General Pharmacology
Learning Objectives
After studying this chapter, you should be able to
1. Define terms related to general pharmacology.
2. List common sources of drugs used in veterinary medicine.
3. Outline the basic principles of pharmacotherapeutics.
4. Define the difference between prescription and over-the-counter drugs.
5. Describe the events that occur after a drug is administered to a patient.
6. List and describe the routes used for administration of drugs.
7. Define biotransformation, and list common chemical reactions involved in this process.
8. List the routes of drug excretion.
9. Discuss in basic terms the mechanisms by which drugs produce their effects in the body.
10. Discuss the mechanisms of clinically important drug interactions.
11. Discuss the different names that a particular drug is given.
12. List the items that should be included on a drug label.
13. List the steps and discuss the processes involved in gaining approval for a new drug.
14. List the government agencies involved in the regulation of animal health products.
15. Describe reasons for dispensing rather than prescribing drugs in veterinary medicine.
16. Discuss the primary methods of drug marketing.
Key Terms
Adverse drug event
Adverse drug reaction
Agonist
Antagonist
Compounding
Drug
Efficacy
Extralabel use
Half-life
Manufacturing
Metabolism (biotransformation)
Parenteral
Partition coefficient
Prescription (legend) drug
Regimen
Residue
Veterinarian–client–patient relationship
Withdrawal time
Introduction
Veterinary technicians are an essential component of the efficient health care delivery team in veterinary medicine. One of the important tasks that veterinary technicians carry out is administration of drugs to animals on the order of a veterinarian. Because this task may have serious consequences in terms of the outcome of a case, it is mandatory that technicians have a thorough knowledge of the types and actions of drugs used in veterinary medicine. They should have an understanding of the reasons for using drugs, called indications, and the reasons for not using drugs, called contraindications (pharmacotherapeutics). They also should know what happens to drugs once they enter the body (pharmacokinetics), how drugs exert their effects (pharmacodynamics), and how adverse drug reactions manifest themselves (toxicity). Because veterinarians dispense a large number of drugs, technicians also must be well versed in the components of a valid veterinarian–client–patient relationship, the importance of proper labeling of dispensed products, and methods of client education on the proper use of products to avoid toxic effects or residue. Finally, technicians should have a basic understanding of the laws that apply to drug use in veterinary medicine and the concept of the marketing of veterinary drugs. In short, veterinary technicians must have a working knowledge of the science of veterinary pharmacology.
Drug Sources
Traditional sources of drugs are plants (botanical) and minerals. Plants have long been a source of drugs. The active components of plants that are useful as drugs include alkaloids, glycosides, gums, resins, and oils. The names of alkaloids usually end in -ine, and the names of glycosides end in -in (Williams and Baer, 1990). Examples of alkaloids include atropine, caffeine, and nicotine. Digoxin and digitoxin are examples of glycosides. Bacteria and molds (e.g., Penicillium) produce many of the antibiotics (penicillin) and anthelmintics (ivermectin) in use today. Animals once were important as a source of hormones such as insulin and as a source of anticoagulants such as heparin. Today, most hormones are synthesized in a laboratory. Mineral sources of drugs include electrolytes (sodium, potassium, and chloride), iron, selenium, and others. Laboratories are one of the most important sources of currently used drugs because chemists are finding methods of reproducing drugs previously obtained through plant and animal sources. Advances in recombinant deoxyribonucleic acid (DNA) technology have made it possible for animal and human products (e.g., insulin) in bacteria to be produced in large quantities.
Inactive Ingredients
Veterinary pharmaceutic products and supplements may contain substances in addition to active ingredients. Inactive ingredients are classified as binders, coatings, coloring agents, disintegrants, emulsifiers, fillers, flavorings, flow agents, humectants, preservatives, sweeteners, and thickeners (Table 1-1).
TABLE 1-1
| INACTIVE INGREDIENT | FUNCTION | EXAMPLES |
| Binder | Holds tablet together | Cellulose, lactose, methylcellulose, sorbitol, starch, xylitol, and others |
| Coating | Protects tablet from breaking, absorbing moisture, and early disintegration | Beeswax, carob extract, methylcellulose, cellulose acetate, acrylic resin, and others |
| Coloring agents | Provide color and enhance appearance | Yellow No. 5, annatto, caramel color, titanium oxide, FD&C Blue No. 1, FD&C Red No. 3, and others |
| Disintegrants | Expand when exposed to liquid, allowing tablets and capsules to dissolve and disperse their active ingredients | Cellulose products, crospovidone, sodium starch glycolate, and starch |
| Emulsifiers | Allow fat-soluble and water-soluble agents to mix so they do not separate | Stearic acid, xanthan gum, lethicin, and vegetable oils |
| Fillers/diluents | Increase bulk or volume | Calcium carbonate, calcium sulfate, cellulose lactose, mannitol, sorbitol, starch, sucrose, and vegetable oils |
| Flavor agents | Create a desired taste or mask an undesirable taste | Beeswax, carob extract, glyceryl triacetate, and natural orange |
| Flow agents | Prevent powders from sticking together | Calcium stearate, glyceryl triacetate, polyethylene glycol, silica, sodium benzoate, and talc |
| Humectants | Hold moisture in a product | Glycerin, glycerol, glycerol triacetate, and sorbitol |
| Preservatives | Prevent degradation and extend the shelf life of a product | Citric acid, glycerol, potassium benzoate, sodium benzoate, and others |
| Sweetening agents | Improve taste | Aspartate, fructose, glycerin, sorbitol, sucrose, and xylitol |
| Thickening agents | Increase the viscosity of a product | Methylcellulose, povidone, sorbitol, and others |
Adapted from ConsumerLab.com: Review article: inactive ingredients in supplements (website). https://www.consumerlab.com/reviews/Inactive_Ingredients_in_Supplements/inactiveingredients. Accessed July 30, 2013.
Pharmacotherapeutics
Veterinarians are challenged by the task of assessing a patient to determine a diagnosis and arrive at a plan of treatment. If the plan of treatment includes the use of drugs, the veterinarian must choose an appropriate drug and a drug regimen. The drug is selected through the use of one or more broadly defined methods called diagnostic, empirical, or symptomatic. The diagnostic method involves assessment of a patient, including a history, physical examination, laboratory tests, and other diagnostic procedures, to arrive at a specific diagnosis. Once the diagnosis has been determined, the causative microorganism or altered physiologic state is revealed to allow selection of the appropriate drug. The empirical method calls on the use of practical experience and common sense when the drug choice is made. In other instances, drugs are chosen to treat the symptoms or signs of a disease if a specific diagnosis cannot be determined. In veterinary medicine, the comparative cost of a drug also may be an important consideration in selection of an appropriate drug. Once the drug to be used in treatment has been decided, the next step for the veterinarian is to design the plan for administering the drug. This plan, called a regimen, includes details about the following:
Every drug has the potential to cause harmful effects if it is given to the wrong patient or according to the wrong regimen. Some medications have greater potential than others for producing harmful outcomes. According to the U.S. Food and Drug Administration (FDA), when a drug has potential toxic effects or must be administered in a way that requires the services of trained personnel, that drug cannot be approved for animal use except when given under the supervision of a veterinarian. In such a case, the drug is classified as a prescription drug and must be labeled with the following statement: “Caution: Federal law restricts the use of this drug to use by or on the order of a licensed veterinarian.” This statement sometimes is referred to as the legend, and the drug is called a legend (prescription) drug. Labels that state “For veterinary use only” or “Sold to veterinarians only” do not designate prescription drugs. Technicians should be aware that prescription drugs often have been approved by the FDA for use in specific species or for particular diseases or conditions. Veterinarians have some discretion to use a drug in ways not indicated by the label, if they take responsibility for the outcome of use. Use of a drug in a way not specified by the label is called extralabel use.
Federal law and sound medical practices dictate that prescription drugs should not be dispensed indiscriminately. Before prescription drugs are issued or extralabel use is undertaken, a valid veterinarian–client–patient relationship must exist. For this relationship to occur, several conditions must be met. These include but are not limited to the following:
Drugs that do not have enough potential to be toxic or that do not require administration in special ways do not require the supervision of a veterinarian for administration. These drugs are called over-the-counter drugs because they may be purchased without a prescription. Drugs that have the potential for abuse or dependence have been classified as controlled substances. Careful records of the inventory and use of these drugs must be maintained, and some of them must be kept in a locked storage area.
When a drug and its regimen have been selected, veterinary technicians often are directed through verbal or written orders to administer the drug. Technicians have several important responsibilities in carrying out these orders:
1. Ensuring that the correct drug is being administered
2. Administering the drug by the correct route and at the correct time
3. Carefully observing the animal’s response to the drug
4. Questioning any medication orders that are not clear
5. Creating and affixing labels to medication containers accurately
6. Explaining administration instructions to clients
Technicians should be aware that even when the correct drug is administered in a correct manner, an unexpected adverse reaction might occur in a patient. All adverse events or reactions should be reported immediately to the veterinarian.
Pharmacokinetics
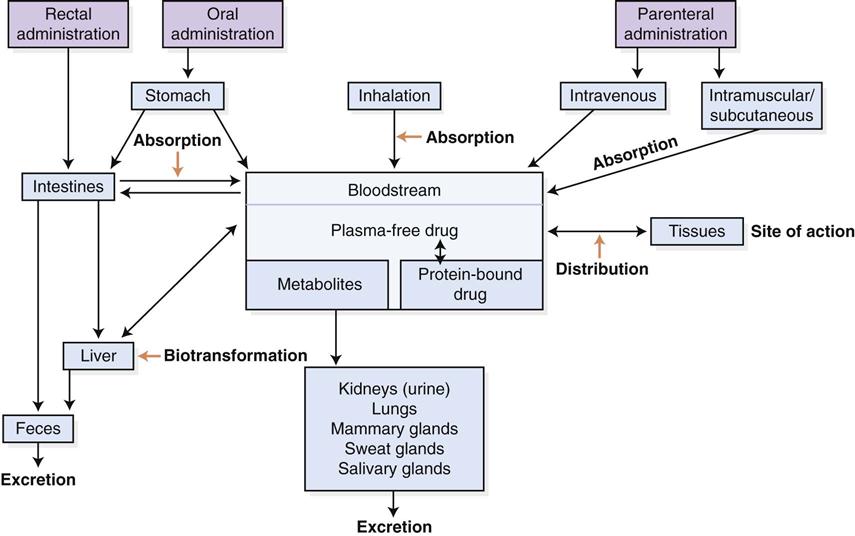
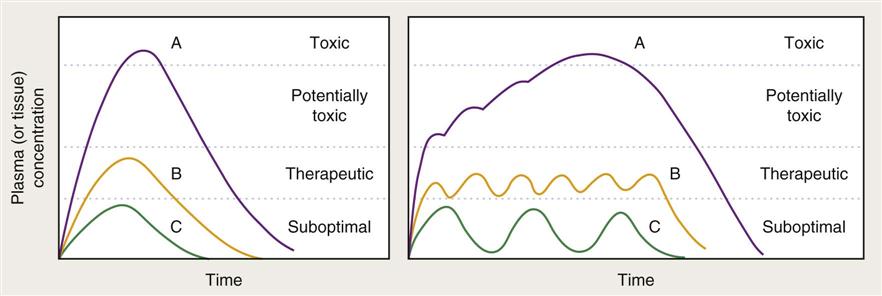
Pharmacokinetics is the complex sequence of events that occurs after a drug is administered to a patient (Figure 1-1). Once a drug has been given, it is available for absorption into the bloodstream and delivery to the site where it will exert its action. After a drug is absorbed, it is distributed to various fluids and tissues in the body. It is not enough, however, for the drug simply to reach the desired area. It also must accumulate in that fluid or tissue at the required concentration to be effective. Because the body immediately begins to break down and excrete the drug, the amount available to the target tissue becomes less and less over time. The veterinarian then must administer the drug repeatedly and at fixed time intervals to maintain the drug at the site of action in the desired concentration. Some drugs are administered at a high dose (loading dose) until an appropriate blood level is reached. Then the dose is reduced to an amount that replaces the amount lost through elimination. Doses of other drugs are at the replacement level throughout the regimen. The point at which drug accumulation equals drug elimination is called the steady state or distribution equilibrium. This equilibrium represents the state where the amount of drug leaving the plasma for tissue equals the amount of drug leaving the tissue for the plasma. Underdosing leads to less-than-effective levels in tissue, and overdosing may result in toxic levels (Figure 1-2). Drug levels can be measured in blood, urine, cerebrospinal fluid, and other appropriate body fluids to help a veterinarian determine whether an appropriate level has been achieved. This procedure, which is called therapeutic drug monitoring, is being used increasingly in veterinary practice. Nonsteroidal antiinflammatory drugs (NSAIDs), cardiac drugs, anticonvulsants, and thyroid drugs are commonly monitored.
The primary factors that influence blood concentration levels of a drug and a patient’s response to it include the following:
These factors are explored after the drug administration routes have been discussed.
Routes of Administration
A drug is of no use unless it can be delivered to the patient in an appropriate form at an appropriate site. The way in which a drug is administered to an animal patient is influenced by several factors:
• Available pharmaceutic form of the drug
• Physical or chemical properties (irritation) of the drug
• How quickly onset of action should occur
• Use of restraint or behavioral characteristics of the patient
The routes of administration of drugs to animal patients are as follows.
Oral
In veterinary medicine, drugs commonly are administered through the oral route. Medications given by this route may be placed directly in the mouth or may be given via a tube passed through the nasal passages (nasogastric tube) or through the mouth (orogastric tube). The mucosa of the digestive tract is a large absorptive surface area with a rich blood supply. Drugs given by this route, however, are not absorbed as quickly as drugs administered by injection, and their effects are subject to species (e.g., ruminants vs. animals with a simple stomach) and individual differences. Many factors may influence the absorption of drugs from the digestive tract, including the pH of the drug, its solubility (fat vs. water), the size and shape of the molecule, the presence or absence of food in the digestive tract, the degree of gastrointestinal (GI) motility, and the presence and nature of disease processes. This route is not suitable for animals that are vomiting or have diarrhea. Drugs given by this route generally produce a longer lasting effect than those given by injection.
Parenteral
Drugs that are given by injection are called parenteral drugs. A drug can be injected via many different routes:
Drugs given by the intravenous route produce the most rapid onset of action, accompanied by the shortest duration. Medications that are irritating to tissue generally are given by this route because of the diluting effect of blood. Intravenous medications should be administered slowly to lessen the possibility of a toxic or allergic reaction. Unless a product is specifically labeled for intravenous use, it should never be given by this route. Oil-based drugs and those with suspended particles (i.e., those that look cloudy or thick) generally should not be given intravenously because of the possibility of an embolism. Special care should be taken to ensure that irritating drugs are injected into the vein and not around it, to avoid causing phlebitis.
The intramuscular route of administration produces a slower onset of action than the intravenous route but usually provides a longer duration of action. The onset of action by this route can be relatively fast with a water-based form (aqueous) and is slower with other diluents (vehicles) such as oil or with other forms such as microfine crystals. When an injectable drug is placed in a substance that delays its absorption, this may be referred to as a depot preparation. Altering the molecule of the drug itself can influence its onset or duration of action. Onset of action usually is inversely related to duration of action. Irritating drugs should not be given by the intramuscular route, and back pressure always should be applied to the syringe plunger before intramuscular administration of a drug to ensure that the injection is not directed into a blood vessel.
The subcutaneous route produces a slower onset of action but a slightly longer duration than the intramuscular route. Irritating or hyperosmotic solutions (i.e., those with a greater number of suspended particles than are found in body fluid) should not be given by this route. (See Chapter 15.)
Quantities of medications that are appropriate for the species or individual being treated should be used to prevent possible dissection of the skin from underlying tissue, which could lead to death or loss (sloughing) of surface skin.
The intradermal route involves injecting a drug into the skin. This route is used in veterinary medicine primarily for testing for tuberculosis and allergies.
The intraperitoneal route is used to deliver drugs into the abdominal cavity. The onset and duration of action of drugs given by this route are variable. This route is used to administer fluids, blood, and other medications when normal routes are not available or are not practical. Problems such as adhesions and puncture of abdominal organs may be caused by this method.
The intraarterial route involves injecting a drug directly into an artery. This route seldom is used intentionally, but this may happen by mistake. Administration of drugs into the jugular vein of a horse must be done with caution to avoid injection into the underlying carotid artery. Intracarotid injection results in delivery of a high concentration of the drug directly to the brain, and seizures or death may result.
Through the intraarticular route, a drug is injected directly into a joint. This method is used primarily to treat inflammatory conditions of the joint. Extreme care must be exercised to ensure that sterile technique is used when an intraarticular injection is given. Technicians usually do not use this route.
The intracardiac route is used to inject drugs through the chest wall directly into the chambers of the heart. This provides immediate access to the bloodstream and ensures that the drug is delivered quickly to all tissue in the body. This method is often used in cases of cardiopulmonary resuscitation and in euthanasia.
The intramedullary route is another route that is seldom used in veterinary medicine. It involves injection of the substance directly into the bone marrow. The bones used most often are the femur and the humerus. The intramedullary route usually is used to provide blood or fluids to animals with very small or damaged veins or for treatment of animals with very low blood pressure.
When spinal anesthesia is provided, drugs may be injected into the epidural or subdural space. The epidural space is outside the dura mater (meninges) but inside the spinal canal. The subdural space is inside the dura mater. Injection of drugs into the subdural space (cerebrospinal fluid) is also called the intrathecal route. A veterinarian usually carries out these methods of drug delivery.
Inhalation
Medications may be delivered to a patient in inspired air by converting a liquid form into a gaseous form through the use of a vaporizer or nebulizer. Examples of drugs that may be given by this route include anesthetics, antibiotics, bronchodilators, and mucolytics.
Topical
Drugs that are administered topically are placed on the skin or on mucous membranes. Drugs generally are absorbed more slowly through the skin than through other body membranes. The rate of absorption may be increased or absorption facilitated by placement of the drug in a vehicle such as dimethyl sulfoxide (DMSO). Medication also may be applied to the mucosa of the oral cavity (sublingual), the rectum (suppositories), the uterus, the vagina, the mammary glands, the eyes, and the ears. In horses, caustic materials may be applied topically to inhibit the growth of exuberant granulation tissue (proud flesh).
Transdermal drug administration is a form of topical administration that involves the use of a patch applied to the skin to deliver a drug through intact skin directly into the blood. This method is used most commonly to administer an analgesic in a slow, continuous manner or to administer compounded drugs to animals when oral administration may be difficult (e.g., cats).
Drug Absorption
Before drugs can reach their site of action, they must pass across a series of cellular membranes that make up the absorptive surfaces of the sites of administration. The degree to which a drug is absorbed and reaches the general circulation is called bioavailability.
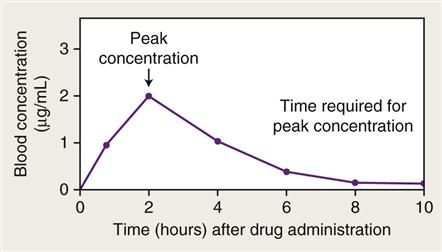
The manufacturing process can have a significant effect on the physical and chemical characteristics of drug molecules that influence their bioavailability. Because of manufacturing differences, the generic equivalent form of a drug may differ somewhat from a trademark form in overall efficacy. Bioavailability often is demonstrated with the use of a blood level curve (Figure 1-3). Factors that may affect the absorption process include the following:
• pH and ionization status of the drug
• Status of the GI tract (motility, permeability, and thickness of the mucosal epithelium)
Drugs pass across cellular membranes through three common methods. Passive absorption (transport) occurs by simple diffusion of a drug molecule from an area of high concentration of drug on one side of the membrane to an area of lower concentration on the other side. This method requires no expenditure of energy by the cell. The drug may pass through small pores in the cell membrane or may dissolve into the cell membrane on one side, pass through the membrane, and exit on the other side. For example, a disintegrated tablet or capsule results in a high concentration of drug in the GI tract. This concentration then passes through the cellular membranes of intestinal villi and adjacent capillaries, and the drug then appears in lesser concentration in the bloodstream. Alternatively, a drug may cross a membrane passively with the help of a carrier.
Drug transporters also play a major role in drug absorption. The best described transporter is the P-glycoprotein (P-gp). P-gp is produced at the direction of the MDR1 (ABCB1) gene. It uses adenosine triphosphate (ATP) as an energy source to pump drugs from cells. It is found in most mammalian tissue and appears to act in a protective manner. It is useful in intestinal, renal, placental, liver, and brain tissue, where it helps to pump transported drugs out of the body or away from protected sites. The protection is achieved by pumping the drugs into the intestine, bile, or urine for elimination or away from the fetus or brain (Boothe, 2012).
Some small drug molecules such as electrolytes may simply move with fluid through pores in cell membranes. Active transport of drugs across cell membranes moves molecules from an area of lower concentration to an area of higher concentration and requires that the cell use energy. This is the usual mechanism for the absorption of sodium, potassium, and other electrolytes. In pinocytosis, a third method of passive transport, cells engulf drug molecules by invaginating their cell membrane to form a vesicle that then breaks off from the membrane in the interior of the cell. The method of absorption that occurs in a particular situation depends on whether the drug is fat soluble or water soluble, the size and shape of the drug molecule, and the degree of ionization of the drug.
Many drugs can pass through a cell membrane only if they are nonionized (i.e., not positively or negatively charged). Most drugs exist in the body in a state that consists of both ionized and nonionized forms. The pH of a drug and the pH of the area in which the drug is located can determine the degree to which a drug becomes ionized and thus is absorbed. Weakly acidic drugs in an acidic environment do not ionize readily and therefore are absorbed well. The absorption of basic drugs is more favorable in an alkaline environment. If a drug is placed in an environment in which it readily ionizes, such as a mildly acidic drug in an alkaline environment or a mildly alkaline drug in an acidic environment, it does not diffuse and may become trapped in that environment.
As the absorptive surface of the area of drug placement increases, so does the rate of absorption. One of the largest absorptive surfaces in the body is found in the small intestine because the efficient design of the villi maximizes the surface area.
At any site of drug administration, as the blood supply to an area increases, so does the rate of absorption of the drug. Drugs are absorbed from an intramuscular site at a faster rate than from a subcutaneous site because of the proportionately greater blood supply to the muscle. Initiating the fight-or-flight response increases blood flow to the muscle but decreases blood flow to the intestines. Heat and massage also increase blood flow to an area. Poor circulation, which may occur during shock or cardiac failure, decreases blood flow, as does cooling or elevation of a body part. These factors then can positively or negatively influence drug absorption.
Another important factor that determines the rate at which drugs pass across cell membranes is the solubility of the drug. The lipid (fat) solubility of a drug tends to be directly proportional to the degree of drug nonionization. As was stated previously, the nonionized form is the one that usually is absorbed. The degree of lipid solubility of a drug often is expressed as its lipid partition coefficient. A high lipid partition coefficient indicates enhanced drug absorption.
Drug absorption rates often depend on the formulation of the drug. Various inert ingredients, such as carriers (vehicles), binding agents, and coatings, are used to prepare dosage forms. These substances have major effects on the rate at which formulations dissolve. Depot and spansule are terms that are associated with prolonged- or sustained-release formulations in veterinary medicine. Subcutaneous implants that contain growth stimulants that break down slowly and release their products over prolonged periods are used in some situations.
When drugs are given orally, the condition of the GI tract can have a major influence on the rate and extent of drug absorption. Factors such as degree of intestinal motility, emptying time of the stomach, irritation or inflammation of the mucosa (e.g., gastritis, enteritis), damage to or loss of villi (e.g., viral diseases), composition and amount of food material, and changes in intestinal microorganisms can affect the rate and extent of absorbance of medications. Another consideration regarding drugs that are absorbed from the GI tract is the first-pass effect. This refers to the fact that substances are absorbed from the GI tract into the portal venous system, which delivers the drug to the liver before it enters the general circulation. In some instances, a drug then is metabolized in the liver to altered forms; this process may make the drug inactive or less active.
The process of combining some drugs with other drugs or with certain foods can negatively affect drug absorption. The availability of tetracycline is reduced if it is administered with milk or milk products. Antacids may reduce the absorption of phenylbutazone or iron products. Technicians always should consult appropriate references about potential interactions before administering new drugs.
Drug Distribution
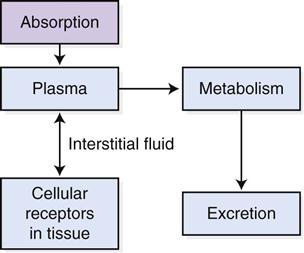
Drug distribution is the process by which a drug is carried from its site of absorption to its site of action. Drugs move from the absorption site into the plasma of the bloodstream, from the plasma into the interstitial fluid that surrounds cells, and from the interstitial fluid into the cells, where they combine with cellular receptors to create an action. Equilibrium soon is established between these three compartments while the drug moves from the blood into the tissue and then from the tissue back into the blood (Figure 1-4). How well a drug is distributed throughout the body depends on several factors.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree