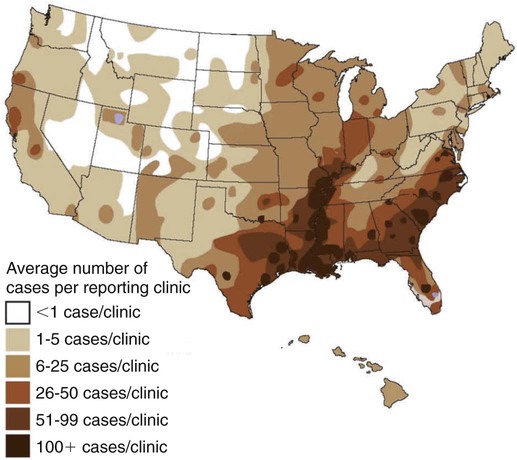
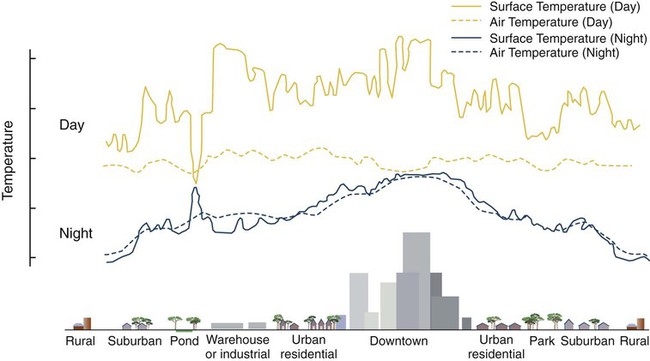
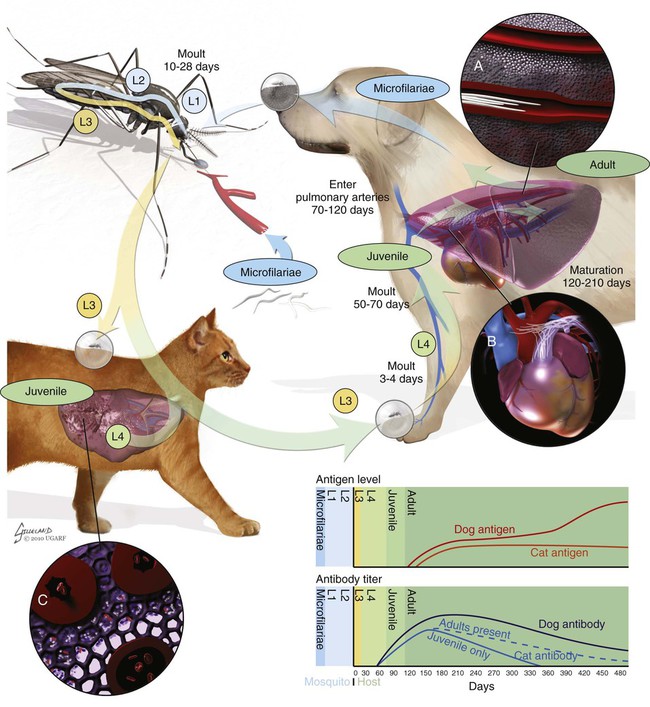
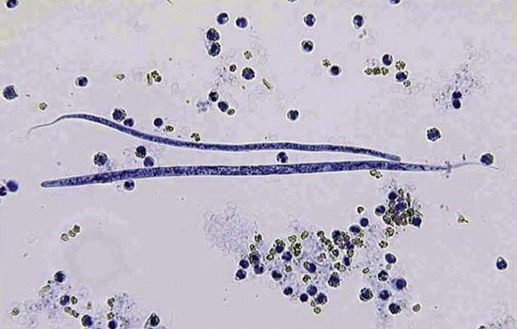
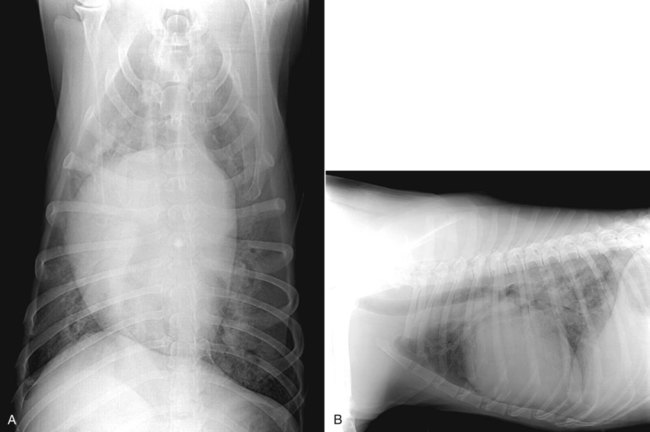
Heartworm disease is caused by the parasitic filarial nematode Dirofilaria immitis, belonging to the superfamily Filarioidea and family Onchocercidae. Nine filarial nematode species infect dogs worldwide, and three have been diagnosed in the United States: D. immitis, Dirofilaria repens, and Acanthocheilonema reconditum (formerly Dipetalonema reconditum). Only D. immitis and A. reconditum are considered endemic, but conditions exist for D. repens to become established. Heartworm was first described in dogs in Italy in 16267 and in the United States in 1847.44 Although canids (domestic dogs, coyotes, foxes, wolves, and other wild canids) are the definitive hosts for heartworms, the parasite has been found in more than 30 species of animals, including domestic cats and wild felids, bears, ferrets, seals, sea lions, and humans. There has even been one confirmed case in a bird.51 Information specific to the cat is presented in the second part of this chapter. Heartworms are endemic in North, Central, and South America; the Caribbean Islands; the coastal regions of Africa, southern Europe, and Asia; Japan; Indonesia; and Australia.49,61 In the United States, heartworm has progressed from being a parasitic disease primarily in the southeast to being diagnosed in all 50 states of the United States (Fig. 83-1). Alaska is the only state in which transmission has not been documented; however, there are mosquito vectors and climatic conditions present in regions of central Alaska to support the transmission of heartworms for brief periods.56 The yearly incidence of identified cases of canine heartworm infection continues to rise despite the availability of many chemoprophylactic drugs. The increased incidence has been prominent in the western half of the United States over the past 30 years. Two factors are likely responsible: (1) heartworm-infected dogs have been relocated to western regions as humans have moved from endemic areas, thus establishing a reservoir of infection, and (2) mosquito habitats have been created as a result of irrigation of newly developed areas and the subsequent influx of suitable vector mosquitoes. A sylvan cycle has been created in the coyote population as the rate of heartworm infection is over 90% in many western areas.50 The prevalence of infection elsewhere in the United States varies by region with over 90% of unprotected dogs along the Gulf Coast, lower Atlantic Coast, and Mississippi River valley being infected.39 The severity of clinical illness also varies by region. The worm biomass in an infected dog is typically higher in dogs coming from the high-prevalence areas just listed. However, there are isolated pockets of infection with high prevalence and severity of infection throughout the United States. There are multiple mosquito vectors in every region of the United States.52 More than 70 species of mosquitoes have been shown to be capable of transmitting heartworms; 22 species have been proven to be significant vectors. Importation of new mosquito species into an area, environmental changes created by humans, and natural climatic conditions can affect heartworm transmission. Aedes albopictus (Asian tiger mosquito), originally introduced into southeast Texas in 1985, has increased the exposure rate of dogs in many regions of the United States.53 This mosquito can now be found in 25 states primarily in the Southeast, the Midwest, and the Atlantic seaboard as far north as New Jersey. This large urban-dwelling mosquito can reproduce in small water-containing vessels such as flower pots. It voraciously feeds throughout the day, not just at dusk and dawn. In many areas, it has displaced Aedes aegypti as the primary heartworm-transmitting mosquito. The female lives for 3 months and is capable of overwintering. A. albopictus was introduced into Italy in 1990 and has spread over much of southern Europe, becoming a major vector of D. immitis and D. repens. Aedes sierrensis (western tree-hole mosquito) has had its geographic range extended in the western United States as a result of the continuing development of previously semiarid areas by the installation of irrigation systems and the planting of trees in new urban home sites. Laboratory studies have determined that a minimum temperature of 57° F is necessary for the development of heartworm larvae to the infective third-stage larva (L3) stage.32 This has led to the common practice of seasonal, rather than year-round, heartworm prevention in states north of the 37th parallel. Unfortunately, the heat island effect (Fig. 83-2) in urban areas can create microenvironments that are capable of supporting heartworm larva development even during winter months in northern states. Furthermore, Culex spp. mosquitoes are competent vectors that are frequently found indoors. Therefore, transmission can continue even during cooler months in large indoor kennels. These factors along with the northward spread of A. albopictus have prompted the American Heartworm Society to recommend year-round administration of heartworm preventive to dogs and cats in the continental United States and Hawaii.40 A working knowledge of the heartworm life cycle is important in developing and implementing prevention and treatment strategies (Fig. 83-3). The life cycle of D. immitis is 7 to 9 months (210 to 270 days). Female mosquitoes feeding on a microfilaremic canine host ingest microfilariae. The ingested microfilariae then undergo a transformation into first-stage larvae (L1) after entering a mosquito.10 The L1 will undergo two molts over the next 2 to 4 weeks, depending on the average ambient temperature, at which time they become infective L3. The L3 are then deposited on the dog’s skin in a droplet of hemolymph by a feeding female mosquito. The L3 enter through the bite wound into the subcutaneous tissue, where most L3 larvae molt to fourth-stage larvae (L4) within a couple of days. The L4 migrates in subcutaneous tissue and muscle toward the thorax, and from approximately 50 to 70 days after initial infection L4 larvae undergo a final molt to juvenile worms. These juvenile worms subsequently penetrate muscle tissue and eventually enter the circulation. Historically, the juvenile worm has been referred to as a fifth-stage larva (L5). Because this stage does not undergo another molt, it should not be considered a larval stage. It is an immature or juvenile worm that matures into an adult over the next several months. After entering a peripheral vein, the blood then carries immature worms to and through the heart and into the pulmonary arteries, arriving as early as 70 days postinoculation (PI).30 By 120 days PI, virtually all juvenile worms have entered the pulmonary vasculature. Final maturation to adult worms and mating occurs in the pulmonary vessels. Microfilariae are then produced, typically around 180 to 210 days (but up to 270 days) PI, completing the life cycle. Mature adults males range from 15 to 18 cm in length and females from 25 to 30 cm with a life expectancy of 5 to 7 years.46 Heartworm infections primarily cause damage to the pulmonary arteries and lungs. Juvenile worms entering the pulmonary vasculature 3 months PI initiate an eosinophilia and eosinophilic pneumonitis.13 Mature worms cause endothelial damage to pulmonary vessels, villous proliferation, and neutrophilic infiltrates in vessel walls. The rickettsial endosymbiont Wolbachia (see Chapter 25) produces interleukin (IL)-8 and has been implicated in this process of the disease. The exact role that these rickettsial organisms have in the pathologic process is uncertain.20a The severity of disease can be affected by the number of worms and the duration of the infection; but of equal importance, if not greater, is the activity level of the dog. Dogs in which 50 worms were surgically implanted and that were exercise restricted took longer to develop clinical disease, and developed less pulmonary vascular resistance, than dogs infected with 14 heartworms that were allowed moderate activity.16 This is also evident in naturally infected dogs in which there is no correlation between the number of heartworms and pulmonary vascular resistance, indicating that the individual host-parasite interaction plays an important role in the severity of disease.16 Results of a subsequent study included similar findings in dogs being treated with melarsomine.24 Whereas live heartworms can cause endarteritis and muscular hypertrophy of arteriole walls, primarily of the caudal pulmonary arteries, the pathologic changes seen in clinical disease are primarily the result of the effects of dying heartworms. As worms die from either natural causes or as a result of administration of adulticidal drugs, they decompose. Small worm fragments lodge in the distal pulmonary arteriole and capillary beds in the caudal lung lobes, blocking blood flow. These worm fragments, along with the inflammation that they elicit and platelet aggregation, result in pulmonary thromboembolism. During periods of increased activity or exercise, the increased blood flow to these blocked vessels can cause fragile capillaries to rupture with resultant pulmonary hemorrhage and subsequent fibrosis.20 This fibrosis, along with the release of vasoactive substances by heartworms, leads to increased pulmonary vascular resistance and subsequent pulmonary hypertension.29 The sequela to these pulmonary vascular events is cor pulmonale and potential right-sided heart failure. Ectopic lesions, due to aberrant migration of worms into the eye, central nervous system, abdominal cavity, and systemic circulation, can occur.10 “Caval syndrome,” which occurs in a small percentage of cases, results from heartworms located in the posterior vena cava and right atrium causing interference with tricuspid valve function. Luminal obstruction, which can result in a peracute hemolytic crisis from traumatic injury to erythrocytes, contributes to the development of overt right-sided heart failure. “Caval syndrome” results from excessively large worm burdens filling the entire pulmonary artery and subsequently extending into the right ventricle and atrium. It may also result from decreased right heart output, secondary to increased pulmonary vascular resistance, which allows the heartworms in the pulmonary artery to move back into the right heart. Glomerulonephropathy may also occur as a result of antigen-antibody complexes leading to proteinuria. Wolbachia surface proteins (WSPs) have been detected in large amounts within glomerular capillaries, suggesting that Wolbachia is associated with this pathologic condition (see Wolbachia Infection, Chapter 25). Many dogs infected with heartworms have no clinical signs, because dogs can tolerate live heartworms very well, especially if they are not very active. Factors that can affect the onset of clinical signs are the number of worms present in relationship to the size of the dog; the duration of infection; the individual host response to the parasite; and, most importantly, the activity level of the dog. Clinical signs usually develop gradually, with a mild cough being the most common sign noticed. This will be followed by exercise intolerance and an unthrifty appearance. Clinical signs associated with more advanced disease can also develop acutely as a result of the natural death and disintegration of a heartworm that leads to sudden pulmonary thromboembolism. As the disease progresses and pulmonary pathology worsens, signs associated with right-sided heart involvement such as abnormal heart sounds and ascites appear.10 Table 83-1 describes four classes of heartworm disease and their associated clinical signs. Classification of disease has been used to determine treatment strategies and prognosis. However, although class 3 patients are statistically at a greater risk for severe complications and death after treatment, class 1 and 2 patients can experience the same levels of complications.40 TABLE 83-1 Summary of Clinical Signs of Canine Heartworm Disease Radiography is an effective way of evaluating the severity of cardiopulmonary disease, and before the availability of serologic testing, it was the method used to diagnose occult (nonmicrofilaremic) heartworm disease. The heart silhouette appears as a reversed “D” and caudal lobar pulmonary arteries are enlarged, tortuous, and often truncated. These vascular changes are accompanied by varying degrees of pulmonary parenchymal disease (Fig. 83-4).33 Microfilaria can be identified microscopically by direct examination of a drop of anticoagulated blood, with concentration techniques using a Millipore filter, or centrifugation using the modified Knott’s procedure. Microfilariae may not be detected in approximately 80% of heartworm-infected dogs. These “occult” infections may occur as a result of single-sex infections, host immune responses suppressing reproduction, and if the dog has been on a heartworm preventive.10 For various reasons, microfilariae may be present in dogs with negative blood antigen result. It is possible for puppies to acquire microfilariae in utero when the bitch has a high microfilaria count. Because microfilariae can survive in the dog’s bloodstream for over 2 years, dogs treated with an adulticide or dogs whose infection has cleared naturally can have microfilariae and have negative antigen test results.46 In less than 1% of cases, microfilariae may precede antigenemia. Heartworm microfilariae must be differentiated from those of A. reconditum (Fig. 83-5 and Table 83-2). TABLE 83-2 Comparison of Microfilaria of Acanthocheilonema reconditum and Dirofilaria immitis Antigen detection, the recommended method for primary diagnostic screening in dogs, is accomplished by using one of several commercially available antigen tests kits (see Web Appendix 6). These are available as point-of-care tests or as well tests that are used by reference laboratories to run large numbers of samples. These tests detect a glycoprotein secreted mainly by adult female heartworms and are the most sensitive diagnostic method currently available. Commercially available antigen tests systems are based on enzyme immunoassay methods that have been adapted to laboratory or point-of-care kits. The sensitivity (95% to 100%) and specificity (100%) of these test systems are excellent in infections when three or more female worms are present. Most point-of-care test kit systems have low sensitivity in dogs with fewer than three female worms. In dogs with one female worm, the sensitivity ranged from 65% to 85%, and with two female worms was 85% and 95%.37 However, when comparing the presence or absence of female worms at necropsy, the sensitivity of one in-clinic instrument-based agglutination immunoassay test system ranged between 50% and 72% compared to 92% and 99.6% for a kit-based enzyme immunoassay.31b False-positive results are rare, and, therefore, it is better to accept rather than reject a positive test result. However, it is prudent to repeat a test when positive results are found for dogs residing in hypoendemic areas, and in animals receiving heartworm preventive. An animal exhibiting clinical signs of heartworm disease that has a negative test result should also be retested. The amount of antigen present in the circulation can be quantitated and does have a direct relationship to the number of female worms, but this is not an accurate determination of the total worm burden. Male and immature female worms could be present but not producing detectable antigen. Likewise, shortly after the death of a female worm, large amounts of antigen are released into the circulation, giving the appearance of a heavy worm burden in quantitative test results.40 Questionable positive antigen test results should be confirmed by either examining the blood for the presence of microfilaria or getting independent confirmation via another antigen test from a reference laboratory. Antigen may be detected as early as 5 months PI and consistently by 7 months PI. Antigenemia may be delayed until 9 months PI in some infected dogs receiving preventives. Microfilaria can be detected microscopically 6 months PI, by examining fresh blood, or with concentration techniques. Screening dogs for the presence of microfilaria is complementary to antigen testing, because it confirms the infection and alerts the veterinarian of a potential adverse reaction that may occur after administration of microfilaricidal doses of macrocyclic lactones. Because of the prepatent period, dogs should not be tested with either microfilariae or antigen tests until they are at least 7 months old.40 Molecular genetic detection methods are being considered for use in diagnosing heartworm infection as they have been important in improving the sensitivity of detecting infection with microbial agents. A multiplex polymerase chain reaction (PCR) has been developed that can detect and discriminate D. immitis from D. repens.25a Successful management and treatment heartworm disease require a thorough understanding of the life cycle of D. immitis, the host-parasite relationship, and the susceptibility of the various life stages to microfilaricidal and adulticidal drugs. The goals of any heartworm treatment are to improve the clinical condition of the animal and to eliminate all adult heartworms and developmental stages with minimal posttreatment complications. Because a heartworm-infected dog can potentially have L3 and L4, juvenile worms, and adults, therapies must be strategically administered to accomplish these goals. Before initiating therapy, a thorough physical exam, clinical laboratory testing, and thoracic radiographs should be completed. Clinical or laboratory problems of concern should be controlled or stabilized before the dog receives adulticidal therapy. This may involve the administration of glucocorticoids to reduce pulmonary inflammation as well as angiotensin-converting enzyme inhibitors to improve cardiac function. In severe cases of right-sided heart failure, abdominal drainage may be necessary to remove ascitic fluid to initially reduce diaphragmatic pressure followed by maintenance therapy with furosemide. Dosages of drugs used to treat canine heartworm disease are summarized in Table 83-3. TABLE 83-3 Therapy for Canine Heartworm Disease IM, Intramuscular; PO, by mouth. aSee Table 93-10 for specific information on each anti-infective drug. bThis drug has a low margin of safety: 3 times the single dose indicated (7.5 mg/kg) can result in severe pulmonary inflammation, edema, and death. Dose calculations must be very accurate and given over the extended period specified. cIvermectin, milbemycin, moxidectin, selamectin: See manufacturers’ dosage recommendations. dDosage is reduced to 0.5 mg/kg every 24 hours for the next 7 days and 0.5 mg/kg every other day for 1 to 2 weeks thereafter. Caval syndrome is a potentially fatal manifestation of heartworm disease that can be observed in dogs that have a high worm burden obstructing blood flow. Severe pulmonary arteriolar hypertension and decreased cardiac output occur. Heartworms can be surgically extracted via a right jugular venotomy and introduction of a flexible snare or brush that is guided into the pulmonary artery using a C-arm imaging device.9a This procedure is done as an emergency measure that is reserved for dogs with this syndrome. Medical management of heartworm disease is used in all other patients as discussed in the following sections. Melarsomine is an arsenical-based drug and is the only adulticidal drug approved by the Food and Drug Administration (FDA) for heartworm treatment in the United States. It is administered by deep intramuscular injection into the epaxial lumbar muscles. Melarsomine has not been shown to be effective against filarial stages less than 4 months old. This presents a problem with the goal of eliminating all the heartworms present. Furthermore, the two-dose protocol recommended on the package insert for treating class 1 and 2 heartworm infections (see Table 83-1) kills only 90% of the adult worms present. The “alternate” or three-dose protocol recommended for treating Class 3 heartworm infections kills 98% of the adult worms. Approximately 50% of the adult worms are killed by the first dose, and most of the remaining worms are killed by the second and third injection administered 4 to 8 weeks later. The three-dose protocol is recommended by the American Heartworm Society as the adulticidal treatment of choice because of its increased safety margin and efficacy. The heartworm preventive products belonging to the macrocyclic lactones contain ivermectin, milbemycin, moxidectin, or selamectin. As previously stated, it is possible for a heartworm-infected dog to have worms that are less than 1 month old to 7 years old. Also, melarsomine is not effective against heartworms less than 4 months old, which creates a treatment gap. This gap can be eliminated by administering a macrocyclic lactone preventive for the 2-month period before administering melarsomine. This will eliminate the tissue stages less than 2 months old. It also allows those stages between 2 and 4 months to reach an age at which they are susceptible to melarsomine. Administration of macrocyclic lactones may cause a rapid decrease in circulating microfilaria. Pretreatment with antihistamines and glucocorticoids should minimize any potential reaction.1
Heartworm Disease
Canine Heartworm Disease
Etiology
Epidemiology
Geographic Distribution
Transmission
Life Cycle
Pathogenesis
Clinical Findings
Early infection
Class 1
No signs
Mild disease
Class 1
Cough
Moderate disease
Class 2
Cough, exercise intolerance, abnormal lung sounds
Severe disease
Class 3
Cough, exercise intolerance, dyspnea, abnormal heart and lung sounds, hepatomegaly, syncope, ascites, and death
Caval syndrome
Class 4
Sudden onset of severe lethargy and weakness accompanied by hemoglobinemia and hemoglobinuria
Diagnosis
Radiography
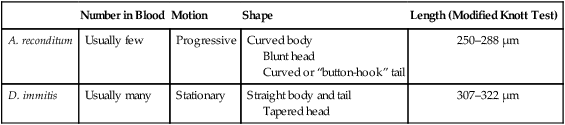
Microfilarial Detection
Number in Blood
Motion
Shape
Length (Modified Knott Test)
A. reconditum
Usually few
Progressive
Curved body
Blunt head
Curved or “button-hook” tail
250–288 µm
D. immitis
Usually many
Stationary
Straight body and tail
Tapered head
307–322 µm
Serologic Testing
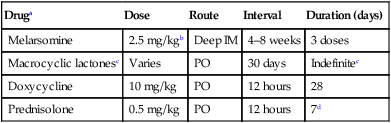
Therapy
Druga
Dose
Route
Interval
Duration (days)
Melarsomine
2.5 mg/kgb
Deep IM
4–8 weeks
3 doses
Macrocyclic lactonesc
Varies
PO
30 days
Indefinitec
Doxycycline
10 mg/kg
PO
12 hours
28
Prednisolone
0.5 mg/kg
PO
12 hours
7d
Melarsomine
Macrocyclic Lactones
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Heartworm Disease