Chapter 16
Anaesthesia of the cat
Introduction
General principles and recommendations for good anaesthetic practice that are discussed in the previous chapter are applicable to anaesthesia of cats. This chapter will focus on aspects of anaesthetic management that are influenced by feline behaviour, anatomy, physiology, pharmacology and disease. Other reading sources include the guidelines published by veterinary organizations with specific interest in the feline species, including the Association for Veterinary Anaesthesia (AVA), the American College of Veterinary Anesthesia and Analgesia (ACVAA), the International Society of Feline Medicine (ISFM) and the American Association of Feline Practitioners (AAFP).
The risk of death from anaesthesia or sedation has decreased. Recent data have led to approximate estimates of mortality of 0.1% in healthy cats and 1.4% in sick cats, with cats apparently at greater risk than dogs (Brodbelt et al., 2008a,b). Administration of anaesthesia in cats is not identical to that in dogs as the behavioural responses to and metabolism of some anaesthetic agents differ between cats and dogs. In a 2-year prospective survey of anaesthesia of 79 178 cats in the UK, physical status, age and weight increased the risk of death. Cats that were sicker, over 12 years of age, and weighing ≤2 kg or ≥6 kg were more likely to die during or after anaesthesia. Furthermore, cats weighing ≤2 kg were nearly 16 times more likely to die than cats weighing between 2 and 6 kg (Brodbelt et al., 2007). As with other species, illness and increasing age may exaggerate or diminish physiological responses to anaesthetic agents as well as decrease the dose rates required. Overweight cats are at increased risk for hypoventilation and hypoxaemia and the dose rates of anaesthetic agents must be decreased for these patients. Small cats are at risk for unintended drug overdose, especially if correct weights are not used. Accuracy of administration of small volumes is improved by use of 1 mL syringes with 24 gauge needles or insulin syringes. Larger needles may contain as much as 0.1–0.2 mL of drug that can increase the dose administered when injected into a small patient. Drugs should be diluted when the volume to be injected is too small to be accurately administered with available syringes/needles. Published factors associated with anaesthetic- and sedation-related deaths included the urgency and severity of the procedure, and use of endotracheal intubation and fluid therapy. Possible explanations are that preanaesthetic evaluation and preparation are curtailed with emergency and out-of-hours surgery. Less or inadequate time is spent restoring fluid and electrolyte balance, and fewer personnel may be available for optimum management. Endotracheal intubation is not quite as easy in cats due to the smaller size of the larynx and increased tendency for laryngospasm to interfere with insertion of the tube. Use of force or repeated attempts may cause trauma leading to laryngeal and tracheal mucosal swelling that obstructs airflow after extubation. Increased risk of death associated with fluid therapy may be a consequence of fluid overload. Not only is the blood volume of a cat smaller than that of a dog on a per kg basis such that a relatively smaller volume of fluid is required, but also accurate assessment of the fluid volume delivered may be difficult when using gravity flow from a 500 mL bag of balanced electrolyte solution, even with a paediatric (60 drops/mL) administration set. The anaesthetist must maintain close control of fluid administration and this can be facilitated by use of an infusion pump or syringe driver. A running tally of all fluid administered should be recorded, including any volumes administered with adjunct therapies such as dopamine or dobutamine and 5% dextrose in water (D5W).
Patient evaluation
Evaluation of the cat before sedation or anaesthesia should include assessment in each of the following areas.
History
The effects and elimination of anaesthetic agents may be altered by specific diseases and anaesthesia may result in a long-term adverse effect on organ function that was already compromised. Drugs administered to control fear or anxiety, such as amitriptyline, paroxetine, sertraline, fluoxetine, or selegiline may have interaction with anaesthetic agents. Although related complications are not documented in cats, there are potential problems with these agents and they have been discussed in the previous chapter. Concurrent administration of drugs to control hypertension may potentiate hypotension during anaesthesia. Frequent measurement of blood glucose perioperatively is important for cats with diabetes and some cats will require infusion of glucose-containing fluid. Knowledge of previous adverse responses to anaesthesia, such as excitement, hypotension, or difficult recovery, is useful so that the current protocol can be modified accordingly.
Physical examination
The physical characteristics of the patient may immediately suggest a potential problem, such as brachycephalic syndrome in Persian and Exotic shorthair cats. With severe brachycephalia, the upper canine teeth rotate from the normal nearly vertically aligned position to pronounced dorsorotation or nearly horizontal position. The greater the degree of brachycephalia and dorsorotation, the narrower the nasal cavity, nasal airways and nares (Schlueter et al., 2009). In the previous study, hypoplasia of the trachea was not present in the brachycephalic cats studied. Brachycephalic cats must not be left alone after premedication for fear of airway obstruction. Obese cats have impaired ventilation and are predisposed to hypoxaemia during sedation and anaesthesia. Dose rates of anaesthetic agents should be calculated on the ideal weights of these patients to avoid overdosage. Very thin cats are at increased risk for hypothermia and warming methods must be used throughout the anaesthetic episode, particularly during the time between induction of anaesthesia and start of surgery, and continued into recovery.
As in other species, age affects aspects of anaesthesia in feline patients. Although the demarcations between life stages are arbitrary, the AAFP and AAHA (American Animal Hospital Association) have determined feline life-stages as follows (Hoyumpa Vogt et al., 2010):
Senior and geriatric cats will have decreased requirements for anaesthetic agents and are more likely than younger animals to develop hypoventilation, hypoxaemia, and hypotension during anaesthesia.
Auscultation of a heart murmur on routine physical examination should be further investigated. Cardiac pathology was identified using echocardiography in 53% of apparently healthy cats with a murmur (Nakamura et al., 2011). A heart murmur may be auscultated in animals without underlying structural heart disease, and in cats that are hypovolaemic, anaemic or febrile, or may be absent despite the presence of significant heart disease such as hypertrophic cardiomyopathy (HCM). Nevertheless, the presence of cardiac disease such as cardiomyopathy, mitral insufficiency, or a patent ductus arteriosus (PDA) requires modification of the anaesthetic protocol to avoid an adverse outcome. The lungs should be auscultated for abnormal or absent air sounds that suggest the presence of pulmonary disease or diaphragmatic rupture. Some breeds are associated with increased incidence of certain diseases, such as the Maine Coon and cardiac disease.
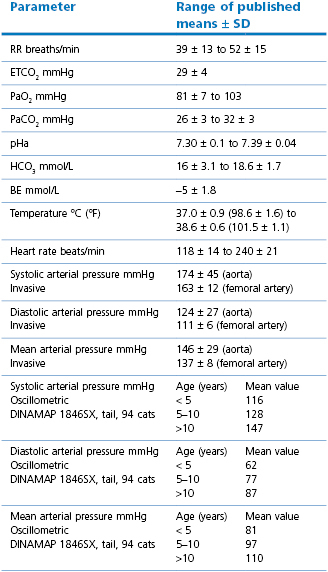
The range of normal values for physiological parameters is wide and the measurements obtained may be influenced by the surroundings and method of monitoring (Table 16.1). Respiratory rates (RR), heart rates (HR), and Doppler systolic arterial pressures (SAP) have been found to be slightly higher when obtained in the hospital compared with the home environment although, even at home, some cats struggle during measurements (Quimby et al., 2011). Cats ≥11 years of age were found to have significantly higher SAP, diastolic arterial pressures (DAP) and mean arterial pressures (MAP) than younger cats (Bodey & Sansom, 1998) and, in a later study, the increases in blood pressures were progressive with increasing age (Sansom et al., 2004). Increased systolic arterial pressures of 160–179 mmHg and DAP 100–119 mmHg carry a moderate risk for organ damage, and SAP ≥180 mmHg with DAP ≥120 mmHg as carrying a severe risk for organ damage (Brown et al., 2007). Cardiac abnormalities are frequent in cats with hypertension. Hypertension is present in 19–65% of cats with chronic kidney disease, although cats with extreme renal compromise may have lower pressures and cats with chronic kidney disease and hypertension may not have azotaemia (Bodey & Sansom, 1998; Brown et al., 2007). Cats with hyperthyroidism may or may not be hypertensive but hypertension has been measured in 50–100% of cats with primary hyperaldosteronism. Hypertension is considered pathological in cats when ocular pathology is also present. Hypokalaemia and muscle weakness are also features of primary hyperaldosteronism. Measurement of blood pressure is advisable in older cats as part of the preanaesthetic evaluation. The cat must be completely relaxed, preferably in its cage, and the final values recorded should be the average of several measurements.
Diagnostic tests
Further information may be desired based on the patient’s history and physical examination. Requirement for preoperative laboratory data is based on the patient’s age, pre-existing disease, and the type and complexity of the planned procedure. Since organ malfunction increases with increasing age, haematological and biochemical measurements should be determined in mature and older animals and the results may provide warning of a problem that requires specific anaesthetic management. Laboratory tests should be performed on all cats with comorbidities. Preoperative test results from healthy or sick cats scheduled for complicated surgical procedures will provide baseline values for postoperative evaluation. Measurement of haematocrit, total protein, and blood urea nitrogen (BUN) is recommended in any dog or cat that has not had laboratory tests within 2–4 weeks of anaesthesia. The clinician has an obligation to determine clinical relevance of an abnormal result and need for further investigation (Epstein et al., 2005; Klein & Arrowsmith, 2010). Administration of sedation or anaesthesia before collection of blood will alter the results (Frankel & Hawkey, 1980; Dhumeaux et al., 2012; Reynolds et al., 2012). Even a small IV dose of ketamine, 2 mg/kg, with diazepam, 0.1 mg/kg, decreased red and white blood cell and platelet counts, albumin and triglycerides (Reynolds et al., 2012). Some laboratory reference values may be different for various breeds. An investigation of 525 Birman, Chartreux, Maine Coon, and Persian cats in France identified clinically relevant differences for creatinine, glucose, and total protein (Reynolds et al., 2010). The Birman cats were found to have higher creatinine concentrations than cats of the other breeds, and plasma glucose concentrations were higher in Chartreux and Maine Coon cats.
Impact of disease
The presence of abnormalities may indicate the need to adjust choice of agents and dose rates or anaesthetic management to improve safety. Cats with kidney disease or urethral obstruction have reduced urine flow and prolonged recovery from anaesthesia will result from use of agents that rely on renal excretion for elimination, specifically ketamine and tiletamine–zolazepam. Azotaemia decreases anaesthetic requirement, therefore, lower doses should be used and the drugs titrated to effect. Cats with chronic kidney disease should be administered balanced electrolyte solution IV during anaesthesia and even preoperatively and through the recovery period to encourage diuresis. The cats should be monitored after anaesthesia until confirmation of urine voiding.
Medications for cardiac disease should be continued on the morning of the day of surgery. Some medications used to treat hypertension increase the risk for hypotension during inhalation anaesthesia and preparations for treatment should be available. Increased HR may adversely impact cardiac function in cats with HCM. Atropine or glycopyrrolate should not be used for premedication in these patients but reserved for therapeutic use if necessary. Vasoconstriction will decrease cardiac output in the presence of mitral insufficiency. Medetomidine and dexmedetomidine or acepromazine should be used cautiously to avoid exacerbation of hypertension or hypotension, respectively. Fluid overloading should be avoided in cats with severe cardiac disease.
Consideration of the results from the physical examination and diagnostic tests should culminate in an overall assessment of the health status of the patient. Commonly, the patient is then assigned a category of 1–5, with 1 representing a healthy animal and 5 moribund, and with the suffix E in situations of emergency anaesthesia (Chapter 1; ASA categories). This is a useful exercise because categories 3–5 have been associated with increased incidence of complications. Potential complications of the proposed medical or surgical procedure are not included with ASA status, therefore, the assigned category does not represent the total risk of anaesthesia and surgery.
Vaccination
The effect of anaesthesia on serological responses to vaccination at the time of neutering has been of concern. One investigation of serological responses to vaccinations before, during or after neutering kittens at 7, 8, or 9 weeks of age measured antibody titres that were comparable to kittens that were not anaesthetized, suggesting that anaesthesia and surgery did not have a significant impact (Reese et al., 2008).
Handling
The ISFM and AAFP guidelines for handling cats encourages the veterinary team to ‘think like a cat’ (Rodan et al., 2011). Since cats often respond to confrontation by avoidance or hiding, allowing a cat to feel hidden using towels or carriers may facilitate handling. Cats should be handled gently (unless unavoidable) using slow steady movements, a quiet voice and avoiding direct eye contact. Spraying synthetic feline facial pheromone in advance into the cage and on towels used for handling may reduce anxiety and fear aggression. Use of pheromones was found to add an additional calming effect in cats given acepromazine and to a lesser extent when cats were not given acepromazine, but had no appreciable effect on the ease of IV catheter insertion (Kronen et al., 2006). When approaching a cat in a cage, the opening should not be completely blocked (which the cat perceives as a threat) and the cat should be allowed to approach the handler. If the cat is reluctant to approach, the top of some carriers can be removed to gain access to the cat. The ISFM/AAFP guidelines recommend that the carrier should not be tipped up and the cat shaken out but to reach in and support the caudal end of the cat and back legs to encourage it to move forward. Sliding a towel around or over the cat before laying on hands may sufficiently relax the cat to facilitate easy removal from the cage. Most cats prefer touch on their heads and neck than other parts of the body. Signs of anxiety or fear include ears that are lowered and swiveled out, head drawn into the body, back slightly hunched, tail moved in to cover the feet, and sweaty paws. Immobility (‘freezing’) may also indicate anxiety.
‘Scruffing’ is a term for a variety of holds on the skin of the cat’s neck. Opinions vary about use of scruffing (Rodan et al., 2011). Some clinicians will never use scruffing, others use scruffing only if necessary to protect a cat or person from injury, while others believe that it is acceptable for short procedures or to prevent escape. A gentle hold is sufficient for most pets. Chemical restraint may be needed when an aggressive response is anticipated. Preanaesthetic medication reduces patient struggling during insertion of an IV catheter before general anaesthesia.
Nets of the clam-shell design with small holes are available commercially to capture cats reluctant to be handled (Fig. 16.1). Any part of the cat is then available for IM injection. Also available are clear plastic boxes with one side that can be moved to immobilize the cat for IM injection.
Sedation and analgesia
The goal for sedation is a quiet tractable animal to facilitate procedures such as examination or IV injection. Sedation will modify sympathetic nervous system stimulation and minimize increases in cardiac output and blood pressure that would increase the dose rate of subsequent anaesthetic agents. High dose rates result in greater cardiopulmonary depression and a narrower margin of safety. Minimizing conflict between cat and handler also minimizes stress on the veterinary personnel and contributes to efficiency by allowing them to stay focused and complete the job in hand more quickly.
Agents
Acepromazine
Acepromazine may be given to cats at dose rates of 0.02–0.1 mg/kg IM, IV or SC (Table 16.2). Acepromazine given alone provides mild sedation that can be intensified by the addition of an opioid. It is used to provide a base for anaesthesia, to increase sedation from an opioid, to decrease signs of CNS stimulation seen with an opioid or ketamine, and to provide a quieter recovery from anaesthesia.
Table 16.2
Injectable drugs for sedation, analgesia, or premedication in cats
| Drug | Dose (mg/kg) |
| Anticholinergic | |
| Atropine | 0.04 IM, SC; 0.02 IV |
| Glycopyrrolate | 0.01 IM; 0.005 IV |
| Phenothiazine | |
| Acepromazine | 0.02–0.1 IM; 0.02–0.05 IV |
| Benzodiazepine | |
| Diazepam | 0.2–0.5 IV |
| Midazolam | 0.1–0.5 IM, 0.1–0.3 IV |
| α2-Agonist sedative | |
| Xylazine | 1–2 IM, 0.2–0.5 IV |
| Medetomidine* | 0.005–0.08 IM |
| Dexmedetomidine* | 0.002–0.04 IM |
| Opioid | |
| Butorphanol | 0.1–0.4 IM, IV, SC |
| Buprenorphine | 0.005–0.02 IM; 0.005–0.01 IV |
| Morphine | 0.2–0.3 IM |
| Meperidine | 2–5 IM, SC |
| Hydromorphone | 0.05–0.1 IM, IV |
| Oxymorphone | 0.05–0.1 IM, IV |
| Methadone | 0.2–0.5 IM; 0.1–0.3 IV |
| Other | |
| Tramadol | 2 SC |
* 0.005 mg/kg = 5 µg/kg, 0.08 mg/kg = 80 µg/kg
α2-Agonist sedatives
Experimental and clinical studies confirm that medetomidine and dexmedetomidine reliably produce sedation and analgesia in cats in 15–20 minutes after IM administration, although pre-injection excitement or aggression may result in sedation of less intensity. Dose rates based on body surface area are available in the product package, indicating that the µg/kg dose rate decreases as the animal’s body weight increases. The duration of sedation is dose dependent. Equivalent sedative doses for dexmedetomidine are approximately one-half those for medetomidine. Analgesia produced by dexmedetomidine, 0.04 mg/kg (40 µg/kg), was reported to be equivalent to that provided by medetomidine, 0.08 mg/kg (80 µg/kg), IM (Slingsby & Taylor, 2008).
Dexmedetomidine given at doses varying from 0.015 to 0.04 mg/kg (15–40 µg/kg), IM induced lateral recumbency in cats in approximately 20–30 minutes (Monteiro et al., 2009; Slingsby et al., 2010). Maximum sedation without responsiveness lasted about 30 minutes from the highest dose and the sedative effects began to wane at 60 minutes after administration (Slingsby et al., 2010). Although times vary somewhat between reports, in general, the duration of maximum sedation was similar for all the doses but the total duration of sedation tended to be longer the higher the dose rate. Experimental tests of analgesia indicated that dexmedetomidine does not produce analgesia at low dose rates but that analgesia is present when the dose of dexmedetomidine is increased to 0.04 mg/kg, IM. Further, the intensity and duration of analgesia from dexmedetomidine, 0.04 mg/kg, was less than that provided by buprenorphine, 0.02 mg/kg (Slingsby et al., 2010). Dexmedetomidine, 0.04 mg/kg, administered by the buccal transmucosal (TM) route achieved the same effect as from IM administration (Slingsby & Taylor, 2009). Onset of sedation was on average 30–35 minutes and duration an average of 98 minutes from both IM and TM routes. Medetomidine, at a lower dose of 0.02 mg/kg (20 µg/kg), produced sedation lasting 30–60 minutes in experimental cats (Lamont et al., 2001).
The cardiovascular effects of medetomidine and dexmedetomidine are similar for all dose rates. After administration of medetomidine, 0.02 mg/kg, HR were decreased by 40% from the preadministration values and, although systemic vascular resistance (SVR) increased significantly at 15 minutes (vasoconstriction), MAP was unchanged. Cardiac output (CO) was decreased by 63% at 15 minutes, an effect due to a combination of decreased HR and decreased stroke index, the latter presumed due to the increase in afterload. No significant changes in blood gas values were noted. Similarly, HR decreased after administration of dexmedetomidine and SAP as measured by Doppler non-invasive blood pressure measurement (NIBP) was unchanged for 60 minutes (Monteiro et al., 2009). Prior administration of atropine, 0.05 mg/kg, IM significantly increased HR and SAP for 45 minutes, although not to the high levels recorded in dogs. Concurrent administration of atropine with these sedatives is not usually recommended.
The sedative effects following administration of xylazine, 1 mg/kg, or romifidine, 0.2 mg/kg, IM are similar although muscle relaxation and analgesia were less with romifidine and were not increased significantly by increasing dose rate (Selmi et al., 2004). Medetomidine and dexmedetomidine are generally chosen over romifidine for use in cats.
α2-Agonist sedatives are commonly administered in combination with an opioid. The addition of butorphanol, 0.2 mg/kg, IM to a low dose of dexmedetomidine, 0.01 mg/kg, significantly increased sedation and muscle relaxation over administration of dexmedetomidine alone (Selmi et al., 2003). The combination of buprenorphine, 0.01 mg/kg, with dexmedetomidine, 0.02 mg/kg, IM produced a similar duration and degree of maximum sedation as dexmedetomidine, 0.04 mg/kg, and a greater duration of analgesia (Slingsby et al., 2010). A combination of midazolam, 0.4 mg/kg, butorphanol, 0.4 mg/kg, and dexmedetomidine, 0.005 mg/kg (5 µg/kg), IM produced deep and long-lasting sedation (Biermann et al., 2012). With this combination, HR decreased, CO decreased by approximately 50%, and blood pressure was unchanged.
The addition of ketamine to the combination of medetomidine or dexmedetomidine and butorphanol (popularly known as ‘kitty magic’) can produce sedation at low doses and anaesthesia at higher doses. The combination of 0.1 mL each of medetomidine (1 mg/mL) or dexmedetomidine (0.5 mg/mL), butorphanol (10 mg/mL), and ketamine (100 mg/mL) mixed in one syringe and administered IM will induce heavy sedation within 5 minutes (Ko et al., 2009). These volumes are calculated for cats weighing 4.5 kg and are equivalent to dexmedetomidine, 0.011 mg/kg, butorphanol, 0.22 mg/kg, and ketamine, 2.2 mg/kg. More or less of the total volume may be administered depending on the desired effect. The volume of each drug may be increased to 0.2 mL/4.5 kg or 0.13 mL/3 kg to induce general anaesthesia for castration and to 0.3 mL/4.5 kg or 0.2 mL/3 kg to produce 40 minutes of anaesthesia suitable for ovariohysterectomy. Administration of this drug combination IV is generally at half the IM dose. Substitution with buprenorphine for butorphanol is not as satisfactory for injectable anaesthesia because the onset time for buprenorphine is long, but is suitable for premedication to inhalation anaesthesia.
The sedative effects of medetomidine and dexmedetomidine may be antagonized by administration of atipamezole IM. The optimal recommended dose for atipamezole is 2.5× the dose of medetomidine, which equals half the volume of medetomidine previously administered, or up to 5× the dose of dexmedetomidine for deeply sedated cats. Allowance should be made for the time elapsed since administration of the α2-sedative, as less atipamezole will be necessary over time. Overantagonism can lead to the cat becoming excessively alert. Antagonism of the sedative will remove analgesia and reveal excitatory effects of ketamine, when present. In an emergency, administration of atipamezole, 5–20 µg/kg has been recommended slowly IV (off label) over several minutes. Atipamezole is not FDA licensed for use in cats in the USA.
Vomiting has been recorded in cats after administration of xylazine, medetomidine, dexmedetomidine, and romifidine in most (Lamont et al., 2001; Selmi et al., 2004; Slingsby & Taylor, 2009; Slingsby et al., 2010; Biermann et al., 2012) but not all reports (Selmi et al., 2003). The cats are usually still standing at this point which decreases the risk of aspiration.
Concerns with use of α2-agonists centre on their sedative and cardiovascular effects. When any of these agents is used for premedication, the dose of subsequently administered anaesthetic agent may be decreased by half or even more, especially when an opioid or a low dose of ketamine has been included. α2-Sedatives profoundly decrease CO and this must be taken into consideration when selecting an anaesthetic protocol for old or sick cats and cats with cardiac disease. Use of medetomidine or dexmedetomidine is not recommended in very young kittens.
Benzodiazepines
Diazepam and midazolam are rarely used alone as they produce minimal sedative effects and may cause agitation or restlessness in healthy patients. Benzodiazepines are commonly administered as adjunct agents to opioids or anaesthetic agents to increase sedation or to block excitatory effects of ketamine. Midazolam, 0.1–0.5 mg/kg, may be administered IM or IV and diazepam, 0.1–0.5 mg/kg, may be administered IV. Oral forms of diazepam are not recommended because of the risk of hepatotoxicity.
Opioids
Factors to be considered when choosing an opioid are the preferred route of administration, speed of onset of effect, duration of effect, efficacy of analgesia in relation to the species and procedure to be performed, and adverse effects.
The speed of onset is of significance depending on the time available between administration and the start of anaesthesia or painful process, with IV and IM administration as the most reliable routes. Onset after IV administration is rapid for butorphanol, hydromorphone, oxymorphone, fentanyl, and methadone. Onset of analgesia following administration of buprenorphine, 0.02 mg/kg, was 15 minutes after IV administration in one study (Steagall et al., 2008a), 35 minutes for both IV and IM routes in another (Slingsby & Taylor, 2008), and 4 hours after IM administration in another (Robertson et al., 2003). Onset of effect after IM administration is relatively rapid with butorphanol and hydromorphone at 15–20 minutes (Lascelles & Robertson, 2004; Wells et al., 2008) and peak effect of hydromorphone, 0.1 mg/kg, was measured at 30 minutes (Robertson et al., 2009). Onset after IM injection of meperidine (pethidine) 5 mg/kg, was approximately 20 minutes with analgesia persisting for 60 minutes (Millette et al., 2008).
Methadone, 0.3 mg/kg, IV quickly causes sedation that lasts 30 minutes. Antinociception was detected 5 minutes after IV administration, was most intense for 60 minutes and a significant effect persisted for 4 hours (Ferreira et al., 2011). In a clinical investigation of cats undergoing ovariohysterectomy, premedication with methadone, 0.6 mg/kg, IM provided pain relief for 4 hours after surgery (Rohrer-Bley et al., 2004). The duration of analgesia from hydromorphone, 0.1 mg/kg, against experimental nociception was twice as long after IM than IV administration, 5.7 hours compared with 2.7 hours, respectively, and this dose rate produced significantly more analgesia than half that dose rate (Lascelles & Robertson, 2004; Wegner & Robertson, 2007). Thermal antinociception was recorded at 4–6 hours after IM injection of morphine, 0.2 mg/kg (Robertson et al., 2003). Morphine may not be as analgesic in cats as in other species because the glucuronidation pathway is less efficient in cats, preventing formation of a morphine active metabolite (Taylor et al., 2001). Evaluations of the duration of antinociception from butorphanol, 0.1–0.8 mg/kg, IM vary from 5 to 480 minutes (Robertson et al., 2003; Wells et al., 2008). A clinical perception is that the duration of analgesia from butorphanol is up to 2 hours and that while analgesia may be satisfactory for soft tissue surgery, butorphanol does not provide sufficient analgesia for orthopaedic procedures. No sedation should be expected after administration of buprenorphine alone. Duration of analgesia from buprenorphine, 0.02 mg/kg, was recorded as 7–8 hours (Slingsby & Taylor, 2008).
Care must be taken when mixing opioids in the same patient as µ agonists, buprenorphine, and butorphanol possess different receptor affinities that may result in diminished analgesia. Administration of hydromorphone and buprenorphine together resulted in minimal analgesia for the first 2 hours followed by onset of analgesia (Lascelles & Robertson, 2004) and, in another investigation, simultaneous administration of butorphanol and buprenorphine resulted in some cats without analgesia (Johnson et al., 2007).
Tramadol, 1 mg/kg, SC was an inefficient analgesic agent in an experimental investigation (Steagall et al., 2008b) whereas 2 mg/kg, administered SC 1 hour before ovariohysterectomy provided adequate analgesia postoperatively in 50% of cats (Brondani et al., 2009). Cats given both tramadol and a non-steroidal anti-inflammatory drug (NSAID) did not need rescue analgesia and did not develop hyperalgesia. A longer elimination half-life for tramadol was measured in cats than in dogs (Pypendop & Ilkiw, 2007).
Routes of administration other than IV and IM are not reliable for all opioids. Subcutaneous injection of hydromorphone has a slower onset of peak effect, a shorter duration of antinociception and is associated with more undesirable side effects (Robertson et al., 2009). Buprenorphine administered SC or by buccal transmucosal (TM) routes produced significantly less analgesia than from IV or IM injection (Slingsby & Taylor, 2008; Giordano et al., 2010), although one investigation using thermal nociception found the efficacy and duration of analgesia from buprenorphine similar after TM and IV administration (Robertson et al., 2005). TM administration is accomplished by squirting the drug from a 1 mL syringe inserted between the teeth and the cheek. Interestingly, administration was found to be significantly easier (without the cat turning its head away) using preservative-free buprenorphine than buprenorphine from a multidose bottle (Bortolami et al., 2012b). The authors proposed that the smell or taste of the chlorocresol preservative in the product intended for multiple injections could be responsible for the aversive behaviour and that the cat’s response to the preservative might result in less precise administration or increased swallowing of buprenorphine. Plasma concentrations of butorphanol after TM administration remained below the concentrations anticipated for effective antinociception (Wells et al., 2008). Butorphanol is not as lipophilic as buprenorphine and the authors proposed that the cats’ oral pH was not high enough to promote maximal absorption. TM administration of methadone resulted in a lower peak plasma concentration than after IV even though the TM dose was double the IV (Ferreira et al., 2011).
Naloxone is an opioid antagonist and will reverse opioid-induced sedation, analgesia and hyperthermia. Naloxone, 0.01–0.02 mg/kg, is administered IM or slowly IV.
Most investigations have described the effects of a reservoir system fentanyl patch using one 25 µg/h patch per cat (see previous chapter for precautions for use and dangers for human toxicity). Despite considerable variability between cats, the increase in plasma concentration of fentanyl is quicker in cats than in dogs reaching a presumed analgesic threshold by 12–18 hours (Lee et al., 2000; Egger et al., 2003). Maximum plasma concentration was measured at 44 hours after application in one group of cats and mean values remained constant for 4 days (Lee et al., 2000). In contrast to dogs, plasma concentrations of fentanyl decreased more slowly in cats after the patches were removed, presumably due to a slow depletion of a cutaneous depot of fentanyl. Differences in skin structure may partly explain interspecies variability in serum or plasma fentanyl concentrations achieved following application or removal of a patch. Arbitrarily decreasing the surface area of the patch by removing part of the adhesive layer was not recommended for the reservoir type patch as that action would not correspond to a predictably linear decrease in fentanyl transfer (Lee et al., 2000). Nonetheless, comparison of full or partial exposure in cats weighing 1.3–4.3 kg anaesthetized for ovariohysterectomy revealed significantly higher plasma concentrations in cats with full exposure (Davidson et al., 2004). The authors proposed a 38% decrease in calculated delivery rate of fentanyl resulting from a 50% decrease in surface area. Dysphoria was observed in several cats that were fully exposed to the patch membrane. Application of a 25 µg/h patch to cats weighing 2.2–5 kg scheduled for anaesthesia and onychectomy resulted in a similar range of plasma fentanyl concentrations without adverse effects (Gellasch et al., 2002). In a study comparing serum cortisol concentrations in conscious cats and during anaesthesia with or without surgery, application of a TD fentanyl patch 12 hours before anaesthesia was associated with significantly decreased cortisol during and after surgery, supporting the authors’ conclusion that the TD patch diminished pain or stress (Glerum et al., 2001). After application of a fentanyl patch, cats’ behaviours should be observed for signs of overdosage, such as dysphoria, lethargy, inappetence. Cats should also be monitored for signs of pain. Adequate analgesia should not be assumed as plasma fentanyl concentrations may be low or undetectable in some cats after application of a patch (Lee et al., 2000).
TD fentanyl results in a small (18%) but significant decrease in isoflurane requirement in experimental cats (Yackey et al., 2004). A decrease in serum fentanyl concentrations after induction of anaesthesia has been measured in cats that developed hypothermia (35°C, 95°F), presumably due to decreased cutaneous blood flow from vasoconstriction (Pettifer & Hosgood, 2003). The mean fentanyl concentrations of all the cats were within values believed to be associated with analgesia so that the decrease may be of clinical relevance only in some cats. A further concern is that plasma fentanyl concentrations may increase when a cat is positioned with the fentanyl patch (and skin) in direct contact with a heating pad or heated cage floor.
Side effects of opioids in cats are excessive salivation, vomiting, euphoria or dysphoria, and increased body temperature, with some variations between agents. Signs of euphoria (behaviour including more than usual meowing, purring, rubbing, rolling, and kneading with forepaws) was present for 2–6 hours after IV methadone and 6–12 hours after TM administration (Ferreira et al., 2011). Euphoria has been observed after administration of other opioids, lasting from 30 minutes with butorphanol to 24 hours with buprenorphine (Gellasch et al., 2002; Robertson et al., 2003; Steagall et al., 2008a; Bortolami et al., 2012a). Euphoria has also been observed after administration of tramadol (Pypendop & Ilkiw, 2007; Steagall et al., 2008b). Dysphoria (staring into space, wary of people, pacing, jumping at the cage walls) has been observed in some cats given oxymorphone, hydromorphone, fentanyl, or tramadol. Mydriasis is induced in cats by µ opioids, butorphanol, and buprenorphine, and dilated pupils may persist for 6–8 hours or even 2–3 days. Cats may vomit or excessively salivate after administration of morphine, hydromorphone, methadone, and tramadol. The addition of acepromazine may decrease the incidence of vomiting. Cats given butorphanol, buprenorphine, or fentanyl are not likely to vomit.
Increased body temperature frequently occurs when cats have been given an opioid. Cats with hyperthermia may pant or behave no differently from the expected effects of the drug(s) administered. Body temperature was monitored for 24 hours in experimental cats with a wireless thermistor implanted within the abdomen that were given different opioids and combinations (Posner et al., 2010). Moderate hyperthermia was defined as 40.1°C (104°F). Hydromorphone, morphine, buprenorphine and butorphanol produced mild to moderate increases in body temperature. Various doses of hydromorphone increased cats’ temperatures to similar extents with a peak around 1.5–2 hours after administration and a return to baseline by about 5 hours. The maximum temperature recorded for any cat in this study was 40.7°C (105.3°F) but temperatures up to 42.5°C (108.5°F) have been reported (Niedfeldt & Robertson, 2006). Greater increases in temperature have been noted after opioid administration in conjunction with inhalation anaesthesia compared with opioid administration without general anaesthesia. It has also been noted that cats that were coldest during anaesthesia developed the highest peak temperatures during recovery (Posner et al., 2010). Increased body temperatures were also recorded for several hours after anaesthesia in cats with fentanyl patches applied for premedication (Lee et al., 2000; Glerum et al., 2001). Mild to moderate increases in temperature should resolve without treatment. Cooling measures should be instituted to treat severe hyperthermia and injection of naloxone may further decrease body temperature.
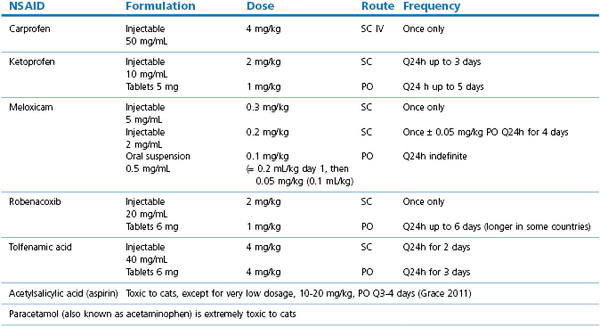
Non-steroidal anti-inflammatory agents
An NSAID will contribute to the comfort of a cat after a surgical procedure. Recommended dose rates for use in cats are given in Table 16.3, however, not all NSAIDs are licensed in all countries and the clinician should refer to local information and regulations (Sparkes et al., 2010). Single doses administered for treatment of acute pain may have a duration of 18–20 hours. Although the risk of acute renal failure is low in healthy cats, the ISFM and AAFP panel recommendations list increased risk of adverse renal effects associated with hypotension during anaesthesia, cats that are dehydrated or hypovolaemic, older cats, cats with concurrent cardiovascular, renal or hepatic disease, and concurrent administration of ACE inhibitors, diuretics, and beta-blocking drugs. NSAIDs should be used with extreme caution if at all in cats with severe liver dysfunction or hypoalbuminaemia.
Controversial issues
This section addresses some areas where there are differences of opinion. One opinion may not be better or safer than another but they illustrate varying practices in different circumstances and the need for further evaluation.
Evaluation of pain
The presence and severity of pain in cats may be difficult to assess. Observation alone appears to be an inadequate assessment of pain and changes in HR or RR may be unrelated. Cats in pain tend to become quiet and less interactive and evaluation of their responses to stroking their fur or handling of the damaged or surgical site may be more informative. Cats that hunch their backs, lower their ears, hiss, or are aggressive may be showing signs of anxiety, fearfulness, or pain. There are many pain scoring systems that may be employed (Chapter 5):
In many studies, these evaluations are performed before anaesthesia to provide a baseline value for comparison with postoperative values. The difficulty in designing a scale that is sufficiently sensitive to detect pain or a difference between two or more analgesic treatments lies in the interpretation of the cat’s behaviour or response and in the relative ranking of the individual components of the scoring system. Healthy cats normally spend 40% of their day sleeping and 20% resting (Robertson & Lascelles, 2010). Thus, sternal recumbency may be normal or a result of agent-induced sedation, and lateral recumbency may be normal or the cat may be lying in this position because sternal position is uncomfortable or painful after a midline incision. A cat that is at the back of the cage, hunched over, tail wrapped around forepaws, eyes closed and ears down may be sedated or exhibiting the effects of residual anaesthetic agents or may be in pain. Cats will tear at bandages and exhibit abnormal behaviour whether they are in pain or not. Palpation of the surgical site followed by the cat moving away from the pressure may be the cat’s normal aversion to being touched or may be a consequence of induced discomfort. Palpation of the wound during clinical evaluation and for experimental studies, use of a calibrated force testing instrument or device on or adjacent to the incision may be a more accurate measure of tenderness of the surgical area and existence of hyperalgesia (Benito-de-la-Víbora et al., 2008; Brondani et al., 2009; Bortolami et al., 2012a).
A further dilemma is the designated threshold of the scoring system at which rescue (interventional) analgesia is administered, that is, the point where there is recognition that the current analgesia protocol is providing insufficient analgesia for the individual cat. A frequently used threshold is a score that is 55–62% of the total points possible, a value that may be too high since, in many publications, the need for rescue analgesia does not fit an intuitive expectation of induced pain and authors have concluded that the assessment system was not sensitive enough to pick up the more subtle signs of pain. A threshold of 33% of the total possible score was utilized in one study for identifying need for rescue analgesia in cats undergoing ovariohysterectomy and this value appeared to demarcate clearly differences between cats that did or did not receive analgesic drugs (Brondani et al., 2009).
Experimental studies of the analgesic effects of drugs employ the application of a noxious stimulus, thermal, mechanical, or electrical, to a leg or the skin over the thorax in stepwise increments before and after administration of the drug to determine the threshold at which the cat responds. The stimulus ceases when the cat responds by turning towards the device or attempts to move away, and has an automatic cut-off to prevent tissue damage in the event the drug has prevented the cat from feeling the stimulus. The nature of these stimuli may not accurately mimic a naturally occurring painful process but allows comparison of drugs. The results have demonstrated that many drugs are associated with wide variations in responses within a cat population.
Pain induced by elective surgery of short duration generally lasts only a few days. Persistence of post-surgical pain occurs in a proportion of patients but, even in human medicine, this condition is under-diagnosed and under-recognized (Schug, 2012). Persistent pain was reported in 40%, 18% with moderate to severe pain, of over 2000 human patients that had surgery 3 months to 3 years previously (Johansen et al., 2012). The survey identified a strong association between persistent pain and the presence and intensity of the immediate post-surgical pain, and many of the patients were experiencing pain before surgery. Hyperaesthesia, indicating sensitization, occurred in most patients but hypoaesthesia, indicating nerve damage, was also often present. Cats clearly experience pain after surgery when not treated with analgesic agents. The incidence of persistent pain is unknown, however, experiences with human patients highlight the need to deal effectively with surgical pain. Preventive analgesia to minimize central sensitization of the spinal nociceptive neurons aims to block noxious stimuli pre-, intra-, and postoperatively and requires use of more than one (‘multimodal’) approach (Katz et al., 2011; Gurney, 2012). Acepromazine, thiopental, propofol, alfaxalone, and the volatile inhalation anaesthetics do not provide analgesia for painful procedures. Thus, the multimodal approach employs combinations of systemic opioid(s), NSAIDs, infiltration or regional nerve blocks with local anaesthetic or other drugs, and an NMDA-receptor antagonist. Effective analgesia is demonstrated by the reduction in pain and analgesic drug use beyond the duration of the drugs administered, defined as 5.5 half-lives (Katz et al., 2011). Long-term pain persisting for more than 2–3 weeks in cats may be associated with signs of decreased mobility, decreased interaction with humans and other animals, poor appetite, and aggression. Long-term pain from non-surgical causes, such as degenerative joint disease, cystitis, neoplasia, and dental disease requires consideration of agents that may ‘reset’ the CNS and agents that are considered to be safe for continued administration in cats, such as tramadol, NSAIDs, and ‘off label’ use of other drugs (Robertson & Lascelles, 2010).
Ovariohysterectomy, castration
Pain scoring has identified that cats exhibit signs attributable to pain after ovariohysterectomy and castration, and pain has been present in some cats for at least 24 hours (Balmer et al., 1998; Slingsby & Waterman-Pearson, 1998, 2002; Glerum et al., 2001; Al-Gizawiy & Rudé, 2004; Rohrer-Bley et al., 2004; Gassel et al., 2005; Grint et al., 2006; Tobias et al., 2006; Benito-de-la-Víbora et al., 2008; Brondani et al., 2009; Steagall et al., 2009b; Giordano et al., 2010; Murison & Martinez Taboada, 2010). A comparison of the flank and midline surgical approaches confirmed that wound tenderness was greater following a flank incision (Grint et al., 2006), as might be expected from muscle involvement in the flank incision. An inexperienced surgeon may cause more tissue trauma than an experienced surgeon, however, a study evaluating postoperative behaviours after ovariohysterectomy in dogs found no significant association with experience of the surgeon or duration of surgery (Wagner et al., 2008).
Frequently, in clinical practice, a sedative such as acepromazine, medetomidine or dexmedetomidine, an opioid and an NSAID are administered in conjunction with anaesthetic agent(s) in order to achieve both intra- and postoperative analgesia (Bortolami et al., 2012a; Mathis et al., 2012). Anaesthesia for ovariohysterectomy may be maintained with an inhalant agent preceded by premedication and induction of anaesthesia with ketamine, ketamine with diazepam or midazolam, tiletamine–zolazepam, thiopental, propofol, or alfaxalone. Anaesthetic protocols using only injectable agents are commonly used when surgery time is likely to be less than 30 minutes (Table 16.4). Published investigations involving cats presented for ovariohysterectomy or castration should be evaluated cautiously because the anaesthetic protocols may have been limited combinations to facilitate study of a single analgesic drug. It is important when selecting analgesic drugs to consider their onset times to avoid start of surgery before onset of analgesia. For example, administration of buprenorphine or an NSAID at induction of anaesthesia does not provide sufficient time for onset of analgesia before the surgical procedure and, in some cases, recovery from anaesthesia. The durations of analgesia of the agents chosen must also be known for scheduling of the redosing interval. Butorphanol and pethidine (meperidine) have short durations of action of about 2 hours and a single administration of these drugs for premedication will be insufficient for postoperative analgesia (Balmer et al., 1998; Slingsby & Waterman-Pearson, 1998). Methadone, hydromorphone, oxymorphone, and buprenorphine may provide analgesia for 4–6 hours. Published research indicates that administration of only an NSAID for analgesia does not provide sufficient analgesia after ovariohysterectomy (Brondani et al., 2009; Murison & Martinez Taboada, 2010) although, in some studies, cats receiving an NSAID had better total pain scores than those receiving an opioid only (Slingsby & Waterman-Pearson, 1998; Gassel et al., 2005). Inclusion of an NSAID was found to decrease incisional hyperalgesia (Al-Gizawiy & Rudé, 2004; Benito-de-la-Víbora et al., 2008) and decrease pain scores when administered in combination with an opioid (Brondani et al., 2009; Steagall et al., 2009b).
Table 16.4
Examples of anaesthetic agent combinations for general anaesthesia in domestic cats
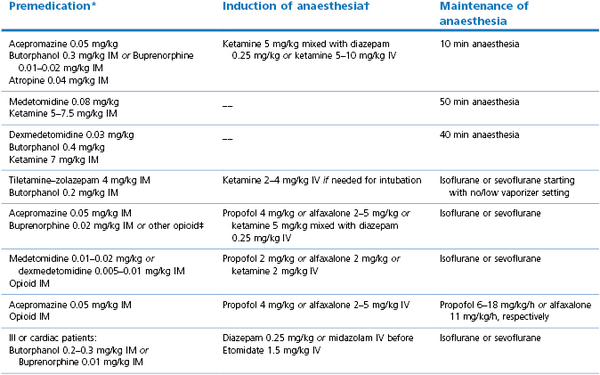
* Onset time depends on drugs chosen;
† Approximate dose rates, drugs should be given ‘to effect’;
‡ Or substitute with IM injection of butorphanol 0.3 mg/kg IM, or oxymorphone 0.1 mg/kg, or hydromorphone 0.1 mg/kg, or methadone 0.3 mg/kg, or morphine 0.3 mg/kg
Verstegen et al., 1990; Ko et al., 2009; O’Hagan et al., 2012.
Rescue (interventional) analgesia is usually in the form of an opioid at the same or reduced dose that is used for premedication, and is commonly administered IM for rapid onset of analgesia. An NSAID (SC administration) may be included if one has not already been administered. Examples of drugs used for rescue analgesia in cats after ovariohysterectomy are:
•Pethidine (meperidine) 4 mg/kg IM
•Buprenorphine 0.01–0.02 mg/kg IM
•Hydromorphone or oxymorphone 0.05 mg/kg IM
Management of anaesthesia in high volume practices and animal shelter programmes may of necessity be more regimented than tailored to the individual patient, and preanaesthetic evaluation of feral cats may not be possible. Guidelines for spay-neuter programmes published by the Association of Shelter Veterinarians are detailed and contain many suggestions for good patient care (Looney et al., 2008). The guidelines stress that a standard volume dose rate of anaesthetic agents for all cats should be avoided and suggest that doses either be calculated on a per kg basis or calculated for cats in several weight ranges, for example <1 kg, 1–2 kg, and 2–4 kg, and the volume of each drug be written out in a chart for easy reference. Anaesthetic protocols should provide analgesia. Reversible drugs are useful, especially in feral cats, but recognizing that reversal of medetomidine or dexmedetomidine removes some of the analgesia. In the context of shelter medicine, an animal with a mild infection or non-infectious medical condition may be anaesthetized because of the likelihood that the animal will not be available for neutering at a later date (Looney et al., 2008). The presence of disease may be responsible for deaths during or following anaesthesia (Gerdin et al., 2011).
Feral cats
Feral cats are defined as free-roaming cats without owners. A feral cat is caught in a special trap that can be adjusted to immobilize the cat for IM injection. The anaesthetic agent combination must reliably induce anaesthesia in unsocialized cats without causing overdosage. A small number of cats may need supplementation with additional anaesthetic agent to provide a sufficient depth of anaesthesia for ovariohysterectomy or castration. In one series of 101 feral cats (Harrison et al., 2011), the weight of each cat was determined by subtracting the known weight of the trap from the combined total weight. Medetomidine, 0.1 mg/kg (100 µg/kg), ketamine, 10 mg/kg, and buprenorphine, 0.01 mg/kg, were administered into the paralumbar muscles. If the cat was still responsive after the first injection, additional medetomidine, 0.02 mg/kg, was administered IM. Ketamine, 2.5 mg/kg, was injected IM if anaesthesia was still insufficient. Atipamezole, 0.125 mg/kg, SC was administered at the completion of surgery and the cats were released the next day. Eleven cats required additional medetomidine ± ketamine and 11 cats required supplemental isoflurane, half of which were after 45 minutes of satisfactory anaesthesia. The initial injection induced recumbency in 5 ± 5 minutes and the median time from injection to start of surgery was 23 minutes. Monitoring the cats immediately after induction and during anaesthesia is important and support treatment must be available. In the previous investigation, significant decreases in SpO2 were observed in cats in the first few minutes of anaesthesia, one cat was apnoeic after induction of anaesthesia, one cat was cyanotic and responded sluggishly to O2 supplementation, one cat had bradycardia (<60 beats/min), and three cats were hypotensive (Doppler SAP <90 mmHg).
An older review of 7501 feral cats anaesthetized for ovariohysterectomy (59%) or castration (41%) utilized a combination of tiletamine–zolazepam, ketamine, and xylazine (TKX) (Williams et al., 2002). A vial of 500 mg of lyophilized tiletamine–zolazepam was reconstituted with 4 mL of ketamine (100 mg/mL) and 1 mL of 10% xylazine (100 mg/mL) so that each mL of solution contained 50 mg tiletamine, 50 mg zolazepam, 80 mg ketamine, and 20 mg xylazine. The average dose per cat was 0.25 mL of the combination injected IM. Using the average weight of 3 kg from 194 cats, the dose rates can be calculated as tiletamine–zolazepam, 8.3 mg/kg, ketamine, 7 mg/kg, and xylazine 1.7 mg/kg. The standard dose of 0.25 mL was adjusted according to the estimated size of the cat and additional small doses were administered when the first dose was insufficient. A single dose provided adequate anaesthesia for 79.5% of cats. Yohimbine, 0.5 mg for adults and 0.3 mg for kittens, was injected at the end of surgery to reverse the xylazine. Twenty-six cats (0.35%) died before hospital discharge, of which 17 were considered to be anaesthetic deaths. In another report, the same anaesthetic drug combination (0.25 mL IM) was administered to 22 male and 67 female feral cats, and a single injection provided sufficient anaesthesia in 92% of all cats (Cistola et al., 2004). The mean time to start of ovariohysterectomy was 28 minutes and surgery time was 11 minutes. All cats survived to discharge but hypoxaemia, detected by pulse oximetry, was prevalent and some cats became hypotensive. These abnormalities are potential causes of anaesthetic death but all the cats in this study survived without treatment. The authors stressed the difficulty of routinely supplying supplemental O2 in a high volume Trap-Neuter-Return programme when, for example, 12 cats are anaesthetized simultaneously. The authors also noted that MAP increased at the start of surgery and questioned the adequacy of the combination for providing postoperative analgesia.
When dealing with a small number of feral cats in a practice environment, addition of pulse oximetry and Doppler or oscillometric arterial pressure monitoring, and supplementation with O2 when needed, is feasible and advisable.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree