Fig. 10.1
Light micrographs of hematoxylin-eosin (HE) staining for paraffin sections of mesenteric lymph nodes prepared by IVCT followed by FS fixation. (a) Lower magnified view of a lymph node showing wide areas from the cortex (Co) to medulla (Me). In the cortex, outer (OC) and inner (IC) cortices are observed. Cp capsule, ALV afferent lymphatic vessel, IS intermediate sinus, LyN lymphatic nodule, and SS subcapsular sinus. The deep areas of dotted lines in this lymph node are showing tissue damage caused by ice crystal formation (b). The panels (b), (c), and (d) are higher magnified views of three parts shown as rectangles in (a). (b) Space of subcapsular sinus (SS) is opening, and the outside is surrounded by a capsule (Cp). Small lymphocytes with dense chromatin in nuclei are localized along inner endothelial cell s of SS (arrowheads in b). (c) An intermediate sinus (IS) is connecting from SS. (d) Medullary cords (MeC) and open medullary sinuses (MeS) are clearly observed in the medulla of lymph nodes in vivo. Lymphocytes are observed in intermediate and medullary sinuses (arrowheads in c, d). Bars = 100 μm (a), 50 μm (b–d)
10.3 LYVE-1 Immunolocalization in Mouse Lymph Node Tissue
The LYVE-1 was immunolocalized in the inner endothelial cell s and reticular cells of subcapsular sinuses, but not in outer ones or in afferent lymphatic vessels, as shown in Fig. 10.2. Immunoelectron microscopy also showed LYVE-1 in both luminal and basal cell membrane surfaces of inner endothelial cells, but immunoreactivity of LYVE-1 was strongly observed in the luminal sides than that of inner sides, as shown in Fig. 10.2j, k. The immunolocalization of LYVE-1 was first identified as a lymph-specific receptor for hyaluronic acid (HA) [1]. In addition, rather than facilitating degradation of HA, it was thought to be involved in HA transportation across lymphatic endothelial cells, specifically movement of accumulated HA from lymphatic sinuses to the lymph node parenchyma [7]. The possible roles of the lymphatic endothelial cells in HA homeostasis are also modified with HA binding by sialylation and self-association [16]. In addition, it was already shown that injected fluorophore-conjugated HA was taken through the endothelial cells of subcapsular sinuses [17]. The flow of lymphatic fluid was examined by injection of fluorophore-conjugated gelatin into the peritoneal cavity [18], showing its movement through endothelial cells to medullary labyrinths. But in the previous studies, by using injection of soluble substances with different molecular weights, the lymphatic parenchyma was impenetrable to those molecules with about 70 kD [19]. The IVCT could be used to keep the morphology in situ of lymph nodes, and dynamic experiments with fluorescence-labeled tracers will be possible to clarify it.
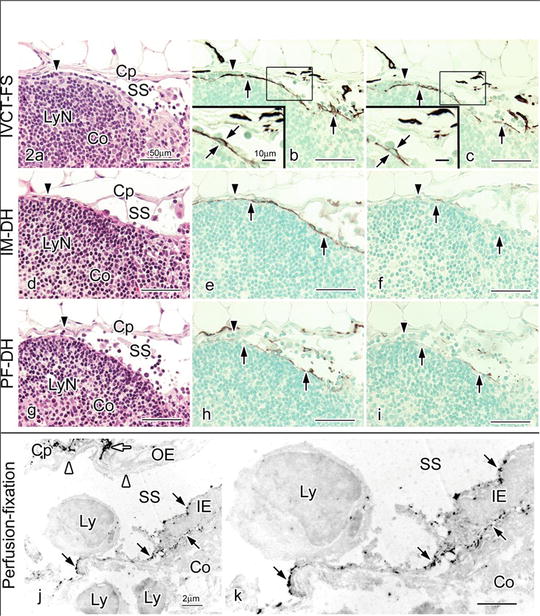

Fig. 10.2
Hematoxylin-eosin (HE) staining (a, d, g) and LYVE-1 immunostaining with different concentrations of primary anti-LYVE-1 antibody (1:300 in b, e, h and 1:7500 in c, f, i) in serial paraffin sections prepared by IVCT-FS (a–c), immersion fixation followed by alcohol dehydration (IM-DH; d–f) or perfusion fixation followed by alcohol dehydration (PF-DH; g–i). Insets in (b) and (c) are highly magnified views of inner endothelial cell s shown by the squares. With IVCT-FS, a row of lymphocytes along subcapsular sinus (SS) is observed in this area (arrowhead in a), compared to IM-DH or PF-DH (arrowheads in d, g). The LYVE-1 immunoreactivity is more clearly detected at a 1:7500 primary antibody dilution in samples with IVCT-FS (c), but it is decreased in samples with IM-DH (f) or PF-DH (i). (j and k) Pre-embedding immunoelectron microscopy for LYVE-1 in a mesenteric lymph node. The LYVE-1 is immunolocalized at both luminal and basal cell membrane surfaces of inner endothelial cells (IE, black arrows) along subcapsular sinus (SS), but not in outer endothelial cells (OE, white arrowheads). The LYVE-1 is also immunolocalized in some cells in the interstitium of capsules (Cp) surrounding SS (white arrow). Co, cortex; Cp, capsule; LyN, lymphatic nodule; Ly, lymphocyte; k, higher magnification of IE. Bars = 50 μm (a–i), 10 μm (insets of b, c), 2 μm (j, k)
Our findings that the LYVE-1 was present at both luminal and basal surfaces of inner endothelial cell s and that the immunolocalization of LYVE-1 is more strongly observed in the apical sides of inner endothelial cells, also interesting to note that many small lymphocytes were attached to inner endothelial cells in subcapsular sinuses, as shown in Fig. 10.2a–c, are well compatible with this functional concept. LYVE-1 immunolocalization is different between inner and outer endothelial cells of subcapsular sinuses, probably reflecting the different functions for the transportation of HA and also the adhesion or migration of CD44-positive lymphocytes [6, 16]. Such different features between inner and outer endothelial cells were already reported by another histochemical approach for hydrolytic enzymes [20].
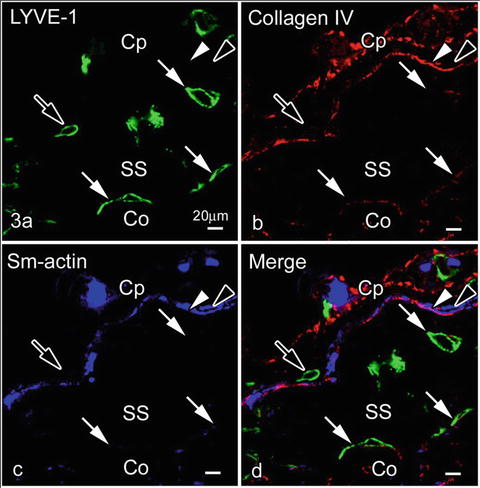
10.4 Relation of Type IV Collagen or Smooth Muscle Actin and LYVE-1 Immunopositive Endothelial Cells in Subcapsular Sinuses
LYVE-1 was not expressed in outer endothelial cell s contacting with type IV collagen and/or smooth muscle cell s , as shown in Fig. 10.3. This finding is also consistent with a study of developing lymph vessels, in which mature lymphatic vessels surrounded by smooth muscle cells usually reduced the LYVE-1 expression [21]. As it was already reported to be expressed in some types of macrophages [22] and other cells [23], identification of such cell types in the capsules will be another topic for future studies.