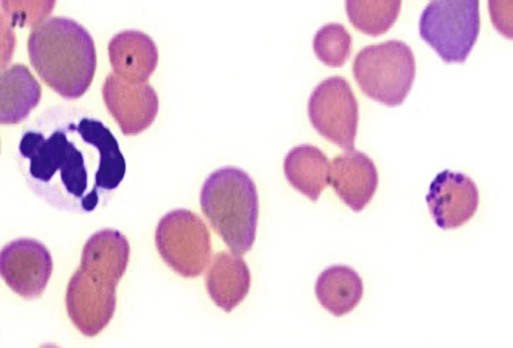
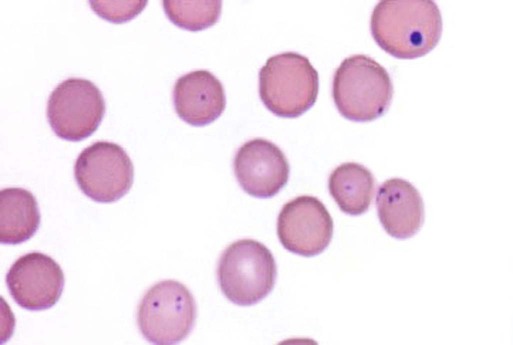
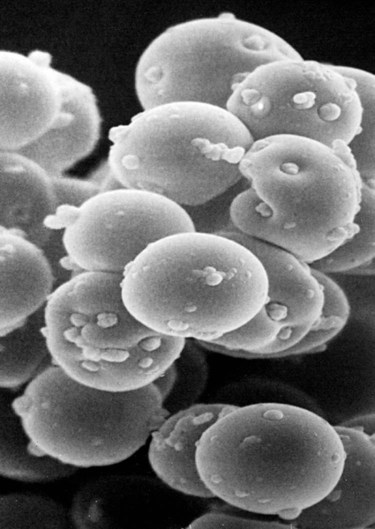
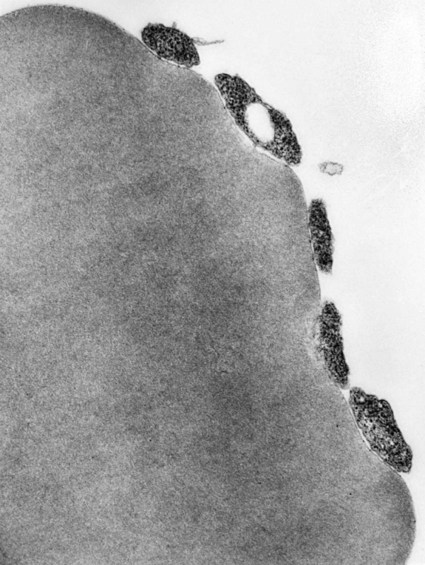
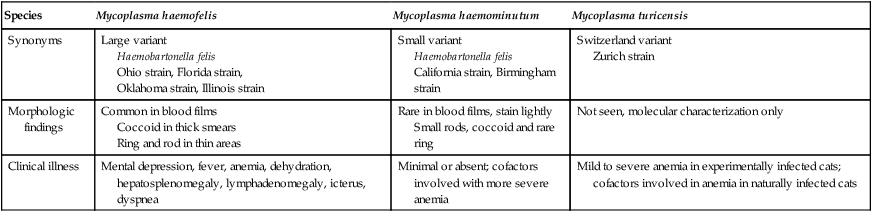
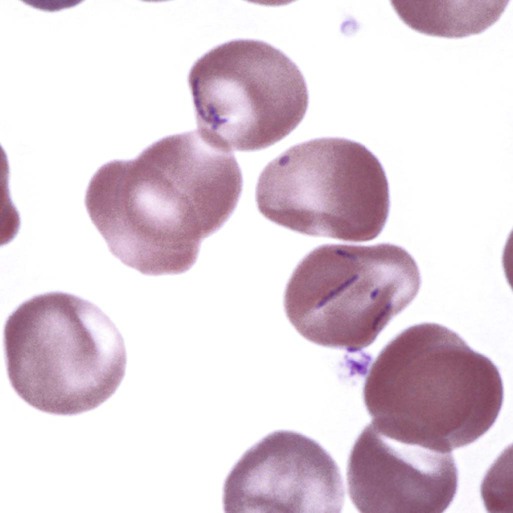
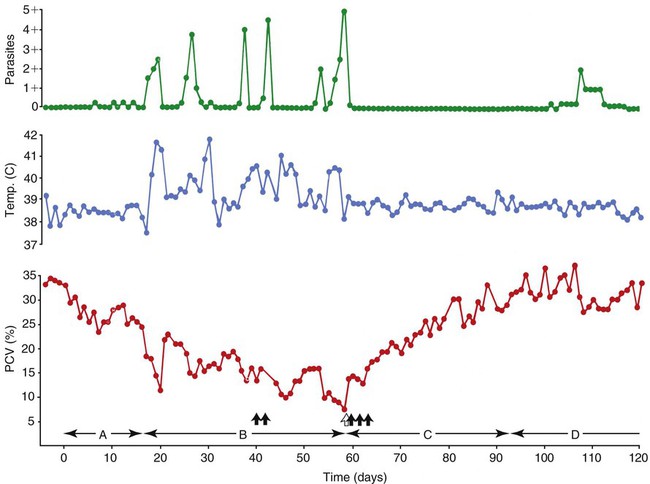
Hemotropic mycoplasmas are wall-less bacterial organisms that attach to and grow on the surface of red blood cells. They are gram-negative and non-acid-fast, and despite numerous attempts, culturing them on artificial media has been unsuccessful. These epicellular organisms were classified as rickettsia in the genera Haemobartonella and Eperythrozoon for many years; however, results from sequencing of the 16S rRNA (ribosomal RNA)20,58,58 and Ribonuclease (RNase) P RNA genes indicate that they are mycoplasmas.67 Consequently, the Haemobartonella and Eperythrozoon genera have been discarded, and these hemotropic organisms have been moved to the genus Mycoplasma. This transfer has generated some controversy. Because of specific biological properties and sequence data, some argue that the hemotropic mycoplasmas should form a separate genus from Mycoplasma.88 Nevertheless, designated species names often include the prefix “haemo-” (e.g., Mycoplasma haemofelis) to identify these unique mycoplasmas that attach to erythrocytes, whereas new, incompletely described taxa are given a Candidatus designation. Hemoplasmas has been proposed as a general name for these hemotropic mycoplasmas. Hemotropic mycoplasmas contain DNA and RNA and replicate by binary fission. They may be rod-shaped, spherical, or ring-shaped and are found individually or in chains on the red blood cell. Three hemoplasmas, M. haemofelis, “Candidatus Mycoplasma haemominutum” and “Candidatus Mycoplasma turicensis,” are known to infect the cat. The similarities of 16S rRNA and RNase P RNA gene sequences between these organisms is only about 83%.21 One organism was previously referred to as the Haemobartonella felis large (Hflg) variant because the organism appeared larger than the second organism, which was referred to as the H. felis small (Hfsm) variant.20 The Hflg organism is now classified as M. haemofelis65 and the Hfsm organism is now classified as “Candidatus M. haemominutum.”21 Organisms previously classified as Florida, Ohio, Oklahoma, and Illinois isolates of H. felis are now classified as M. haemofelis isolates, and organisms previously classified as California and Birmingham isolates of H. felis are now classified as isolates of “Candidatus M. haemominutum” based on similarities in the sequence of the 16S rRNA gene (Table 31-1).82 An Australian strain of M. haemofelis has been reported with a 16S rRNA gene sequence identical to the Ohio and Florida isolates.13 “Candidatus M. turicensis,” a third hemoplasma was reported in a Swiss cat. The 16S rRNA gene sequences of this organism had an 88% and 83% similarity to M. haemofelis and “Candidatus M. haemominutum,” respectively.95 TABLE 31-1 Comparison of Hemotropic Mycoplasmas of Cats In polychrome-stained blood films, hemotropic mycoplasmas appear as small dark blue-staining rods, as cocci (0.3 to 0.8 µm), or as slight larger ring forms that are usually attached to erythrocytes. The genome size of M. haemofelis is about 1200 kb2, a finding supported by the construction of an optical map of its whole genome. In thick blood films or thick areas of thin films, nearly all organisms appear as cocci. Ring- and rod-shaped organisms are seen more readily in thin blood films or the feathered edges of thick blood films (Fig. 31-1). “Candidatus M. haemominutum” organisms are rarely observed in stained blood films from infected cats. When seen, “Candidatus M. haemominutum” appear as small rods or coccoid organisms and infrequently ring-forms; low numbers of organisms are detected per red blood cell, which stain less densely and measure about half the size (approximately 0.3 µm) of M. haemofelis (Fig. 31-2).20,21,21 However, this reported size difference has been challenged by the finding of a “Candidatus M. haemominutum” isolate in Great Britain that is about 0.6 µm in diameter.82 Morphologic appearance is not reliable in distinguishing isolates, and genetic analysis is needed for specific identification. In none of the cats infected with “Candidatus M. turicensis” have hemoplasmas been definitely identified by light or electron microscopy.95 The genomic sizes of “Candidatus M. haemominutum” and “Candidatus M. turicensis” have not been reported. The epicellular nature of M. haemofelis on erythrocytes is readily apparent by scanning electron microscopy (EM) examinations (Fig. 31-3). Organisms appear to be partially buried in indented foci on the surface of the erythrocytes. Discoid, conical, coccoid, rod-shaped, and doughnut-shaped organisms have been observed. Parasitized erythrocytes may lose the normal biconcave shape and become spherocytes or stomatospherocytes. Transmission EM (Fig. 31-4) further supports the epicellular nature of this organism.73 A 15- to 25-nm clear zone separates the organism from the erythrocyte membrane, with delicate fibrils from the organism appearing to extend through this zone, attaching the organism to the erythrocyte membrane at intermittent contact points. Although smudging of the erythrocytic membrane in association with organism attachment has been reported, complete erosion of the membrane has not been documented. A single membrane surrounds the organisms. The cytoplasm of organisms is composed of granules that vary in size and density; however, no cytoplasmic organelles have been recognized. Electron-lucent areas (vacuoles) appear to be present in some organisms. “Candidatus M. haemominutum” is ultrastructurally similar to M. haemofelis and other mollicutes. Although somewhat smaller, it has a limiting plasma membrane with no cell wall or internal membranous structures.21 Mycoplasma haemocanis (formerly Haemobartonella canis)54,60 is considered to be the causative agent of hemotropic mycoplasmosis in dogs; however, the sequence of the 16S rRNA gene of M. haemocanis was remarkably similar (99% homology) to that of M. haemofelis, suggesting that these agents might be different strains of the same organism.10 Other studies evaluating RNase P gene sequences demonstrated a lower degree of sequence homology between the two organisms (about 95%), indicating that the organisms may be different species.7 M. haemocanis differs morphologically from M. haemofelis in that it more commonly forms chains that extend across the surface of affected erythrocytes (Fig. 31-5). However, individual organisms may also appear as small cocci, rods, or rings. A novel small hemoplasma, “Candidatus Mycoplasma haematoparvum,” which is genetically more closely related to “Candidatus M. haemominutum” than to M. haemofelis, was isolated from the blood of a splenectomized dog with hematopoietic neoplasia.74 In a study involving real-time polymerase chain reaction (PCR) of blood from dogs in the south of France, this novel organism was more prevalent than M. haemocanis.42 Using the same detection method, a low prevalence of canine hemoplasma infections was found in Switzerland.92 A study based on conventional PCR in free-roaming dogs in the Sudan found high hemoplasma infection rates.37 Using PCR-based assays, investigators in the United States38 and Spain15 reported finding about 30% of anemic cats suspected of having hemotropic mycoplasmosis to have positive results for hemotropic mycoplasmas by PCR, with a majority of cats having positive results for M. haemofelis alone or in combination with “Candidatus M. haemominutum.” Additionally, about 14% of healthy control cats in the U.S. study38 had positive PCR results for hemotropic mycoplasmas. Of these PCR-positive-result healthy cats, 19 were infected only with “Candidatus M. haemominutum” and one was infected with both variants.38 Some caution is needed in interpreting these results, because only one variant was subsequently identified by PCR in some cats experimentally infected with both variants.93 In another study in Spain, positive results were found in 3.7% of cats for M. haemofelis and 9.9% of cats for “Candidatus M. haemominutum,” while a small number of cats had combined infections.70a A positive correlation was found between hemoplasma infection and outdoor access, feline immunodeficiency virus (FIV) co-infection, and male sex. In Canada, subclinical hemoplasma infections were found in 12% of shelter cats and 4% of client-owned cats; however, no risk factors were found to correlate with the rate of infection.65a In a survey of domestic cats in Japan suspected of having a hemoplasma infection, 67% had positive PCR results for M. haemofelis, 22% had positive results for M. haemominutum, and 11% were co-infected.91 In Australia, the prevalence rates of infection in flea-infested cats were 0.9% for M. haemofelis and 15.3% for “Candidatus M. haemominutum.”2a In anemic and nonanemic cats examined by PCR at a veterinary teaching hospital on the west coast of the United States, the prevalence of hemoplasma infections was 16% for “Candidatus M. haemominutum,” but only 0.4% for both M. haemofelis and “Candidatus M. turicensis.” The possibility of a fourth hemoplasma in cats, an “M. haematoparvum”-like organism, was also identified in 0.7% of the cats.75 In a survey of British cats, less than 2% of ill cats (anemic or nonanemic) and less than 2% of healthy cats were infected with M. haemofelis. The prevalence of “Candidatus M. haemominutum” was about 20% in ill cats (anemic or nonanemic); however, only 8% of healthy cats in this study were infected with “Candidatus M. haemominutum.”77 In a survey of feral cats in northern Florida, 8% had PCR positive results for M. haemofelis and 12% had positive PCR results for “Candidatus M. haemominutum.”55 As in other reports, males were more commonly infected than females, and a positive correlation was found between hemotropic mycoplasmal infections and FeLV and FIV infections. “Candidatus M. turicensis” infections have been compared in Switzerland, the United Kingdom, the United States, Australia, and South Africa.70a,97 The numbers of cats (clinically ill and healthy) having positive real-time PCR test results were 1%, 2.3%, 10%, and 26% in these respective countries.96 In another study, in Eastern Australia, the prevalence rate of infection in flea-infested cats was 0.9%.2a In Spain, the prevalence of infection in cats (clinically ill and healthy) was 7.9%.70a M. haemocanis has been reported in both North American and European dogs. Blood samples from dogs living in the south of France showed 9.6% were infected with “Candidatus M. haematoparvum,” whereas only 3.3% were infected with M. haemocanis. However, in Switzerland only 1.2% of dogs had positive real-time PCR results for the canine hemoplasmas. The prevalence in Europe is higher in the Mediterranean countries.96b A study using conventional PCR testing of free-roaming dogs in the Sudan found much higher hemoplasma infection rates.37 It is postulated that the presence or absence of an appropriate vector for transmission of the organisms may explain these differences.92 Based on the morphologic appearance of the organisms, clinical signs, and laboratory findings, it appears that most publications concerning feline hemotropic mycoplasmal infections published before 1998 involved M. haemofelis. The Florida and Ohio strains of the organism used in experimental studies cited in this chapter were shown to be M. haemofelis by the PCR and sequencing of the 16S rRNA gene.70 M. haemofelis often produces anemia and clinical signs of disease, whereas “Candidatus M. haemominutum” generally results in inapparent infections and a minimal change in hematocrit (HCT), unless complicated by feline leukemia virus (FeLV) infections or another concurrent disease.20,21,23,77,93 The following discussion focuses on the pathogenesis of disease associated with M. haemofelis infection. Experimentally, M. haemofelis infection has been transmitted by intraperitoneal and intravenous injection and oral administration of infected blood. Dissemination of infection by blood-sucking arthropods such as fleas is considered by many to be the primary mode of transmission; however, such transmission has been shown experimentally in only one cat, and the infection was transient without clinical or hematologic changes.98 It has been suggested that M. haemofelis can be transmitted from queens with clinical disease to their newborn offspring in the absence of arthropod vectors. Whether or not this transmission occurs in utero, during parturition, or via nursing is unknown, because experimental studies examining this mode of transmission are lacking. Iatrogenic transmission of M. haemofelis can occur by the transfusion of blood from clinically healthy carrier cats. The severity of disease produced by M. haemofelis varies, with some cats having mild anemia and no clinical signs and others having marked depression and severe anemia leading to death. To facilitate our understanding, the disease has been divided into preparasitemic, acute, recovery, and carrier phases, or stages (Fig. 31-6).33 The preparasitemic phase is generally about 1 to 3 weeks after intravenous injection. The acute phase of disease represents the time from the first to the last major parasitemia. This phase often lasts a month or more, but occasionally cats die quickly from massive parasitemias and precipitous decreases in HCT early in the course of the disease. Organisms generally appear in the blood in a cyclic manner within discrete parasitemic episodes (see Fig. 31-6). The number of organisms generally increases to a peak value over 1 to 5 days, followed by a rapid decline. The synchronized disappearance of organisms from the blood can occur in 2 hours or less. Few if any organisms are seen in blood films for several days after parasitemic episodes, although low numbers of organisms can still be detected in blood using PCR.4,83 Repetitive parasitemic episodes appear to cause progressive erythrocyte damage and shortened erythrocyte life spans. Some damage to erythrocytes may be caused directly by the organism, but immune-mediated injury appears to be more important. Coombs’ (direct antiglobulin) tests (37° C) may have positive results within a week after the first parasitemia, and Coombs’ test results generally remain positive during the acute stage of disease, regardless of whether organisms are present. Erythrocyte-bound antibodies reacting at 4° C (both IgM and IgG) appeared between 8 and 22 days after experimental infection with M. haemofelis and persisted for 2 to 4 weeks, whereas warm reacting antibodies (37° C) appeared later, between days 22 and 29, and persisted up to 5 weeks.87 The authors of this study proposed that erythrocyte antibodies arose as a sequel to M. haemofelis infection and/or hemolysis rather than mediating the anemia. The attachment of organisms to erythrocytes either exposes hidden erythrocyte antigens or results in altered erythrocyte antigens, with a subsequent host response of producing anti-erythrocyte antibodies. Specific antibodies against M. haemofelis antigens have been detected in serum within 14 days after experimental infections.1 However, another possible mechanism of immune-mediated injury should also be considered. If antibody-mediated complement fixation occurs, the erythrocytic membrane may be damaged as an “innocent bystander.” However, minimal intravascular hemolysis occurs in this disorder. The anemia occurs primarily as a result of extravascular erythrophagocytosis by macrophages in the spleen, liver, lungs, and bone marrow. In contrast to M. haemofelis infection, no cold- or warm-reactive erythrocyte-bound antibodies have been found in cats with “Candidatus M. haemominutum” and “Candidatus M. turicensis” infections.87 As a lymphocyte- and macrophage-rich blood filter, the spleen is of primary importance in the clearance of bloodborne particulate antigens and the elaboration of specific immune responses to these antigens. Results of quantitative PCR indicate that splenic and pulmonary tissues had more M. haemofelis than others in relation to blood levels.86 However, rapid replication rather than release from sequestration sites was presumed to cause the cyclic increases in parasite numbers.86 In animals other than the cat, splenectomy is generally required before clinical disease is produced by the various species-specific hemotropic mycoplasmal organisms. M. haemofelis organisms are removed less readily in splenectomized cats, resulting in parasitemias that last about twice as long as those in intact cats. However, splenectomy performed before infection does not appear to affect the incubation period or severity of disease in cats. Splenectomy after cats have recovered results in transient reappearance of blood organisms, but in most animals the HCT does not drop to a clinically significant level. Cats that recover from acute infections with M. haemofelis remain chronically infected for months to years, if not for life. Although an extracellular organism should be eliminated by immune mechanisms, intact organisms have been reported within phagocytic vacuoles of splenic and pulmonary macrophages. Some organisms may survive within these cells, which would account for the indefinite, chronically infected state (although in situ hybridization studies of an infected cat using a specific DNA probe demonstrated organisms only on erythrocytes).6 A report demonstrating the intracellular location of another hemoplasma species is intriguing, because this site might also afford protection and account for the development of a chronic infection.25 Carrier cats (those with latent infections) appear clinically healthy. They may have an HCT within reference limits or mild regenerative anemia. Low numbers of organisms are regularly observed in some cats, but in others no organisms are visible in blood films for weeks. Carrier cats appear to be in a balanced state in which replication of organisms is balanced by phagocytosis and removal of organisms. M. haemofelis may be an opportunistic agent that exists in healthy cats and produces disease when the cat is stressed by other diseases or surgical procedures; however, some cats with hemotropic mycoplasmosis do not have identifiable predisposing disease or stress conditions. Cat-bite abscesses appear to be the most frequent disorder recognized to precede hemotropic mycoplasmosis by a few weeks.24 Although an abscess is undoubtedly stressful, the possibility of transmission of the disease through a cat bite should be considered. Studies have shown that M. haemofelis could not be detected in saliva or salivary gland samples from infected cats.17 Thus, social contact and grooming behavior are unlikely to result in transmission; however, this still does not exclude the possibility of transmission by aggressive interactions associated with bleeding or perhaps percutaneous inoculation of contaminated flea feces from an infected cat’s haircoat. Other factors associated with an increased likelihood of finding M. haemofelis organisms in stained blood films include male sex, lack of vaccinations, outdoor roaming, and positive FeLV and FIV tests.24,28 Of particular interest is the possible interrelationship between M. haemofelis and FeLV-produced disease. About 40% to 50% of cats with clinical hemotropic mycoplasmosis based on microscopic findings are FeLV positive.24,28,28 FeLV can suppress the normal immune response to unrelated antigens; thus this viral infection might increase the susceptibility of cats to hemotropic mycoplasmosis or convert a latent hemotropic mycoplasmal infection into a patent infection with clinical disease. However, FeLV-infected cats in Brazil were not shown to be at greater risk than FeLV-negative cats for M. haemofelis infection.56 Experimental studies have demonstrated that the opposite can occur—M. haemofelis infection can increase the susceptibility of cats to FeLV infection.44 FeLV and FIV infection of cats in the United States was significantly associated with M. haemofelis infection and may support this possible relationship.76 Regardless of how the concurrent infections occur, infection with M. haemofelis and FeLV generally results in more severe anemia and clinical signs than occur with infection by either agent alone.8,28,28 In contrast, concurrent infection with FIV and M. haemofelis does not appear to cause more severe anemia than does M. haemofelis infection alone.28,68 A growing body of information is available concerning the pathogenesis of “Candidatus M. haemominutum.” Infection with this hemoplasma often results in no clinical illness or significant anemia, and a carrier status is commonly encountered.81 Interestingly, substantial parasitemias can occur in blood with little change in HCT.20,75,83,87,93 However, in one study, a slight decrease in HCT values was demonstrated after experimental “Candidatus M. haemominutum” infection, suggesting that this organism may induce erythrocyte destruction.80,81 The measured copy numbers of organism DNAs in these experimentally infected cats peaked around 30 days, but no marked variations or cycling of numbers were observed. There are also reports of “Candidatus M. haemominutum” as the only recognizable cause of anemia in some naturally infected cats.69 Cofactors may be involved in producing clinical illness with “Candidatus M. haemominutum” infection. In one study, older age, outdoor exposure, cutaneous squamous cell carcinoma, and stomatitis were identified as risk factors for infection.75 Several studies have shown that FIV-infected pet and feral cats are at greater risk for having an infection.55,46,46 As in M. haemofelis–infected cats discussed previously, cats co-infected with FeLV and “Candidatus M. haemominutum” have more severe anemia than cats infected only with “Candidatus M. haemominutum.”23 In addition, the stress of infection with “Candidatus M. haemominutum” may induce the development of myeloproliferative disease in FeLV-infected cats.23 Although some investigators indicate that infection with “Candidatus M. haemominutum” is protective against challenge with the virulent M. haemofelis, other investigators report that cats chronically infected with “Candidatus M. haemominutum” are not protected against M. haemofelis infection.21,93 This latter group of investigators provides evidence that the anemia occurring with combined infections is more severe than the anemia occurring in cats infected only with M. haemofelis. Previous infection with one strain might predispose cats to having heightened immune-mediated responses when they become infected with a second strain.6 Limited information is available concerning the pathogenesis of “Candidatus M. turicensis,” a unique hemoplasma isolated in a blood sample collected from a cat with severe hemolytic anemia.95 Its transmission by IV inoculation to susceptible cats resulted in a moderate to severe anemia. Peak parasitemia as measured by real-time PCR occurred 16 to 18 days after the experimental infection, but no overt cycling of the hemoplasma load was evident. Characteristic hemoplasma-like inclusions have not been identified on blood smears. In naturally infected cats, a low copy numbers of “Candidatus M. turicensis” is characteristically found in the blood.95 It appears that spontaneous elimination of “Candidatus M. turicensis” infection may be possible without treatment in infected cats.95 “Candidatus M. turicensis” also has been detected in the saliva and feces of infected cats using molecular techniques.96 However, inoculation of hemoplasma-positive saliva by oronasal and subcutaneous routes failed to lead to infection in recipient cats.62 Studies suggest that cofactors, such as concurrent FIV infection and immunosuppression, may increase the pathogenic potential of this organism. “Candidatus M. turicensis” infections often have been associated with co-infection with other hemoplasma species, especially “Candidatus M. haemominutum.”67
Hemotropic Mycoplasmosis (Hemobartonellosis)
Etiology
Hemotropic Mycoplasmas in Cats
Species
Mycoplasma haemofelis
Mycoplasma haemominutum
Mycoplasma turicensis
Synonyms
Large variant
Haemobartonella felis
Ohio strain, Florida strain,
Oklahoma strain, Illinois strain
Small variant
Haemobartonella felis
California strain, Birmingham strain
Switzerland variant
Zurich strain
Morphologic findings
Common in blood films
Coccoid in thick smears
Ring and rod in thin areas
Rare in blood films, stain lightly
Small rods, coccoid and rare ring
Not seen, molecular characterization only
Clinical illness
Mental depression, fever, anemia, dehydration, hepatosplenomegaly, lymphadenomegaly, icterus, dyspnea
Minimal or absent; cofactors involved with more severe anemia
Mild to severe anemia in experimentally infected cats; cofactors involved in anemia in naturally infected cats
Hemotropic Mycoplasma Organisms in Dogs
Epidemiology and Pathogenesis
Prevalence of Infection
Cats
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine