Chapter 6
General pharmacology of the injectable agents used in anaesthesia
Introduction
Injectable anaesthetic agents may be used either just to induce anaesthesia prior to maintenance with an inhalant, or as the only anaesthetic agent for as long as required. In human anaesthesia, such use is almost always by intravenous (IV) injection, duration being extended by infusion. This is termed ‘total intravenous anaesthesia’ or TIVA. Many injectable agents are poor analgesics so additional analgesia is usually necessary. While in veterinary anaesthesia achievement of TIVA by infusion is ideal (see Chapter 3), practicality may mean that prolongation is by bolus injections. Some agents can be given by the intramuscular (IM) route and, in veterinary practice, may be used (often with sedatives or analgesics) at a dose which gives anaesthesia of sufficient duration for some surgery. This is only practicable with agents with minimal cardiac depressant effects, such as ketamine. Other routes which have been employed with suitable agents include subcutaneous (SC), intraperitoneal (not recommended for routine use), transmucous membrane and even oral or rectal. The IV route gives the anaesthetist some control over anaesthetic depth; the other routes do not.
Injectable anaesthesia is often claimed to be ‘easy’ and to require less equipment than do inhalant anaesthetics, but this is not so. Some factors that the anaesthetist has to consider concerning the drugs they are using are listed in Box 6.1. Not all agents given IV produce loss of consciousness in one injection site–brain circulation time and, if not, it becomes more difficult to titrate the dose to the animal’s requirements. Once the agent has been injected, it cannot be removed other than by the metabolic process so there is no margin for error. Duration of action depends both on redistribution and on metabolism (see Chapter 3) and agents which are short acting with a single injection given for induction of anaesthesia may become long acting when used for TIVA as context-sensitive half-life increases. Table 6.1 gives relevant comparative pharmacokinetic data for humans for the drugs discussed in this chapter. However, there are many species differences, not all of which are well documented. In particular, cats, because of their inability to conjugate many agents (see Chapter 16) may metabolize the drug and/or the solvent differently. Knowledge of pharmacodynamics of the agents is also essential for safe use. Almost all of the injectable agents cause respiratory depression, so endotracheal intubation, oxygen and the ability to ventilate should still be available. It is more difficult to compensate for cardiovascular effects resulting from overdose. Specific side effects, for example myoclonus, may be more problematic in different species. For example, a quiet calm non-ataxic recovery is essential for horses so only agents (or combinations) that will ensure this should be used. Most IV anaesthetics are used in perioperative combination with sedatives and analgesics to overcome the shortcomings of the anaesthetic agent, but the combinations may further alter the pharmacodynamics.
Table 6.1
Pharmacokinetic parameters of IV anaesthetic agents in humans as there is good comparative data available
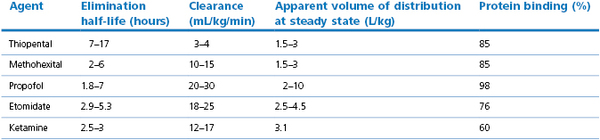
These values differ in other species (see text). Note the wide variation in some reported values.
After Hijazi & Boulier (2002), Evers et al. (2006); Reves et al. (2012).
Formulation of injectable anaesthetic agents
There is not yet an ideal injectable anaesthetic and the search continues for new drugs, or new, improved formulations of existing drugs. The technology involved in formulation (or pharmaceutics) is advancing rapidly and a full resume is beyond the scope of this chapter. Useful reviews include Baker & Naguib (2005) and Loftsson & Brewster (2010). MacPherson (2001) also explains the various preservatives that may be added, their potential side effects and neurotoxicity. Formulations must allow the drug to be available once injected; even small changes can change bioavailability. They should not be irritant if injected extravascularly, not cause pain on injection, have no cardiopulmonary effects themselves, not be toxic, and should not release histamine or cause anaphylactic reactions. A further consideration is to ensure that the final product remains sterile in use; this is of particular importance in small animal practice where economics prefer the use of multidose bottles. Generic versions of drugs may differ in formulation from the original branded product. The following are the most common methods of formulating IV anaesthetic drugs.
The agent may be presented as a ‘salt’ to improve solubility. Examples include thiopental (as the sodium salt) and ketamine (the hydrochloride). Such solutions may or may not have additive preservatives and antibacterial agents.
Many drugs used in anaesthesia use lipid emulsion carriers, the most notable being propofol. The emulsions used may vary in their source and percentage of lipid, each component having a technical reason for its presence, and in their droplet size. Macroemulsions (droplet size 0.1–100 µm) such as used for Diprivan® (the original propofol) appear white while microemulsions (droplet size >0.1 µm) are translucent or opalescent. Lipid emulsions are an excellent growth medium for bacteria, and this has caused clinical problems (Bennett et al., 1995). Some formulations of propofol now contain EDTA or sodium metabisulphite to retard (but not prevent) bacterial growth. Lipid infusions may cause hyperlipaemia. This might contribute to the propofol infusion syndrome (see below). Cats do not metabolize lipids as rapidly as other species (see Chapter 16).
These are complex polysaccharides, derived from starch, that have a hydrophobic centre and ‘wrap round’ lipophilic drugs – described as ‘guest–host complexes’ (Sneyd, 2004). There are a number of different forms which are suitable for solubilizing a wide range of drugs. In veterinary anaesthesia, they are the carrier for alfaxalone (see below) but are/have been trialled for several other IV anaesthetic agents including propofol. Although non-toxic, the concern in human use is that they may possible trigger anaphylactic reactions; time will tell.
This is used as a solvent for a number of agents in anaesthesia, these being etomidate and diazepam. It is miscible with water at all concentrations and also has some preservative action. It does cause pain on injection and a high incidence of thrombophlebitis.
Nanotechnology to produce polymeric micelle formulations of a number of drugs, including propofol, has been investigated. The micelles are so small that solutions appear clear.
Cremophor EL, which is polyethylated castor oil, was tried as a solvent for a number of anaesthetic drugs, but can cause anaphylaxis and, since the withdrawal of the steroid combination Saffan® or Althesin® (see steroid anaesthesia below), is now no longer used.
Polysorbate 80, derived from sorbitol, was considered a possibility, but propofol in polysorbate 80 caused major cardiopulmonary depression when tested in goats (Bettschart-Wolfensberger et al., 2000).
Sites of action of injectable anaesthetic agents
The state of consciousness we term ‘general anaesthesia’, and the receptor theories of how drugs cause this are discussed in Chapter 1. To summarize an extremely simplified view, the injectable (and some inhalation) anaesthetics that cause dose-dependent hypnosis are agonists at the GABAA receptors, but do not necessarily effect the same site on that receptor. They may also act at other central and peripheral ion channels and/or receptors; hence the difference in properties between the agents. The dissociative agents, such as ketamine, act primarily as antagonists at NMDA receptors. It is also possible to obtain a state resembling anaesthesia by the combination of opioid agonists, α2-agonists and dopamine antagonists (Brown et al., 2011); these agents are discussed in Chapters 4 and 5.
IV agents acting primarily at the GABAA receptors
The barbiturates
Currently, four barbiturate drugs are used in veterinary medicine. Phenobarbital is very long acting and is used as an anticonvulsant. Within anaesthesia, agents used are pentobarbital, thiopental and methohexital. All three exert their hypnotic properties primarily by actions at the GABAA receptor (see Chapter 1). Properties in common are that all are very respiratory depressant and are poor analgesics. All are metabolized by the liver, with species differences in rate.
Thiopental
Thiopental (thiopentone) was introduced into veterinary practice in the 1930s and over the next 60 years became the most widely used agent for induction of anaesthesia. Thiopental was originally licensed at a dose of up to 30 mg/kg, now considered a massive overdose, but which gave prolonged hypnosis. Previous editions of this book review the veterinary studies. Thiopental’s primary use in human and in veterinary anaesthesia is for the induction of anaesthesia. However, all barbiturates are neuroprotective, are excellent antiepileptics and infusions of thiopental still have place for prolonged sedation, for example in poisoning cases causing fits and, in humans, in cases of head injury (Majdan et al., 2013).
Thiopental sodium is presented as a yellow solid, to be dissolved in water before use, the concentration to be made being as low as practicable in relation to volume required. Thus, 1.25% or less is recommended for cats and small dogs, 2.5% for larger dogs and up to 10 % for horses and cattle. The preparation contains anhydrous sodium carbonate to prevent precipitation of the insoluble free acid by atmospheric CO2. Aqueous solutions are strongly alkaline and are incompatible to mix with many analgesics and sedative drugs, but do seem compatible with propofol (Chilvers et al., 1999; Ko et al., 1999). Depending on concentration, the solution is highly irritant. It can only be used IV and even 1.25% causes skin sloughs if accidentally injected extravascularly; 10% solutions can result in major damage to surrounding tissues. Solutions of thiopental may be kept for several days; bacterial growth does not easily occur (Strachan et al., 2008).
The pharmacokinetics of thiopental are typical of a cumulative agent. Following a single IV injection, blood and brain levels rise then fall rapidly as the drug is redistributed into other tissues, in particular fat. From a single ‘induction’ dose of thiopental, recovery of consciousness is as rapid as for propofol as both are very lipophilic and have a large volume of distribution. However, for thiopental, metabolism by the liver is slow, so if larger or multiple doses are given, it accumulates in the tissues; recovery now depends on metabolism and can be very prolonged. With only an induction dose of thiopental, there is residual sedation following recovery. In animals with little fat, or in which liver enzymes may not be fully functional (e.g. greyhounds; neonates), recovery is even slower (Sams et al., 1985; Robinson et al., 1986). In mixed-breed dogs, the half-lives of elimination have been reported as from approximately 2.5 to 7 hours and plasma clearances as from 1.5 to 97 mL/kg/minute (Brandon & Baggot, 1981; Ilkiw et al., 1991). It has always been claimed (and is the author’s experience) that barbiturates are shorter acting in herbivores than in cats and dogs but, with the range of reported values from different laboratories, it is not possible to find evidence to prove this (Ilkiw et al., 1991; Abass et al., 1994). Thiopental is highly protein bound (more than 75%); it is the non-bound fraction that is active, so animals with hypoproteinaemia (including anaemia) are very sensitive to its effects. The drug also is partially ionized and acts as a weak organic acid; the dissociation constant is such that a small change in pH will markedly affect the degree of ionization, and this in turn can alter distribution.
When thiopental is injected IV, it results in a smooth induction of anaesthesia within a circulation time: in the unpremedicated animal, this is very rapid (with fast injection, it can be as little as 20 seconds in a horse). If premedication reduces cardiac output (as do the α2-agonists), then speed of induction will be slowed. The induction dose of thiopental required for endotracheal intubation in an unpremeditated animal (most domestic species) can be over 15 mg/kg; after acepromazine premedication with rapid injection in dogs, the dose is 7–10 mg/kg and, after α2-agonists, the dose reduces further depending on the sedation achieved (Young et al., 1990; England & Hammond, 1997). Doses used to induce anaesthesia in horses premedicated with acepromazine are 11 mg/kg, while after moderate α2-premedication, 7 mg/kg suffices (Clarke & Gerring, 1990). Previously, the recommendation (for humans and dogs) was to give thiopental very rapidly, but current advice for small animals is that the drug should be injected more slowly, over around one minute, in order to reduce the initial cardiorespiratory depression. A major reason for fast administration was to avoid an excitement phase at induction; effective sedative premedication negates this problem.
Thiopental is very respiratory depressant. Rapid IV injection of the drug causes apnoea and a fall in blood pressure, even in normovolaemic animals, through a decrease in systemic vascular resistance. In hypovolaemic animals, rapid injection of thiopental can be fatal. After the initial fall, in normovolaemic animals, the blood pressure returns to about the normal level but there is a persistent tachycardia. The drug has a dose-dependent direct depressant effect on the myocardium but, at normal induction doses, cardiac output is maintained by the increase in heart rate. Thiopental is a poor analgesic; very deep levels of anaesthesia are necessary to prevent response to noxious stimulation so, if used other than as an induction agent, additional analgesia, such as fentanyl, is required. Muscle relaxation is poor, possibly partly the effect of increased PaCO2 through respiratory depression. Shivering is common in all species of animal in the recovery period and may be due to persistent cutaneous vasodilation in a cold environment.
The only absolute contraindication to the use of thiopental as an induction agent is porphyria, a disease characterized by progressive acute demyelination of nerves, that is well documented in humans. Porphyria is very rare but has been diagnosed in cattle, pigs and cats (Tobias, 1964). Partial contraindications for thiopental include neonates, caesarean section with a live fetus, very thin animals, uraemia, untreated hypovolaemia and hypoproteinaemia. In small animals, there are several agents available which are preferable to thiopental for longer periods of anaesthesia, although the drug can be useful for this purpose in small ruminants.
Thiopental’s current limited availability relates to its replacement in human anaesthesia by propofol (see below) but, where available, it remains a useful agent in veterinary anaesthesia.
Methohexital
Methohexital sodium is a racemic mixture of the α-d and a-l isomers of sodium 5-allyl-1-methyl-5-(1-methyl-2-pentynyl) barbiturate, and differs from thiopental in having no sulphur in the molecule. It is supplied as a colourless solid and, when dissolved in water, forms a colourless solution which is stable for at least six weeks kept at room temperature.
Methohexital is approximately twice as potent as thiopental, and it is less irritant if accidentally given outside the vein. As for thiopental, when given by rapid IV injection, it rapidly crosses the blood–brain barrier and causes anaesthesia in a circulation time. As for thiopental, initial lowering of plasma levels and awakening is due to redistribution, but it differs from thiopental in that metabolism is much faster, is relatively non-cumulative and complete recovery is rapid, seldom exceeding 30 minutes even after prolonged infusion. Hepatic metabolism breaks methohexital down to non-active metabolites which are rapidly excreted. Cardiorespiratory actions and lack of analgesia are similar to those of thiopental. Sedative premedication avoids muscle tremors during the induction of and recovery from anaesthesia. Suitable doses will be discussed in the relevant species chapters.
Methohexital has been superseded by propofol for most indications but, where it is still available, it can prove a useful agent both for anaesthetic induction and for the maintenance of hypnosis.
Pentobarbital sodium
Pentobarbital sodium in the form of a racemic mixture of sodium 5-ethyl-5-(1-methylbutyl) barbiturate is marketed as a sterile 6.5% solution containing propylene glycol. Pentobarbital has been, and in some areas still is, used to provide total injectable anaesthesia. It is relatively non-irritant and, although the best results are obtained with IV administration, it has been used by intraperitoneal injection in laboratory animals. In humans, it can be used for sedation by the oral or rectal routes. When given IV, it is slow to cross the blood–brain barrier, so anaesthetic induction is slow. The usual mode of administration is to give two-thirds the calculated dose rapidly, so that there is not an excitement phase on induction (one of its isomers has an excitatory effect). Following induction, the anaesthetist must wait some time (a minute or more) to ensure full effect before giving the next increment, or overdose may occur. Doses in unpremedicated dogs and cats to give anaesthesia long enough for 30 minutes of surgery (with additional analgesia) are up to 30 mg/kg IV. Full recovery is accompanied by excitement and, in dogs, vocalization, and takes up to 24 hours. Premedication reduces the dose needed, reduces the chance of excitement at induction or recovery, and speeds recovery. Use of the intraperitoneal route, which is not recommended, often results in a major excitement reaction during the induction period.
The side effects of pentobarbital anaesthesia are as of thiopental; dose dependent respiratory depression, hypotension, and myocardial depression although, at minimally effective dose rates, an increased pulse rate may sustain cardiac output. Analgesia is very poor. Contraindications and partial contraindications are as for thiopental.
As for thiopental, pentobarbital appears to be metabolized more rapidly in horses, sheep and goats than in pigs, dogs and cats, although the authors have been unable to locate evidence to confirm this. Pentobarbital is used to control seizures (e.g. caused by poisons of unknown origins). It is used also in very concentrated forms for euthanasia. The euthanasia solutions contain preservatives and must not be used for anaesthesia, even in large animals. Many of the euthanasia solutions (e.g. Somulose®) are combined with an agent to stop the heart. They should be given very slowly to ensure that the pentobarbital has time to cross the blood–brain barrier and induce anaesthesia before cardiac arrest occurs. In pregnant animals, anaesthesia prior to administration of such euthanasia solutions is preferable to ensure that the fetus is anaesthetized prior to the cardiac arrest in the dam, as it is probable that transport of pentobarbital across the placenta is slow.
Other barbiturates
Thiamylal closely resembles thiopental in chemical structure except that while the latter is the ethyl derivative of the series, the former is the allyl compound. It is also similar to thiopental in its actions as an anaesthetic. It is no longer available.
Phenols
Propofol
The active ingredient of propofol, 2,6 di-isopropylphenol (Fig 6.1), exists as an oil at room temperatures. Propofol is presented for anaesthetic use as a free flowing oil-in-water milk-coloured macro-emulsion, the original format (Diprivan®) containing 1% w/v soya bean oil, 1.2% w/v purified egg phosphatide and 2.25% w/v glycerol (see ‘Formulation’ above). Contamination of syringes of propofol was implicated in a high incidence of postoperative infection (Bennett et al., 1995) and so some preparations now contain preservatives. The first clinical trials of propofol were reported for humans in 1977, for dogs in 1984 (Hall, 1984) and 1987 (Watkins et al., 1987) and for cats in 1988 (Brearley et al., 1988). Since this time, propofol has become the IV induction agent most widely used in human anaesthesia, as well as being used for TIVA and for sedation in intensive care.
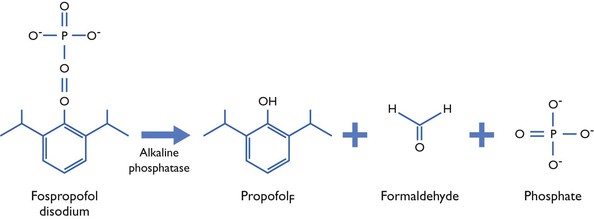
Figure 6.1 Chemical structure of fospropofol disodium and the scheme of its enzymolysis to propofolF.
Propofol is licensed for IV use as an anaesthetic agent in humans, cats and dogs. There is occasionally pain on injection. Although propofol is non-irritant, it can only be used IV as its elimination is too rapid for use by other routes. A number of generic versions of propofol are available. Currently (2012) in the USA and in Europe, there are two formats with veterinary licences. Propoflo® is similar to the original, and vials are for single use only. Propofol™ Plus (PropoFlo™28) contains benzyl alcohol as preservative, and its multidose bottles may be used for up to 28 days after the vial is first broached. PropoClear™ was a formulation as a micelle but, unfortunately, in clinical use, it proved painful on injection (Michou et al., 2012) and possibly caused local tissue damage and has been withdrawn.
Following IV injection of propofol, hypnosis is induced in a circulation time with anaesthetic depth reaching maximal after around 90 seconds (Reves et al., 2012). Recovery is extremely rapid and complete. The initial recovery from a bolus injection is through redistribution, as is thiopental. Also, as for thiopental, propofol is highly protein bound. The difference between the two agents is that the clearance of propofol as it is released from these tissues is very rapid (see Table 6.1), so residual sedation is not seen. Propofol is highly lipophilic and rapidly metabolized primarily to inactive glucuronide conjugates, involving cytochrome P450 systems, the metabolites being excreted in the urine. However, clearance can be greater than liver blood flow, and extrahepatic mechanisms (e.g. lung and kidney) are thought to contribute.
Pharmacokinetic studies in animals have shown species, breed and individual variations, and also variations depending on other anaesthetic and sedative agents given, in speed of elimination of propofol. In dogs, half-life of elimination, depending on other drugs administered, has been reported as between 75 and 486 minutes, volume of distribution as from 3.7 to 6.5 L/kg and clearance as between 34 and 58 mL/kg/minute (Nolan et al., 1993; Reid & Nolan, 1993, 1996; Zoran et al., 1993; Hall et al., 1994; Hughes & Nolan, 1999); the values of clearance and volume of distribution being considerably higher than the human values (see Table 6.1). Zoran et al. (1993) compared the pharmacokinetics of propofol in greyhounds with mixed breed dogs; half-life of elimination was longer (175 minutes cf 122 minutes) and clearance less (54 cf 115 mL/kg/minute) in greyhounds. In vitro studies have shown breed and gender differences in metabolic utilization of propofol (Hay Kraus et al., 2000) and also suggest that the canine kidney (in contrast to humans) does not metabolize the drug (Soars et al., 2001). In the goat, half-life of elimination is very rapid (15 minutes) and clearance very high (275 mL/kg/minute) (Rigby-Jones et al., 2002).
The cat, however, because of its inability to conjugate phenols, metabolizes both propofol and the lipid solvent more slowly than other carnivores. Table 6.2 gives data from the original developmental work (JB Glen, personal communication to LWH), and demonstrates that the utilization of propofol is very slow in cats. Very recent, as yet unpublished (2012) work has investigated the pharmacokinetics of a single dose of 8 mg/kg propofol in the cat. Early analysis of the results (a one compartmental model) suggests that the volume of distribution is 14 ± 6 L/kg; the half-life of elimination 5.9 ± 3.6 hours, and the clearance 115 ± 45 mL/kg/minute (Khursheed Mama, also on behalf of colleagues, personal communication to KC, October 2012).
Table 6.2
Mean utilization rate of propofol
| Species | Mean utilization rate (mg/kg/min) |
| Mouse | 2.22 |
| Rabbit | 1.55 |
| Rat | 0.61 |
| Pig | 0.28 |
| Cat | 0.19 |
Data supplied to L Hall by JB Glen
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


