highest dilution of serum that results in specific fluorescence is reported to the clinician as the antibody titer to the infectious agent of interest. Examples of the use of indirect IFA for serologic testing in dogs and cats include quantitative serology for some tick-borne infectious diseases (e.g., Ehrlichia canis, Anaplasma spp.). More recently, veterinary immunofluorescence assays have been marketed that allow multiple antigen (or antibody) targets to be mounted on novel platforms, such as fluorescent beads or wells on the surface of a spinning silicon disc that resembles a compact disc. Automated silicon disc–based assays allow rapid and simultaneous analysis of hundreds of specimens, with dramatic reduction in labor associated with traditional IFA or ELISA assays, and reduced chance of false-positive or -negative assay results due to human error. A bead-based assay is currently available in the United States for detection of antibody to Borrelia burgdorferi (see Chapter 51). A silicon disc–based assay is available in the United States for detection of antibodies to B. burgdorferi, E. canis, and Anaplasma phagocytophilum and antigen to Dirofilaria immitis (Accuplex 4, Antech Diagnostics, Irvine, Calif). The performance of these assays in the field is under investigation.
Immunoassays
Introduction
Specimen Collection and Transport
Diagnostic Methods
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


