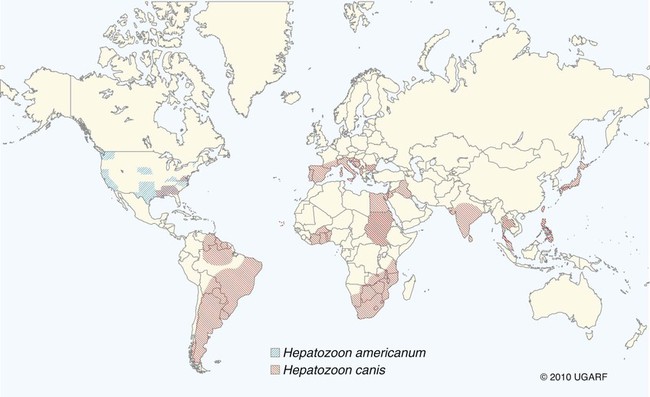
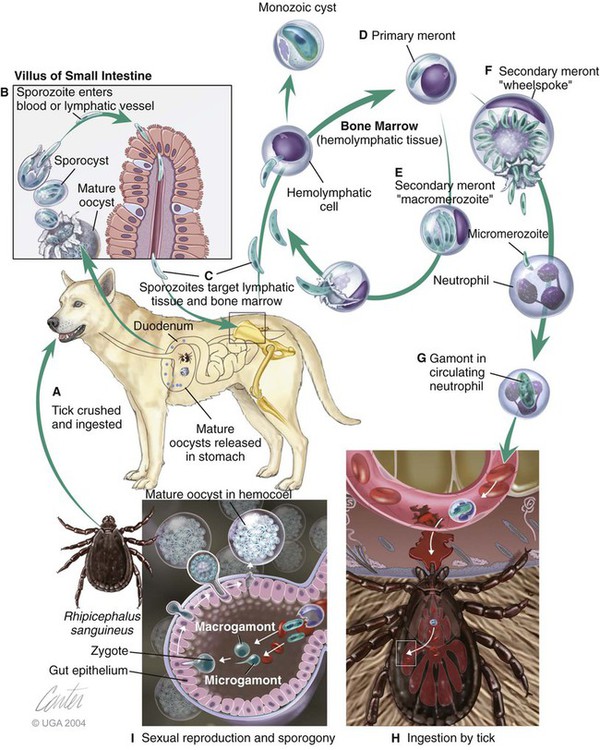
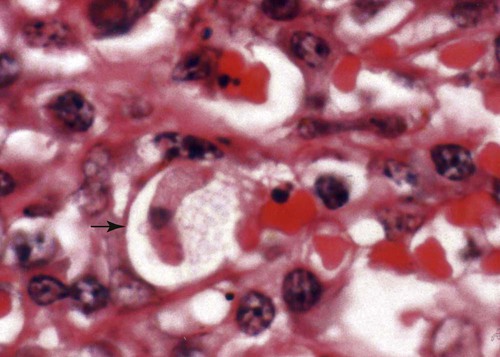
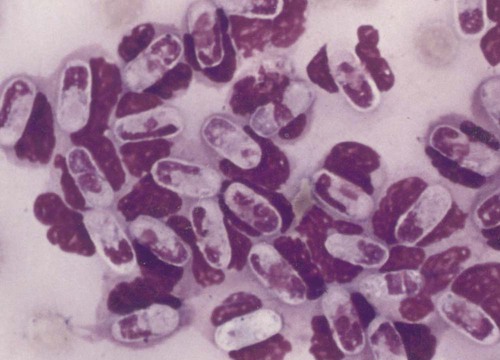
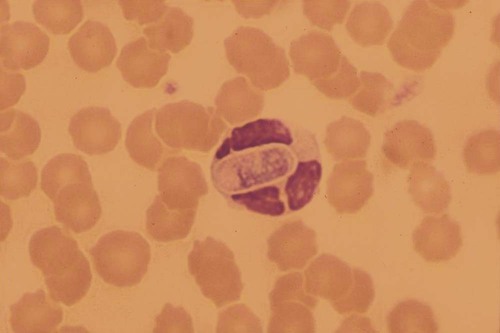
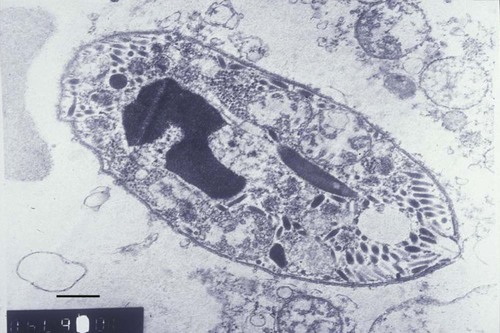
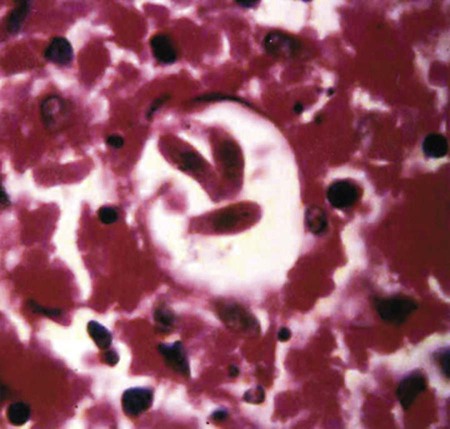
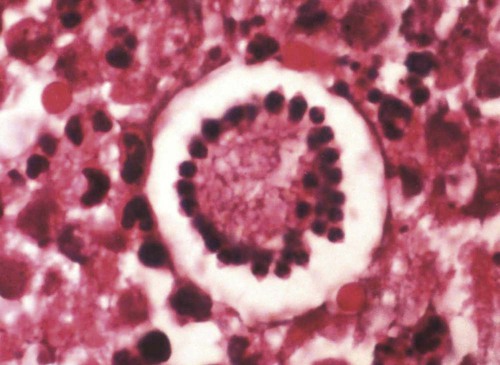
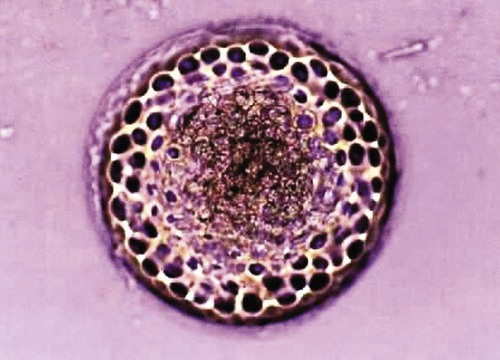
Hepatozoonosis is an arthropod-borne infection caused by apicomplexan protozoa from the family Hepatozoidae in the suborder Adeleorina.18 Based on DNA analysis of the gene encoding for 18S ribosomal RNA (rRNA) (also known as ribosomal DNA [rDNA]), and morphologic features, parasites in the genus Hepatozoon are believed to be most closely related to other apicomplexan parasites such as Plasmodium spp. and piroplasms.19 More than 300 different species of Hepatozoon have been described in amphibians, reptiles, birds, marsupials, and mammals.141 Of these, more than 120 species infect snakes, and approximately 50 have been reported in mammals. The genus was named Hepatozoon because merogonic development of the type strain Hepatozoon muris was observed in the livers of rats.101 However, the liver is not necessarily a major target tissue for other Hepatozoon spp. Hepatozoon spp. that infect amphibians, reptiles, and avian hosts parasitize mainly erythrocytes, whereas gamonts of Hepatozoon spp. infecting mammals are found primarily in leukocytes. Hepatozoon spp. have a basic life cycle that includes asexual development with merogony followed by gamontogony in a vertebrate intermediate host such as the dog and sexual development leading to sporogony in a hematophagous invertebrate definitive host such as a tick. A variety of blood-sucking arthropod vectors serves as definitive hosts for different Hepatozoon spp. These hosts include ticks, mites, mosquitoes, sandflies, tsetse flies, fleas, lice, and reduviid bugs.141 Unlike many vector-borne protozoal and bacterial pathogens that are transmitted via the salivary glands, Hepatozoon transmission takes place by ingestion of the definitive host, an invertebrate that contains mature oocysts, by the intermediate vertebrate host. Two species that use domestic dogs as the intermediate host, Hepatozoon canis and Hepatozoon americanum, have been identified. Infections caused by H. canis and H. americanum are discussed in two separate sections in this chapter. Hepatozoon spp. infection in cats is discussed separately. H. canis infection (HCI) in dogs was first described from India in 1905,63 and until 1997, the presumption was that canine hepatozoonosis was caused by a single species. However, research elucidating the pathologic and clinical syndromes associated with hepatozoonosis,16,43,88,116,151 its transmission by tick species, the parasite life cycle,10,11,92,93 and genetic and antigenic characterization of Hepatozoon isolates5,95 has led to the recognition that two distinct Hepatozoon spp. infect dogs. This finding resulted in naming the parasite that infects dogs in the southern United States H. americanum151 and separating it from the previously described H. canis.9 The discussion in this first section focuses on H. canis infection. The host range of H. canis in mammalian carnivores other than the domestic dog has not been clarified. H. canis or Hepatozoon spp. morphologically resembling H. canis have been reported from several wild canine species, from domestic and wild felines, and from other carnivore species, including the red fox (Vulpes vulpes),24,29,89,90 crab-eating fox (Cerdocyon thous),1 black-backed jackal (Canis mesomelas),97 golden jackal (Canis aureus),138 African wild dog (Lycaon pictus),96,147 coyote (Canis latrans),99 hyena (Crocuta crocuta),37,97 palm civet (Paradoxurus hermaphroditus),79 cheetah (Acinonyx jubatus),97 leopard (Panthera pardus),97 lion (Panthera leo),36 Bobcat (Lynx rufus),99 Pallas’s cat (Felis manul),17 ocelot (Leopardus pardalis),100 little spotted cat (Leopardus tigrinus),100 leopard cat,77,133 Iriomote wildcat (Felis iriomotensis),78 Tsushima leopard cat (Felis bengalensis euptilura),78 and flat-headed cat (Prionailurus planiceps).132 Although the suspicion that H. canis may infect other animals species is high, particularly those that are closely related to the domestic dog, this has not been demonstrated by experimental studies. Reports before 1997 of H. canis–like organisms in North American wild carnivores are possibly related to the species known today as H. americanum (see the following section) or to a species other than H. canis. For a further discussion on feline hepatozoonosis, see the later section. HCI is prevalent in regions of tropical, subtropical, and temperate climate. HCI has been reported from the domestic dog (Canis familiaris) from most continents and numerous countries, including Greece,76 Italy,53 France,127 Spain,64 Portugal, Croatia,152 Kosovo, Albania,81 and Bulgaria145 in southern Europe; Israel72 and Egypt46 in the Middle East; South Africa,97 Sudan,114 and Nigeria45 in Africa; India,63 Sri Lanka, Singapore,79 Malaysia,126 The Philippines,111 Thailand,140 Japan,109 and Turkey71 in Asia; and Brazil,54 Argentina, and Venezuela in South America (Fig. 74-1). HCI was also found in dogs from the Caribbean island of Grenada158 and the Cape Verde Islands in the Atlantic Ocean.56 H. canis was reported to be prevalent in the southeastern United States in some of the areas where H. americanum is present, and co-infections with H. americanum and H. canis in the same dogs have been detected by molecular genetic techniques (see Fig. 74-1).2,84 In addition, imported cases of HCI in dogs brought into nonendemic areas have been reported in several countries, including Germany and The Netherlands.33,47 The genetic similarity of different H. canis isolates using comparison of conserved DNA sequences has not been studied thoroughly. Sequence identity in 18S rDNA fragments reported from dogs infected by H. canis ranges from 97% to 100%, whereas the diversity among dogs infected with H. americanum is considerably higher and ranges from 92.7% to 99.6%.2,84 Phylogenetic analyses of partial sequences of the 18S rDNA from Hepatozoon isolates from dogs in Japan and Brazil indicated that these isolates had a 99% identity with H. canis sequenced from Israel and were more distant to H. americanum.62,128 The prevalence of HCI in different regions ranges considerably. Different diagnostic techniques may influence the observed prevalence rates. Detection of gamonts by microscopy in stained blood smears is considerably less sensitive than detection of Hepatozoon DNA by polymerase chain reaction (PCR) in blood,71,130 whereas serology is indicative of exposure to hepatozoonosis but not of the current presence of parasite circulating in the blood. A comparative study of 349 dogs from Turkey found that 10.6% had positive test results using blood smear evaluation, 25.8% by PCR and 36.8% by indirect fluorescent antibody (FA) testing.71 Circulating H. canis gamonts were detected by blood smear evaluation in 39% of the dogs surveyed in rural areas of Rio de Janeiro state in Brazil,112 in 22% of dogs surveyed in Zaria in Nigeria,45 and in 1.2% in Malaysia.126 Molecular surveys based on detection of fragments of the 18S rDNA revealed infection rates of 3.3% in Barcelona, Spain,144 11.8% in Croatia,152 20.3% in Nigeria,134 42.3% in a village population in Sudan,114 45% in Venezuela,27 58.7% in southeastern Brazil,142 and 64% in the Cape Verde Islands.56 Serologic studies of HCI revealed that 33% of the dogs surveyed in Israel14 and 4.2% in the Yamaguchi region in Japan61 had been exposed to the parasite, as indicated by the presence of anti–H. canis antibodies demonstrated by indirect FA testing. As found also for other tick-borne diseases, including canine ehrlichiosis, babesiosis, and Lyme disease, the exposure rate for HCI in endemic areas is often much greater than the prevalence of clinical disease. Most dogs that are infected with H. canis probably undergo a subclinical infection. Among the dogs surveyed for H. canis antibodies in Israel, 3% of the 33% dogs with positive serum antibody titers had detectable blood gamonts, and only 1% had severe clinical signs associated with the infection.14 The distribution of HCI is tied closely to its acarine definitive hosts. The primary vector of H. canis is the brown dog tick, Rhipicephalus sanguineus.10,11,23,156 It is a three-host tick that is considered to be the most widely distributed tick species in the world.154 R. sanguineus is adaptable to different environmental conditions and is found in warm and temperate regions, making the potential distribution of HCI widespread. H. canis is transmitted transstadially from the nymph to the adult stage in R. sanguineus. Possible transovarial transmission through the tick’s ovary and eggs could not be demonstrated under experimental conditions.10 R. sanguineus ticks can be infected experimentally through percutaneous injection of blood gamonts, allowing researchers to study the disease in ticks.10,11 Another tick species, Amblyomma ovale, has been shown to be a vector of H. canis in Brazil by experimental transmission and in studies of naturally infected dogs and ticks (Fig. 74-2).49,131 Rhipicephalus boophilus, whose preferred host species is cattle, has been found to be infected with H. canis in Brazil,33a and further epidemiologic and transmissions studies are warranted to determine its significance. Other tick species such as Haemaphysalis longicornis and Haemaphysalis flava are potential vectors and were reported to have been removed from dogs with HCI in Japan and identified with oocysts resembling those of Hepatozoon in their hemocoels.107 In addition to infection by ingesting ticks that contain mature oocysts, alternative modes of HCI transmission to dogs should be considered when studying the epidemiology of this disease. As for other apicomplexan parasites, including Toxoplasma gondii and Neospora caninum (see Chapter 79), horizontal transmission through the uterus from dam to its offspring has also been demonstrated in HCI.50,106 Naturally infected pregnant bitches were allowed to give birth in a tick-free environment. Meronts were found in the spleen of a pup that died 16 days after birth, and blood gamonts were detectable as early as 21 days in other pups.106 Another likely mode of transmission that has not yet been demonstrated in HCI is predation by a canine host on another intermediate or transport host. Experimentally, infection did not result from parenteral inoculation of tissues or blood from infected dogs but from inoculation of emulsified tick tissues.49,157 However, H. americanum is transmitted by dog predation on wild rodents and rabbits harboring the tissue cyst stages of this parasite,69,70 and some other species of Hepatozoon that infect snakes, lizards, and frogs have also been shown to be transmitted through predation and ingestion of tissue cysts found in intermediate host tissues.141 No gender or breed predilection for HCI has been noted. HCI was reported in all age groups, from pups younger than three months of age to old dogs3,14,16,51,134 In a case series of dogs from Greece, the female-to-male ratio was 1.2 : 1, and the mean age was 4.2 years.76 Dogs with HCI are more likely to be from a rural community as compared with an urban setting, which is probably the result of a higher exposure rate to ticks.16 In a study of dogs from Brazil, the presence of R. sanguineus ticks on dogs in the household was associated with at least one H. canis–infected dog per household.142 Most HCI cases are detected during the warmer months of the year, when tick vectors are more abundant. In a case-control study of 100 dogs with HCI from Israel, 77% were admitted during the warm period of May through November.16 A follow-up study on the periodic appearance of H. canis gamonts in the blood of dogs in Japan indicated that the peak parasitemia was from spring to autumn.108 However, HCI is also diagnosed during the colder months, when transmission by vector ticks is less likely, caused by chronic persistence of the infection.16 The presumption is that most dogs become infected with H. canis through grooming ticks from their haircoats or feeding on prey infested with parasitized ticks. R. sanguineus is a three-host tick that drops off its host when engorged with blood and, after molt, seeks another animal on which to feed. An adult tick stage might, therefore, acquire HCI as a nymph feeding on a parasitemic dog, and then after molting, attach and feed on a new host, not necessarily a dog. Ticks may also acquire infection during feeding at the adult stage as shown experimentally for A. ovale in Brazil.131 Experimental evidence has shown that both male and female adult R. sanguineus can harbor Hepatozoon oocysts and are potentially infective to dogs.10 The life cycle of H. canis involves the sequential formation of several distinct life forms in each of its two hosts, the dog serving as intermediate host and the tick as definitive host (Fig. 74-3).11 Some confusion has been caused by synonymous use of terms relating to life-cycle stages of H. canis. Some studies describe schizonts and gametocytes, whereas other texts commonly use the terms meronts and gamonts, respectively. When the dog ingests the vector tick or tick parts, H. canis sporozoites are released in the intestine and penetrate the gut wall. The sporozoites invade mononuclear cells and disseminate hematogenously or via the lymph to hemolymphatic target organs that include the bone marrow, spleen, and lymph nodes and to other internal organs such as the liver, kidney, and lungs. Respectively, hepatitis, glomerulonephritis, and pneumonitis may develop. Meronts in which asexually dividing merozoites develop are formed in the dog’s tissues in the process of merogony. Two types of meronts are found in infected tissues, one containing approximately 20 to 30 slender micromerozoites and another containing up to four larger macromerozoites. Merozoites are released from mature meronts, invade neutrophils and monocytes, and develop into gamonts in the process of gamontogony. Alternatively, merozoites can produce secondary meronts in the target tissues. Small monozoic cysts of H. canis containing a single parasite have been described in tissues of naturally and experimentally infected dogs (Fig. 74-4).12 The role of these cysts in the life cycle of H. canis has not been clarified. However, they resemble cysts described in H. americanum and also in lizards and snakes affected by other Hepatozoon species in which transmission by predation has been shown.70,141 The tick, which serves as the definitive host, becomes infected by ingesting leukocytes containing gamonts when feeding on a parasitemic dog. Morphologically indistinguishable male and female H. canis gamonts are released from the dog leukocytes within the tick gut, associate in syzygy, and differentiate in the process of gametogenesis to distinct gametes. After fertilization, the zygote divides, and sporogony takes place with the formation of oocysts that are released into the tick’s hemocoel. The oocysts are large, spherical forms consisting of a membrane that envelops hundreds of sporocysts in which the infective sporozoites are found.11 Sporozoites do not migrate to the feeding parts or salivary gland of the tick; therefore, the tick must be ingested to infect the dog. The life cycle of H. canis can be completed within 81 days including both tick and dog parts of the cycle.11 In an experimental transmission study, adult-stage R. sanguineus ticks were infective by ingestion to dogs 53 days after the ticks fed as nymphs on a naturally infected dog. Meronts were first detected in the experimentally infected dog’s bone marrow 13 days postinoculation, and gamonts appeared in the blood, thereby completing the life cycle in 28 days.10,11 The pathogenesis of HCI is influenced by immunodeficient conditions, an immature immune system in young pups, a congenital defect, or concurrent infectious agents. Conditions that weaken the immune responses increase the susceptibility to new infection with H. canis or allow existing infections to reactivate. Co-infections with Toxoplasma, Leishmania, Babesia, or Ehrlichia predispose to clinical illness. In a litter of Dalmatians that were diagnosed with H. canis parasitemia, pups that developed parvoviral enteritis demonstrated a significantly higher parasitemia than did their littermates that did not become ill.3 In addition, treatment of experimentally infected dogs with an immunosuppressive dose of prednisolone was followed by the appearance of parasitemia.10 Infection with H. canis elicits a distinct humoral immune response during the early stages of the disease.10,13 No information is available on the cellular response to HCI; however, because H. canis is an intracellular parasite, cell-mediated immunity probably plays a major role in the immune mechanism mounted by the host against HCI. A variety of clinical presentations is associated with HCI, ranging in severity from an incidental hematologic finding in an apparently healthy dog to a debilitating and life-threatening illness. A low level of H. canis parasitemia with gamonts found in less than 5% of neutrophils is the most common presentation of HCI. It is usually associated with an asymptomatic to mild disease. A high parasitemia sometimes approaching 100% of neutrophils with a leukocytosis is usually associated with a severe disease. High parasitemia rates are frequently found concurrent with extreme leukocytosis, reaching as high as 150,000 leukocytes per microliter of blood.7,16,16 The mechanism causing this leukocytosis, most commonly involving neutrophils, has not been elucidated. In a case-control study of 100 dogs with HCI, dogs were categorized into low and high parasitemia groups. Eighty-five percent of the dogs had a low parasitemia level and 15% had a high number of circulating parasites.16 Dogs with low parasitemia were more anemic and had lower platelet counts than did the control dogs with other disease conditions. However, dogs with high circulating parasite numbers suffered mainly from fever, lethargy, weight loss, anemia, and hyperglobulinemia. A study of canine hepatozoonosis from Turkey also found an association between the severity of clinical signs and the level of parasitemia. Dogs with severe disease had higher parasitemia levels in comparison to dogs showing moderate or mild clinical signs.71 High numbers of circulating H. canis gamonts, in some cases ranging between 50,000 and 100,000 gamonts per microliter of blood, are indicative of the large extent of tissue parasitism present in highly infected dogs.16 Tissue meronts produce the numerous merozoites that eventually invade leukocytes. The load of blood and tissue parasites present in dogs with high parasitemia levels takes its toll by demanding nutrients and exerting tissue injury. This finding explains the weight loss leading to cachexia and the profound lethargy observed in this subgroup of infected dogs. Concurrent infections involving H. canis and other canine pathogens are common. Some of the reported co-infecting organisms, including Ehrlichia canis51,56,122,136,145 (see Chapter 26) and Babesia canis21,51,56,76,136 (see Chapter 76), are transmitted by the same tick vector, R. sanguineus, and are likely to be found in dogs with tick infestations in areas where these diseases are endemic in the dog population. Other pathogens reported to be involved in concurrent infections include canine parvovirus, canine distemper, Anaplasma phagocytophilum, Anaplasma platys,56,76 T. gondii,57 and Leishmania infantum.76,127,127 Young puppies with hepatozoonosis and other concurrent infections may develop more severe disease.51,56,56 Co-infections may influence the susceptibility to establishing a new infection or the progression of an existing one.3,53,53 In the case of concurrent infection, clinical signs attributed to HCI must be interpreted cautiously and separated from manifestations of the concomitant pathogen. The pathogenesis, vectorial capacity, tissue tropism, and clinical signs associated with HCI differ from H. americanum infection (see later discussion). H. canis is found primarily in hemolymphatic tissues, whereas H. americanum infects mainly muscular tissues, causing myositis and lameness. The principal differences between these two infections are summarized in Table 74-1. TABLE 74-1 Comparative Characteristics of Hepatozoon canis and Hepatozoon americanum Infections ELISA, Enzyme-linked immunosorbent assay; FA, fluorescent antibody; PCR, polymerase chain reaction. Anemia is the most common hematologic abnormality in HCI and has been reported in the majority of HCI cases.* The anemia is, in most cases, normocytic normochromic and occasionally regenerative. The leukocyte count is usually within reference limits when parasitemia is low and is elevated in dogs with high parasitemia. Extreme neutrophilia reaching 100,000 neutrophils per microliter of blood occurs in some cases with high parasitemia (Fig. 74-5). Thrombocytopenia is present in approximately one third of the dogs with HCI and is, in some cases, associated with concurrent canine ehrlichiosis. Serum chemistry abnormalities include hyperproteinemia with hyperglobulinemia and hypoalbuminemia and increased creatine kinase (CK) and alkaline phosphatase (ALP) activities. Electrophoresis of serum proteins from the sera of hyperglobulinemic dogs revealed polyclonal gammopathy.16,53 Microscopic detection of H. canis gamonts in Giemsa- or Diff Quik–stained blood smears is the most common method for diagnosis of HCI. The concentration of organisms increases with the severity of the illness. The gamonts are ellipsoidal in shape and measure approximately 11 µm × 4 µm (Fig. 74-6) and are found in the cytoplasm of neutrophils and rarely in monocytes. Gamonts are enveloped in a thick membrane (Fig. 74-7) and are often situated in the center of the neutrophil and compress its lobulated nucleus toward the cell membrane.11,155 Examination of buffy-coat smears is more sensitive than routine blood smears in detecting the organism; however, PCR methods (see later discussion) are even more accurate.135 H. canis meronts can be detected in histopathologic specimens or in cytologic preparations made from aspirates or touch impressions of hemolymphatic tissues. Meronts are round to oval and are approximately 30 µm in diameter. Immature meronts appear as round opacities filled with globular foamlike material. As the meront matures, basophilic-staining chromatin material forms as either 2 to 4 larger macromerozoites (Fig. 74-8) or more than 20 micromerozoites (Fig. 74-9). The shaping micromerozoites align in a circle close to the meront wall around a central opaque core. The mature meront visualized in histopathologic specimens creates a typical “wheel spoke” form when the circle of micromerozoites is incised in cross-section through their mid-shaft (see Fig. 74-9).11 H. canis can also be identified in vector ticks. Mature oocysts can be seen even without magnification as small white globular forms in wet preparations of the hemocoel. The observation of oocysts is verified and differentiated from the tick’s organs and salivary glands by microscopy of hemocoel smears as unstained wet preparations or as Giemsa-stained specimens (Fig. 74-10). Oocysts are enveloped in an easily torn membrane and contain hundreds of smaller oval sporocysts. Free sporocysts are often present scattered outside the oocyst, and infective sporozoites are packed as elongated slender forms within the sporocysts (see Fig. 74-10). The indirect FA test and the enzyme-linked immunosorbent assay for the serodiagnosis of HCI using gamont antigen have been developed and are used mainly for epidemiologic studies.* Antibodies of the IgM and IgG classes were detectable in the sera of experimentally infected dogs as early as 16 and 22 days postinoculation, respectively, peaked at 7 to 9 weeks, and persisted for more than 7 months.6,13,55,139 PCR assays for the detection of Hepatozoon DNA have been mostly based on amplification of fragments of the 18S rDNA.5,30,31,62 Some of the PCR assays originally developed to detect piroplasms such as Babesia spp. also amplify Hepatozoon DNA; however, the amplified products are of different size, and their identities can be further verified by sequencing of their nucleotides.114,143 Studies that compared the detection of Hepatozoon by blood smear microscopy and conventional PCR from the same blood samples indicated that PCR was considerably more sensitive with 2.6% positive result rate in smears versus 11.4% by PCR in one study,65 10.6% versus 25.8% in another study,71 and 11.3% versus 53.3% in a third study.130 Quantitative PCR assays for Hepatozoon are very sensitive and specific and can provide an estimate of the parasite load in the sample.27,83 Pathologic descriptions of infected dogs range from reports on infrequent tissue meronts termed as incidental findings72 to severe and occasionally fatal multiple-organ involvement.7,16,57,58,97 A considerable variation is found in the spectrum of lesions and the number of parasitic life forms. The major macroscopic lesions found in dogs with heavy infections include splenomegaly and hepatomegaly, with a diffuse pattern of small white necrotic foci 1 to 2 mm in diameter.7,58 Necrotic foci may be larger and nodular in appearance and are also found in other tissues, including the pancreas and on the pleura.7,16,16 Pneumonia may be evident, and lymph nodes are typically enlarged. Histopathologic findings include a varying number of developing or mature meronts with their “wheel spoke” pattern in the affected tissues. These meronts are associated with a mild inflammatory response in some cases, ranging to severe responses in others.97 Focal splenic necrosis is associated with H. canis merogony in red and white pulp regions, with white pulp necrosis located primarily in lymphoid follicles.58 Hepatitis with Kupffer cell hyperplasia and mononuclear and neutrophil infiltration is associated with developing meronts in the liver.16,97 The presence of H. canis in the lung is associated with interstitial pneumonia and the thickening of alveolar septa with inflammatory cell infiltrates. Renal lesions include glomerulonephritis and interstitial nephritis with multifocal necrosis. Mild to extensive parasitism with meronts and developing gamonts is described in the lymph nodes and bone marrow.16,97
Hepatozoonosis
Hepatozoon Canis Infection
Etiology
Dogs
Cats and Wild Carnivores
Epidemiology
Geographic Distribution and Prevalence
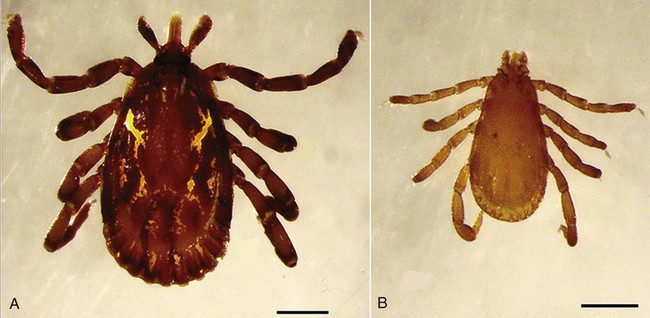
Transmission
Epidemiologic Characteristics
Pathogenesis
Clinical Findings
Dogs
Diagnosis
Variable
Hepatozoon canis
Hepatozoon americanum
Geographic distribution
Asia, southern Europe, Africa, South America, southern United States
Southern United States
Principal vector ticks
Rhipicephalus sanguineus; Amblyomma ovale
Gulf Coast tick, Amblyomma maculatum
Major target organs
Spleen, bone marrow, lymph nodes
Skeletal muscles, myocardium
Common clinical examination findings
Fever, lethargy, weight loss
Gait abnormalities, fever, muscle hyperesthesia, mucopurulent ocular discharge, lethargy
Severity of clinical disease
Usually mild when parasitemia is low; a severe disease with large numbers of circulating gamonts
Severe
Hematologic findings
Anemia; extreme neutrophilia is infrequent and found in dogs with large numbers of circulating gamonts; circulating blood gamonts are common and found mainly in neutrophils and rarely in monocytes; parasitemia ranges from 1% to 100% of the neutrophils
Anemia; neutrophilia is common and often extreme; circulating blood gamonts are infrequent and usually found in less than 0.1% of the leukocytes
Radiographic findings
Nonspecific
Periosteal proliferation
Histopathologic findings
Hepatitis, splenitis, nephritis, and pneumonia associated with presence of H. canis meronts
Pyogranulomatous myositis
Unique morphologic features
“Wheel spoke” meront; small monozoic splenic tissue cysts
Large muscle “onion skin cysts”
Main diagnostic procedure
Demonstrating gamonts in blood or buffy coat smear
Muscle biopsy demonstrating tissue cysts and pyogranulomas associated with developing parasitic stages, quantitative PCR
Serologic tests
Indirect FA test, ELISA (gamont antigens)
ELISA (sporozoite antigens)
Molecular tests
18S rRNA gene (rDNA) PCR; quantitative PCR
18S rRNA gene (rDNA) PCR; quantitative PCR
Clinical Laboratory Findings
Organism Identification
Serologic Testing
Molecular Genetic Testing
Pathologic Findings
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Hepatozoonosis