5 Hemostatic Abnormalities
Goals of Hemostasis Testing
Hemostasis and Diagnosis of Disorders
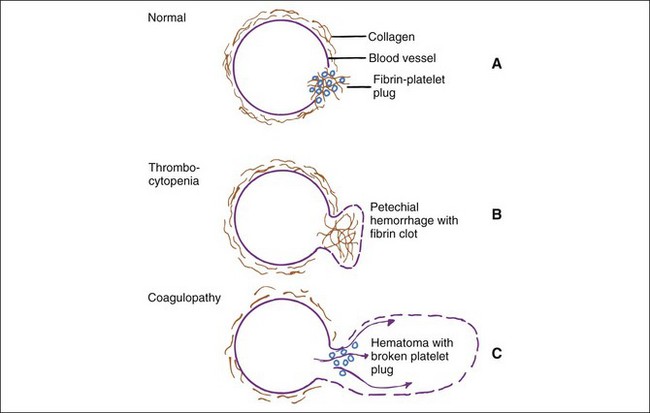
Evaluation of the cause of bleeding varies from handling of overt problems (e.g., severe epistaxis), to detection of suspected problems (e.g., von Willebrand’s disease [vWD] in a Doberman pinscher), to investigation of unexpected problems found during laboratory examination (e.g., mild to moderate thrombocytopenia, evidence of hepatic disease). Signs of bleeding problems may include prolonged bleeding after parturition, estrus, or minor trauma (e.g., venipuncture, loss of deciduous teeth), or spontaneous hemorrhages (i.e., petechiae, ecchymoses, hematomas). Gastrointestinal tract bleeding may appear as fresh red blood or dark tarry stools and is often occult. Bleeding into joints and muscle may cause lameness. Bleeding into body cavities may be diagnosed from fluid cytology. Small hemorrhages such as petechiae and ecchymoses suggest a platelet or vascular defect (Figure 5-1). Epistaxis is often associated with platelet defects, perhaps because of the paucity of tissue between the vessels and the nasal mucosa. In contrast, coagulation defects are characterized by large, deep hemorrhages (e.g., hematomas, hemarthroses).
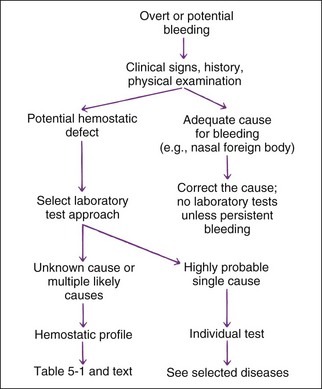
A simple approach for test selection is shown in Figure 5-2. If physical examination shows no cause of the bleeding or bleeding seems disproportionate to the injury, the expense of hemostatic tests (relatively expensive testing) is warranted to document and localize the defect. Some situations require only a single test (e.g., von Willebrand’s factor [vWF] assay or capillary bleeding time as a presurgical screen for elective surgery on a Doberman due to their high incidence of having vWD). Similarly, PT is the preferred single test for suspected ingestion of warfarin-type rodenticide. However, a profile of tests performed the same day is recommended to localize an unknown bleeding defect (Table 5-1; Table 5-2). Some hemostatic disorders affect multiple areas of hemostasis, so evaluation of only one or two tests may lead to an incomplete or erroneous conclusion. Variation in production and inactivation of coagulation and anticoagulation factors and variation in production and removal of platelets and effects of treatment confuse diagnosis; therefore, performing a complete profile of tests concurrently at initial presentation permits a more likely correct diagnosis. Normal results in a profile exclude some diseases from consideration.
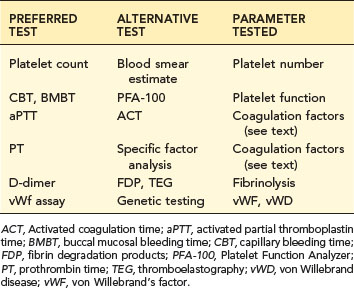
TABLE 5-1 HEMOSTATIC SCREENING PROFILE
| PREFERRED TEST | ALTERNATIVE TEST | PARAMETER TESTED |
|---|---|---|
| Platelet count | Blood smear estimate | Platelet number |
| CBT, BMBT | PFA-100 | Platelet function |
| aPTT | ACT | Coagulation factors (see text) |
| PT | Specific factor analysis | Coagulation factors (see text) |
| D-dimer | FDP, TEG | Fibrinolysis |
| vWf assay | Genetic testing | vWF, vWD |
ACT, Activated coagulation time; aPTT, activated partial thromboplastin time; BMBT, buccal mucosal bleeding time; CBT, capillary bleeding time; FDP, fibrin degradation products; PFA-100, Platelet Function Analyzer; PT, prothrombin time; TEG, thromboelastography; vWD, von Willebrand disease; vWF, von Willebrand’s factor.
A reasonable profile includes a platelet count, a test of platelet function, the aPTT to assess intrinsic and common coagulation pathways, the PT to assess extrinsic and common pathways, and a test of fibrinolytic activity such as D-dimer (see Table 5-1). Alternative and additional tests are used in certain situations (see section on Laboratory Tests).
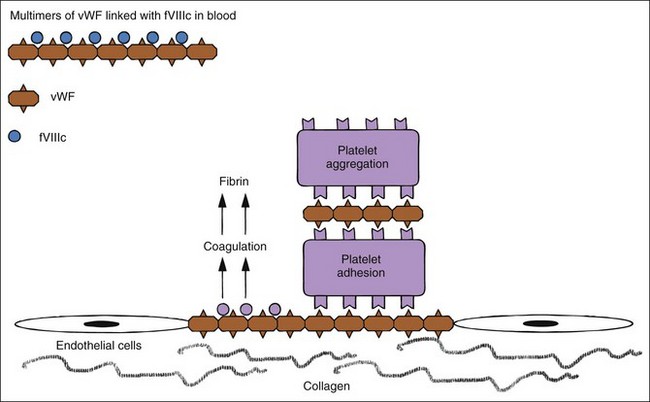
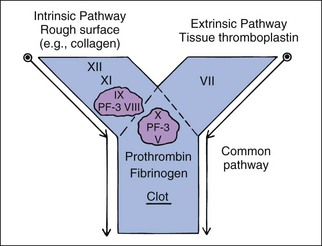
Hemostatic defects should initially be localized to a general area of the hemostatic mechanism, which has four main components: the blood vessels, the platelets, coagulation factors, and the fibrinolytic system (Table 5-2). A plug of aggregated platelets initially seals damaged vessels after circulating platelets come into contact with exposed subendothelial collagen (primary hemostasis). Platelets bind to collagen and each other with the help of vWF (Figure 5-3; see Figure 5-1). The coagulation factors stimulated by the collagen, tissue thromboplastin, and platelets form fibrin strands to stabilize the platelet plug (secondary hemostasis). The fibrinolytic system degrades the clot to reopen the vascular lumen to blood flow while the vessel heals. A platelet plug forms in coagulopathies, but easily breaks down and allows rebleeding because it is not stabilized by fibrin strands. A severe deficiency of one or more coagulation factors slows the formation of a clot, which should form rapidly after contact with the subendothelial collagen. During this time, larger sized hemorrhages can occur before a fibrin clot is finally formed or pressure of adjacent tissues stops the bleeding (see Figure 5-1).
Components of Normal Hemostasis
Platelets and Primary Hemostasis
Platelets are small cytoplasmic fragments shed from megakaryocytes in the bone marrow. They circulate for approximately 5 days in dogs and about 30 hours in cats. Increased thrombopoiesis is simulated by decreased total circulating platelet mass (measured by plateletcrit) and not necessarily reduced platelet count (thrombocytopenia). For example, Cavalier King Charles spaniels with a hereditary dysplasia with fewer but larger platelets have a normal platelecrit.11,54 Plateletcrit, like hematocrit, is the percentage of blood volume composed of platelets. Thus the platelet count, though universally used to judge the adequacy of platelets for hemostasis, is not as physiologic a test as the plateletcrit.
Platelets adhere to a defect in a vascular wall and clump together as a platelet plug. Platelets then enhance and accelerate the coagulation cascade and formation of stabilizing clots. vWF is needed for primary hemostasis and can affect some tests of platelet function. The binding of platelets results in a conformational change to expose previously internalized phosphatidylserine (formerly known as PF-3, a potent receptor for factor X and prothrombin; Figure 5-4), which causes platelet activation, release of platelet granule contents, aggregation of more platelets, and initiation of secondary hemostasis by formation of a procoagulant surface of fibrinogen receptors on the primary plug. Release of adenosine diphosphate (ADP) from the dense granules causes synthesis of thromboxane A2, a prostaglandin that causes irreversible platelet aggregation and viscous metamorphosis and local vasoconstriction. Primary hemostasis requires adequate numbers of platelets that function adequately.
Coagulation Factors
Coagulation factor interactions are much more complex than this description, but may be divided into three parts—(1) intrinsic pathway, (2) extrinsic pathway, and (3) common pathway—for diagnostic purposes. These three parts are diagrammed as a letter Y (see Figure 5-4). The intrinsic pathway includes factors XII, XI, IX, and VIII and is initiated by contact activation. Factor XII is activated when it contacts a nonendothelial surface such as collagen outside a blood vessel.
To keep coagulation localized to the defect, once procoagulant factors have been activated, they are inhibited by anticoagulant factors such as antithrombin III (AT III, complexed with heparin) and removed by the phagocytic system. Those that escape AT III will bind with thrombomodulin on endothelial cells, which activates protein C. Protein C is an anticoagulant and profibrinolytic factor that, with protein S as a cofactor, specifically hydrolyzes factors V and VIII and inhibits thrombin formation.37 Several factors (II, VII, IX, X and protein C) are dependent on vitamin K. Vitamin K antagonists (i.e., warfarin) interfere with hemostasis by interfering with these factors. These factors have different half-lives, which affects which of them become deficient earliest during warfarin poisoning. Factor VII and protein C become deficient earliest, and lack of protein C can lead to often unexpected hypercoagulability early in warfarin poisoning or high-dose warfarin treatment.
Heparin inhibits thrombin and other procoagulant factors indirectly by activating AT III, a circulating natural anticoagulant that is the main physiologic inhibitor of thrombin. Because AT III is usually decreased in DIC, heparin therapy may be ineffective. Heparin has been used clinically to treat hemostatic abnormalities associated with severe gastrointestinal disease, septicemia, and endotoxemia.36 Decreased AT III occurs in hepatic insufficiency, owing to decreased synthesis, and in glomerular disease because of increased loss through the glomerulus. Availability of the AT III assay varies. Automated assays with chromogenic substrates are used by some veterinary laboratories and allow routine testing. However, tests of AT III function, protein C, and protein S are expensive to maintain in terms of time, quality control, and reagents, so they are available only at a few hemostasis reference laboratories. Lack of information about the activity of these anticoagulant factors impedes ability to fully understand the balance of hemostasis in a patient.
Laboratory Tests
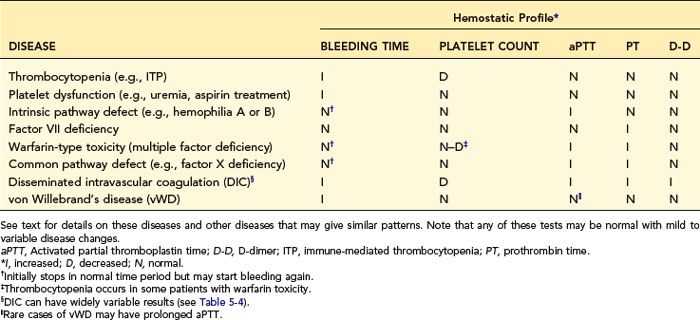
When a profile of tests is used, one should interpret each test individually and list the individual conclusions of what is normal or abnormal. Then one makes a disease diagnosis based on the total laboratory pattern and clinical evidence. Table 5-3 lists patterns expected in various diseases and may aid in diagnosis or understanding of the diseases discussed in the next section. As with all such tables, exceptions occur in the complexity of real cases.
Blood Collection for Hemostatic Testing
The citrated plasma-clotting tests (i.e., aPTT, PT, fibrinogen, thrombin time [TT], FDP) require nine parts fresh whole blood and one part 3.8% trisodium citrate anticoagulant to separate plasma from cells and platelets, which must be done within 30 minutes of sample collection. Citrate or oxalate blood collection tubes must be filled completely to a mark on the tube’s label to avoid errors in dilution. Plasma can be stored at 4° C for 48 hours or 20° C for 6 hours, or frozen for transport, to preserve factors VII and VIII. Furlanello has stated that samples were reasonably stable for 48 hours at room temperature and so may be sent to a laboratory.15 Certain tests require use of special tubes containing various reagents, so one should ask or read information from the laboratory before collecting the sample. Plastic tubes should be used because routine glass surfaces activate coagulation.
Platelet Enumeration
Hematology instrument platelet counts are accurate, sensitive, and precise methods for detecting and evaluating thrombocytopenia and should be used when available. However, blood smear estimates are often adequate for initial quick diagnosis of severe thrombocytopenia. Severe thrombocytopenia is a very common cause of bleeding, and an estimate may be used during off hours or when instruments are not available. After routine preparation and staining of a blood smear, one should determine the average number of platelets in 5 to 10 microscope fields using the 100× oil objective (i.e., 1000× magnification) to estimate platelet numbers.52 Dogs normally have about 8 to 29 platelets/field, and cats should have 10 to 29/field. If the thrombocytopenia is severe enough to cause bleeding, only 0 to 3 platelets/field are expected. Platelets should be counted in the thin “monolayer” area. On most smears, this is where the red blood cells (RBCs) infrequently touch each other, and central pallor in canine RBCs is prominent. Anemic, bleeding animals have fewer RBCs per field in the proper area, so it is easy to look too deeply into the thicker areas of a smear. An alternative is to consider each platelet/100× oil immersion field as approximately equal to 15,000 to 20,000 platelets/µl. There is variation to the number of platelets seen per field of view depending on the microscope objective. Better objectives have a larger field of view.
Feline platelets have a strong tendency to clump, frequently causing inaccurate and lower platelet counts. Prostaglandin E1 (PGE1) will prevent platelet aggregation.53 If added immediately to a feline blood sample or preferably included already in the tube, PGE1 will allow accurate platelet counts that on average are 60% higher than those without PGE1. Optical counts (in instruments with laser systems) in routine feline patients can be 50% to 62% higher than impedance counts because impedance-type instruments fail to identify large platelets, which are excluded from their platelet counts.29 Thus the combination of missing large platelets with impedance hematology instruments and not using PGE1 as an anticoagulant supplement will combine for substantial errors in feline platelet counts.53 PGE1 is expensive and must be frozen until just before use, but need only be used in cats where thrombocytopenia is likely or other dogs with persistent platelet aggregation of blood samples. Fortunately true and severe thrombocytopenia is rare in cats. In feline myeloproliferative disorders, huge numbers of large and bizarre platelets may not be counted. Other particles (e.g., ghost cells: partially lysed RBCs) may be erroneously counted as platelets. Ghost cells are not uncommon problems in immune-mediated hemolytic anemia (IMHA) samples. An abnormal appearance of platelet dot-plots from the Advia 2120 or Sysmex XT-2000iV suggests this error (Figure 2-6).51 Small RBCs in iron deficiency anemia can be counted as platelets, especially with impedance-type instruments. This “small red” error may be detected by inspecting the platelet-RBC histogram and noting a failure of separation of the two peaks representing platelets (left peak) and RBCs (right peak) (see Figure 2-3) or by instrument error flags.
Manual counts with a glass counting chamber (hemocytometer) may be used if automated hematology cell counters are not available or, rarely, if the automated count is in question (e.g., if ghost RBCs are counted as platelets). Manual platelet counts are imprecise and labor intensive but include large platelets and are more similar to optical instrument platelet counts than impedance feline platelet counts.29 The hemocytometer must be clean and free of scratches. Because platelets are the size of dust particles, nicks and scratches in the glass may cause misidentification of dust as platelets. One very good hemocytometer should be reserved just for platelet counts. A phase-contrast microscope helps to differentiate platelets from other particles. Lowering the condenser on a regular microscope also aids in visualizing the relatively transparent platelets. A platelet stain improves platelet detection in a hemocytometer but is usually unnecessary. If the blood sample is poor (e.g., has clots or platelet aggregates), it is not appropriate to request a manual platelet count, because it too will be affected by uneven platelet distribution in the blood.
Blood must be diluted such that the number of cells in the hemocytometer is easy to count. A dilution system with plastic containers is easiest. Becton-Dickinson (BD) discontinued their Unopette System and now recommends, as an alternative, similar products of Bioanalytic GmbH (www.bioanalytic.de). Bioanalytic’s Trombo-tic is advertized to lyse RBCs, disaggregate platelet clumps, and make platelets more rounded for better identification.
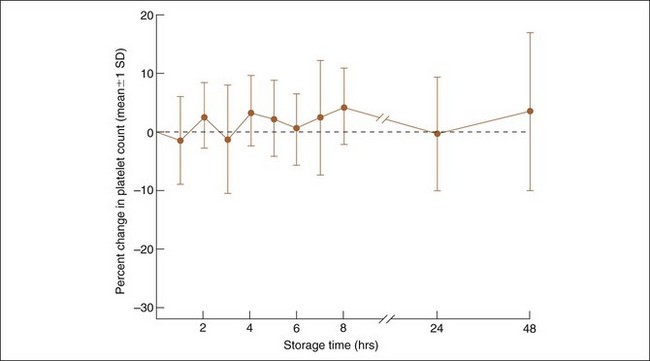
Delayed analysis favors platelet clumping and can lead to a lower platelet count. Various references suggest 30 minutes to 6 hours as a maximum tolerable limit. This may vary with the method, because an evaluation of 21 dogs with the Bayer H-1 hematology analyzer (precursor to the Advia 2120) suggested that clinically accurate platelet counts could be obtained with analysis of EDTA blood up to 2 days old (Figure 5-5). This suggests that EDTA blood sent to laboratories, remaining in the mail for 1 or 2 days, may still have clinically acceptable platelet count results if platelet clumping is absent or minimal.
Platelet Morphology
Platelets may be characterized in different ways by various instruments, including some of those mentioned in this section. Blood smears should be evaluated routinely to ensure that the platelet count appears reasonable. Large platelets are usually seen on blood smears of dogs with thrombocytopenia. However, neither increased numbers of large platelets, increased mean platelet volume (MPV), nor abnormal platelet distribution width (PDW) have been shown in the dog to differentiate among any of the causes of thrombocytopenia or other diseases. Larger platelets and increased MPV consistently are inversely correlated to platelet numbers, which thus may assure the clinician that a thrombocytopenia in the patient is real and not a laboratory error. Large platelets are more functionally active, which may explain why some dogs with platelet counts less than 10,000/µl do not bleed excessively. Cavalier King Charles spaniels often have a hereditary dysplasia with large but fewer platelets. This macrothrombocytopenia is due to a defect in beta1-tubulin.11 They have no signs of bleeding, though impedance platelet counts are often less than 10,000/µl. The Cavalier King Charles spaniels and Norfork terriers have a normal plateletcrit (percentage of blood volume, similar to hematocrit) despite variably low platelet counts (see Figure 2-7).54 Plateletcrit (platelet mass) is a more physiologic measurement than platelet count, but nobody can interpret plateletcrit in % blood volume or L/L. Plateletcrit is directly measured by the IDEXX VetAutoread Hematology Analyzer (QBC) but converted to a platelet number, which most veterinarians can interpret. The QBC measures plateletcrit directly by measuring the width of the platelet layer in a centrifuged tube. The Advia 2120 performs a plateletcrit that works fairly well even in Cavalier King Charles spaniels with their very large platelets (unpublished data). Other instruments enumerate platelet numbers well, but most have problems with accurate measurement of MPV, especially when distribution of platelets by size is not typical. Plateletcrit equals platelet count multiplied by MPV multiplied by a factor.
Large, RNA-rich platelets are named the high fluorescent platelet fraction (HFPF) in the Sysmex XT-2000iV instrument. These HFPF platelets were shown to be equivalent to reticulated platelets.41 HFPF platelets are consistently increased during active thrombopoiesis due to thrombocytopenia or treatment with thrombopoietin. The HFPF is greater in number in thrombocytopenia due to DIC, IMHA, and ITP than in dogs with thrombocytopenia due to leukemia.17,41 This suggests that enumerating the absolute number of large RNA-rich platelets may be useful in differentiating causes of thrombocytopenia (“regenerative” thrombocytopenia), pending further studies. However, greater numbers of large, RNA-rich platelets (HFPF) are seen in hereditary macrothrombocytopenia in Cavalier King Charles spaniels and Norfork terriers, which do not have increased thrombocytosis, and these large platelets are large throughout their lifespans.17
Platelets often have pseudopodia and an irregular shape on blood smears that suggests platelet activation during collection and handling of the blood. Decrease in the Advia 120 and 2120’s mean platelet component (MPC) concentration is an indicator of platelet activation. MPC was more decreased in dogs with IMHA then normal dogs and sick dogs without IMHA, and more decreased in those dogs with IMHA who died.61 Primary platelet disorders such as thrombasthenic thrombopathia in otter hounds, foxhounds, and Scottish terriers are rare causes of large and morphologically bizarre platelets.3,5 Ehrlichia platys may be visible in platelets.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree