Fig. 1.
(a) Table with information relating to the biological features of syngeneic mouse GBM models including effective therapies. (b) Diagram depicting the stereotactic coordinates for injection of syngeneic mouse glioma cell lines as well as the cell number. (c) Kaplan–Meyer survival curve of mice bearing syngeneic intracranial brain tumors. C57BL/6 or VM/Dk mice implanted in the striatum with Gl26, SMA560, B16-f10, or GL261 cells.
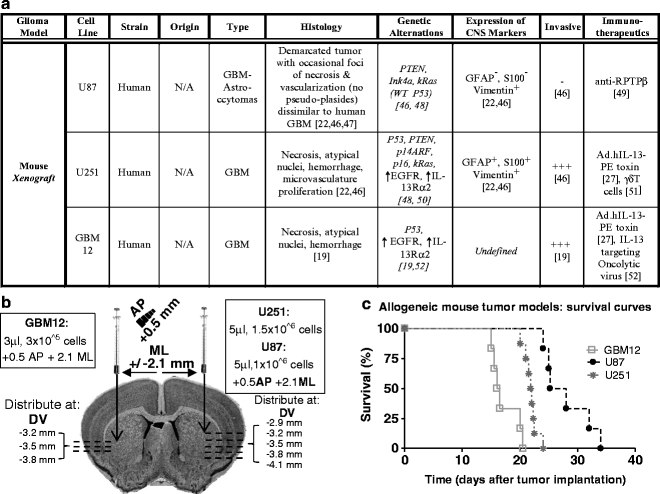
Fig. 2.
(a) Table listing the biological features of human xenograft GBM models including effective therapies. (b) Diagram depicting the stereotactic coordinates for injection of human glioma cell lines as well as the cell number. (c) Kaplan–Meyer survival curve of mice bearing syngeneic intracranial brain tumors. Rag1-deficient mice were injected in the striatum with U87, U251, and GBM12 cells.
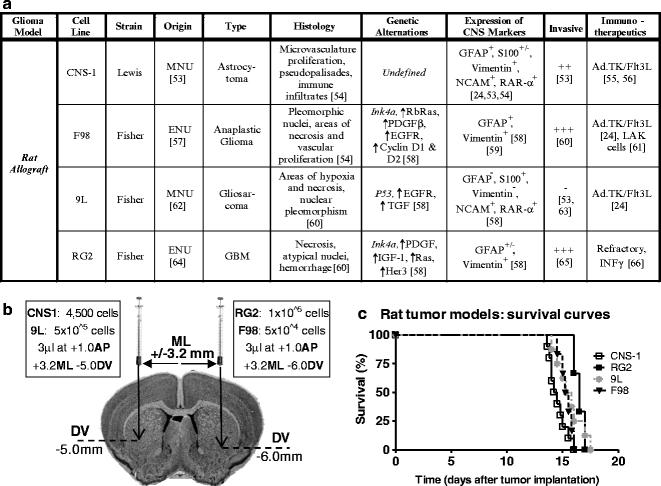
Fig. 3.
(a) Table with information about the biological features of syngeneic rat GBM models including effective therapies. (b) Diagram depicting the stereotactic coordinates for injection of human glioma cell lines as well as the cell number. (c) Kaplan–Meyer survival curve of rats bearing intracranial tumors. Lewis or Fisher rats were injected in the striatum with CNS-1, F98, 9L, and RG2 cells.
Stereotactic surgery is a well-established technique for targeting precise anatomical structures within the brain. This technique can be used to implant glioma cells in the brain and quickly generate tumor-bearing animals to study various aspects of neuro-oncology, such as angiogenesis, invasiveness, tumor-induced immune suppression, and efficacy of novel therapeutics. This protocol will describe how to perform intracranial implantation of cultured tumor cells in mice and rats using a stereotactic apparatus as well as the steps necessary for maintaining primary human tumor cultures.
2 Materials
1.
Glioma cell line of interest resuspended PBS or serum-free media (injectable volume ~1–5 μl). Cells can be kept for 2–3 h on ice during surgery.
2.
Adult rat (250 g body weight. Fisher or Lewis Strain. Charles River Inc., http://www.criver.com).
3.
Adult female mouse (20–25 g weight. 6–8 weeks old C57Bl/6, VM/Dk, Rag1−/− strain, Athymic Balb/c. Jackson Labs Inc., http://www.jax.org).
4.
Drugs:
5.
Betadine (Bruce Medical, cat# FR-2100-88).
7.
Warm Lactated Ringer’s solution (Hospira Inc., cat# 0409-7953-30).
8.
Tissue culture:
(a)
Accutase™ Enzyme Cell Detachment Medium (eBiosciences Inc., cat# 00-4555-56)
(b)
Dulbecco’s PBS, pH 7.4, without Ca or Mg (MediaTech Inc., cat# 21-040-CM)
(c)
Dulbecco’s modified Eagles media (DMEM), supplemented with 10% fetal bovine serum (MediaTech Inc., cat# 10-017-CM and Omega Scientific, cat# FB-01)
(d)
T75 Flasks (VWR, cat# 82050-856)
(e)
Matrigel™ (BD Pharmingen, cat# 356231)
(f)
Hemocytometer (VWR, cat# 15170-263)
9.
Stereotactic frame with rat and mouse adapters and blunt ear bars (Stoelting Inc.).
10.
Stereomicroscope (e.g., Zeiss Stemi 1,000 zoom) equipped with 16× eyepieces and 0.4× auxiliary objective lens, and mounted on hinged coupling arm on a heavy foot stand (or equivalent).
11.
Electric drill with 1.75-mm and 0.6-mm drill bit (Stoelting Inc., cat# 58610).
12.
Digital scale for animal weight (Harvard Apparatus, cat# 724586).
13.
Fiber-optic illuminator with twin goose-neck pipes (Leica Inc.).
14.
10-μl, 26-G Hamilton syringe with needle (Fisher, cat# 701RN).
15.
5–10 μl, 33-G Hamilton syringe with needle (Fisher, cat# 75RN).
16.
3-0 Nylon sutures (Ethicon Inc., cat# 1663H).
17.
Petri dish, plastic (BD Pharmingen, 353002).
18.
Surgical equipment:
(a)
Surgical shavers (Stoelting Inc., cat# 58610)
(b)
Scalpel and blades (Cardinal Health, cat# D2862-15)
(c)
Skin retractors (Fine Science Tools Inc., mouse cat# 17000-03, rat cat# 17000-04)
(d)
Cotton swabs (VWR, cat# 89031-270)
(e)
Curved and straight forceps
(f)
Holding scissors
(g)
Sharp scissors
(h)
Sterile gauze (VWR, cat# 95038-720)
(i)
Bead sterilizer (VWR, cat# IS-350)
(j)
Red lamp (Sylvania, cat# 14663)
(k)
Optional: infant incubator for recovery of nude mice
3 Methods
3.1 Glioma Cell Lines: Maintenance and Passage
Most of the tumor cell lines presented in the chapter (Figs. 1–3) can be passaged indefinitely in tissue culture and cryopreserved for long-term storage with the exception of GBM12. While the genetic features of the original human GBM specimen are maintained with serial tumor passage in vivo (19, 25), GBM12 cell passage in vitro for long periods of time results in loss of EGFR amplification and gain of MGMT promoter methylation (26). Thus, this human tumor must be serially transplanted in the flanks of immune-deficient mice, such as Rag1−/− mice or athymic nude mice. Short-term GBM12 cultures can be derived from flank tumor in order to perform in vitro studies or to establish intracranial tumors (26). To achieve highly reproducible intracranial GBM12 xenografts, it is necessary to implant the same number of cells in the brain of all the animals. Short-term GBM12 cells growing in a monolayers can be harvested and counted for intracranial implantation (see below) (19, 26, 27).
1.
Swab the skin with Betadine to minimize the risk of infection and excise the tumor from the flank of the mouse using a sterile scalpel and place in a Petri dish with 4 ml of DMEM.
2.
Mince the tumor into small 1–2 mm pieces using razor blade until tissue can pass through a 1 cc syringe. Draw up and expel the tissue through the syringe to break up the tumor into even smaller pieces.
3.
At this point, the cells can be cultured in vitro or injected into the flank of new RAG1-deficient mice for in vivo propagation. Short-term cultures can be used for in vitro experiments or in preparation for intracranial implantation. Minced tissue preparations are difficult to count and inject accurately, therefore, they must be cultured in vitro first. GBM12 cultures can be harvested using trypsin and cells can be counted and resuspended in the concentration required to yield accurate survival curves when implanted in the brain (19, 26, 27).
4.
To culture the cells, collect and seed in Matrigel™-coated T75 flasks with DMEM containing 10% fetal calf serum. Cells grow in monolayer and can be passaged for up to 1 month before injecting them into the brain. Their ability to grow in vivo decays and the genetic alterations present in the original human GBM specimen are lost when maintained in culture for longer periods of time.
5.
Following short-term culture (2–3 weeks), the cells are harvested with Accutase™ Detachment Medium. To prepare cells for injection, obtain a count using a hemocytometer and resuspended in DMEM (serum free) at 1 × 105 cells/μl (3 μl/mouse).
6.
To propagate the tumor, transfer the minced tissue into a conical tube and allow the material to settle. Remove the excess media and add Matrigel™ to the wet tissue in a 1:1 ratio.
7.
200 μl of this mixture is injected into the flank of 2–3 immune-deficient mice using 1 cc syringes with 21 G needle. Once the tumors reach 1–2 cm in diameter, usually 1–2 months after tumor implantation, the animal is sacrificed and the flank tumor is excised to be re-implanted in the flank or cultured in vitro.
3.2 Glioma Models: Intracranial Grafts in Rodents
3.2.1 Animals and Surgical Preparation
1.
Prior to performing any procedures involving an animal, the operator should ensure that the surgical area is clean, well organized, and contains all required instruments. Tools can be sterilized by placing them in a flash bead sterilizer for a short time. This sterilization technique is sufficient for rodent surgery but not for larger animals.
2.
Lay out all the surgical instruments on an absorbent under pad in the order in which they will be used. Inspect the stereotactic frame to ensure that it is in proper operating condition. Ensure that all the manipulator arms move freely and smoothly with little to no sideways movement in the syringe holder. Direct the light beam at the ear bars and position the microscope such that the surgical area is visible through the eyepiece.
3.
Place the mouse (female 6–8 weeks) under anesthesia by intraperitoneal (IP) injection of ketamine (75 mg/kg) and Dexmedetomidine (0.5 mg/kg). Before commencing surgery, administer Carprofen subcutaneously (5 mg/kg) as an analgesic and ensure that the animal is completely sedated by pinching the footpad or tail.
– Rat Anesthesia dosage is as follows: Ketamine (75 mg/kg IP), Dexmedetomidine (0.25 mg/kg IP), and Carprofen (5 mg/kg SQ)
4.
Using surgical shavers remove enough fur from the head of the animal to allow for aseptic procedures.
5.
Carefully mount the animal onto the stereotactic frame and use the incisor bar to loosely immobilize the skull. Raise the animal to the level of the ears bars. Proceed by supporting the head of the animal and slide one of the ear bars into the ear canal and tighten in place. While keeping the head supported, slide the other ear bar into the proper position. The ears bars are meant to prevent any mediolateral head movement while maintaining the dorsoventral axis rotation free (see Note 2).
6.
Use blunt forceps to open the mouth of the animal and insert the incisor bar. Press down gently to ensure the incisors are well seated. Swing the nose clamp over the nose of the animal and tighten gently. Ensure that there is no disruption in respiration. (see Notes 3 and 4).
3.2.2 Stereotactic Injection of Tumor Cells
7.
Disinfect the incision area using alcohol wipes followed by Betadine. Apply ophthalmic lubricant to each of the eyes so they remain moist during surgery (see Note 4).
8.
Using size 15 scalpels make a midline incision along the cranium roughly 1–2 cm in length beginning slightly posterior to the eyes and ending at the ears. Use the skin retractor to hold back the skin on both sides of the incision. The incision length for a rat is longer than a mouse as is the skin retractor.
9.
Direct the light beams onto the exposed skull and focus the microscope on bregma; the junction of the sagittal and transverse sutures which should be visible under direct light. Use the blunt end of a scalpel and gently depress the parietal or frontal skull bone to assist in finding the junction (bregma) of the two bones (Fig. 4).
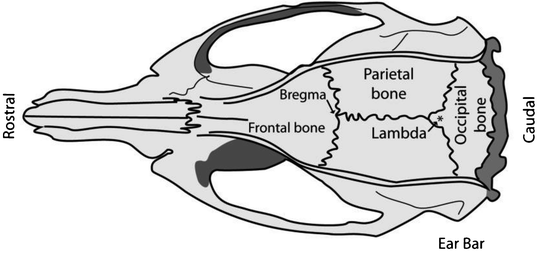

Fig. 4.
Schematic of a rat skull depicting the position of bregma relative to the frontal and parietal skull bones and the position for placing of the ear bars. Adapted from: The Rat Brain in Stereotaxic Coordinates, Academic Press, New York, 1998.
10.
Position the syringe using the manipulator arms so that the needle tip is directly over bregma. Take the anteroposterior (AP) and mediolateral (ML) coordinates of bregma as these will serve as our starting coordinates.
11.
Determine the coordinates of the injection site by adding or subtracting the appropriate lateral and anterior/posterior values. To inject into the striatum of a mouse move +0.5 mm AP then +2.2 mm ML from bregma. Coordinates for the rat striatum are +1.0 mm AP, +3.2 mm ML (see Note 5).
12.
Visualize the injection site through eyepiece and use the drill to itch a small mark in the skull where the needle will penetrate. This mark is used as a guide for the operator to drill in the correct position. The needle can be lifted so that it does not obstruct the area.
13.
With caution, drill a small burr hole with a 0.6-mm bit for mice and 1.75-mm bit for rats. This must be performed with extreme precaution as to avoid severing any blood vessels. Use a bent 28 G needle to ensure penetration of the dura mater. The rat skull is thicker and therefore more difficult to drill. For this purpose, use a 1.75-mm drill bit and drill in short bursts to avoid generating heat. Also, wet the area frequently with fresh saline solution, to avoid excessive heat (see Notes 8 and 9).
14.




Load the Hamilton syringe (33 G needle for mice, 26 G for rats) with 1–5 μl of cells of interest and expel a small amount onto a kimwipe to ensure that the syringe was properly loaded. Lower the needle such that it is level with the bur hole. Again read the dorsoventral coordinates and calculate the desired coordinate of the injection site. For injecting syngeneic mouse cells in the striatum slowly, move the needle down 3.5 mm and leave the needle in place for 1 min, then back up 0.3 mm to allow for a small pocket in which the cells can rest (−3.2 mm DV final). These coordinates will vary depending on the animal and cell line used. We have included the injection coordinates used by our laboratory to generate these glioma models in Figs. 1–3 and Notes 1 and 10.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


