Gastrointestinal Surgery in Alpacas and Llamas
Gastrointestinal (GI) diseases are reportedly considered the leading causes of camelid deaths.1–3 Increasing evidence suggests that some of these deaths likely have surgically correctable lesions that are unresponsive to medical treatment alone.3–9 As in other species, the success of GI surgery relies, in part, on early recognition and timely surgical intervention.10 Unlike in other species such as equine species, the signs and severity of GI disease in camelids tend to be vague and less frequent.7,10,11 Furthermore, the opportunity and usefulness of rectal palpation is often limited by relatively small size of camelids and the location of the lesion.10 Transabdominal ultrasonography, combined with rectal examination and abdominocentesis, likely improves the early detection of surgical lesions.3,10
Not surprisingly, the scientific literature has lagged behind the relatively rapid rise in camelid popularity. Individual case reports predominate the literature on the surgical management of GI lesions in camelids.3,4,9,12 Case management for successful outcome of GI surgery includes the preoperative, intraoperative, and postoperative periods. Knowledge of surgical anatomy and physiology, clinical signs, clinical pathology, transabdominal ultrasonography, anesthesia, surgical approaches, surgical treatments of the forestomach, small and large intestines, complications, and outcome are important to the clinician’s ability to assess and respond to patient and client needs.
Camelids have a unique GI system that is, in some ways, similar to, but also distinctly different from, that of traditional four-chambered ruminants.13 Termed pseudoruminants, adult camelids rely on forestomach protozoal and bacterial fermentation to break down plant material to digest nutrients, can eructate and ruminate, and have a three-chambered stomach that is partitioned into anatomically separate and functionally distinct areas, named C1, C2, and C3. All three compartments have the ability for secretion and absorption. C1 is the largest of the three compartments and is loosely considered the equivalent of the rumen in the cow. Ideally, the normal pH of C1 fluctuates between 6.5 and 7.5. Like the rumen in ruminants, C1 can absorb water and volatile fatty acids. However, prominent papilla are lacking, unlike in ruminants. C1 contents do not stratify in the normal healthy individual, and rows of saccules are present along the caudoventral aspect. These saccules are believed to aid in microfermentation and secrete bicarbonate directly into the C1 lumen. The role of C2 is believed to mimic the reticulum and the omasum in ruminants. C3 is considered the “true” stomach, since it functions similarly to monogastrics and the abomasum in ruminants by secreting hydrogen (H) and chloride (Cl). Anatomically, C3 most closely resembles the equine stomach, since the proximal 80% is non–acid secreting, and the distal 20% is acid secreting. Furthermore, the junction between these two regions is the typical location for ulcers to develop, especially along the lesser curvature. As in neonatal ruminants, the forestomachs in the neonate camelid (cria) initially resemble monogastrics’ the most during the first 2 to 3 months of life when the cria is primarily consuming a milk-based diet. Over several months, the forestomachs gradually increase in size and change in function to resemble the adult ruminant forestomachs more closely. As with all ruminating species, the fermentation process is continuous and the forestomachs should never be empty of feed.
After the forestomachs, the GI tract of the camelid has certain other unique characteristics. A prominent duodenal ampulla is located immediately aborad to the pylorus. The function of this ampulla is not clear, but this dilatation should not be mistaken as abnormal during diagnostic procedures or surgical exploration. Unlike ruminants, camelids do not have a gallbladder. Although camelids have a spiral colon, the proximal loop is longer in comparison to the size of the spiral colon as compared with that of ruminants. The omentum in ruminants is very prominent, whereas in camelids, the omentum is much less robust. Furthermore, the omentum attaches to the C1 along its transverse pillar and does not attach to the dorsal body wall.3,13
Clinical Signs of Surgical Gastrointestinal Disease
As with other species, signs of colic may arise from disorders of other organ systems.13 Causes of abdominal pain include ulceration of C3, enteritis, intestinal wall perforation, intussusception, volvulus of the mesentery, meconium impaction, impaction of the cecum or ascending colon, mesenteric or umbilical abscesses, toxicities, and urinary rupture.3,8,9,11,13–15 Clinical signs of abdominal pain (colic) in camelids include vocalizing (groaning), bruxism, restlessness, getting up and down, refusing to stand, rolling, kicking or looking at the belly, peculiar stance, kyphosis, anorexia, tenesmus, decreased fecal output, regurgitation, and apparent depression.3,7–9,12,13,15,16 Findings on physical examination similarly has a wide range of abnormalities including pyrexia, tachycardia, tachypnea, tense or painful abdomen, distended abdomen, pollakiuria, and C1 atony.3,7–9,12,13,15,16 Signs of colic in camelids tend to be vague and infrequent, more closely resembling sings in cattle than in horses; therefore, the camelid patient should be closely and frequently observed.7,8,11,16
Forestomach lesions tend to have more vague clinical signs such as depression and anorexia, presumably because of the greater capacity of the forestomachs to sequester fluid.3 In comparison, the severity of clinical signs associated with small intestinal obstruction is associated with their anatomic location and includes abdominal distention, abdominal pain, recumbency, pyrexia, anorexia, tachycardia, tachypnea, decreased fecal output, dehydration, and regurgitation, and are rarely violent.3,12 In our experience, the severity of clinical signs associated with lesions affecting the large intestine tend to be more subtle compared with those affecting the small intestine.
In crias, abdominal masses may be felt during deep palpation of the abdomen.9 Although rectal palpation is possible in adult camelids, the usefulness of this procedure is likely limited to lesions affecting the distal GI tract and the spleen.11,16
The incidence of complications during and after exploratory laparotomy is infrequent in camelids. Thus, early surgical intervention, including exploratory laparotomy, should be performed in a timely manner when the cause of abdominal pain is in doubt.8,9,11 Early intervention is expected to improve outcome.3,9,16 Exploratory laparotomy or diagnostic laparoscopy may be considered as extensions of the clinical examination and diagnostic workup of cases without a definitive diagnosis. The normal laparoscopic anatomy of the llama has been described and may provide a minimally invasive alternative to exploratory laparotomy for the acute abdomen of unknown etiology in selected cases when the abdomen is not markedly distended.12,17
Diagnostic Testing
A wide variety of diagnostic tests are available to aid in differentiation of abdominal diseases affecting the GI tract, liver, spleen, urinary tract, reproductive tract, and abdominal compartment.19,20 Of special interest for surgical diseases are hematology, serum biochemistry, first forestomach compartment (C1) chloride concentration, ultrasonography, and abdominocentesis. Although helpful in selected cases, radiography and computed tomography (CT) are not routinely performed. Unlike ruminants, camelids have a greater capacity to absorb water and chloride through their forestomach wall and may be less prone to fluid sequestration in the forestomachs causing dehydration and hypochloremic metabolic alkalosis.13,18 GI disorders in camelids causing metabolic alkalosis have been inconsistent and do not necessarily reflect the duration of clinical signs.3,12 Decreased potassium concentrations are consistent in proximal and distal obstructive lesions.3,12 C1 chloride analysis may help determine whether a proximal small intestinal obstruction is the underlying cause of forestomach distension. Elevated C1 chloride concentration (>40 milliequivalents per liter [mEq/L]) has been reported previously in proximal obstructions but not in distal obstructions.3,11,12 Abdominocentesis is recommended, when possible, and may help differentiate between medical and surgical conditions.3,21–23 Abdominocentesis may be contraindicated in camelids with distended abdomens.8,9 However, the safety margin may be improved when combined with transabdominal ultrasonography. Furthermore, transabdominal ultrasonography–guided abdominocentesis has yielded consistently high-quality samples compared with the blind, ventral, or paramedian approaches.24 In normal camelids, the peritoneal fluid has a low cell count and low protein concentrations.25 A large amount of cellular or proteinaceous fluid or a high cell count (>3000 nucleated cells per microliter [µL]) and protein concentration (>2.5 grams per deciliter [g/dL]) likely indicates the presence of gastrointestinal disease.3,9,11,16,25,26
Radiography and transabdominal ultrasonagraphy has been used clinically as an aid to differentiate between medical and surgical GI diseases, including, but not limited to, peritonitis, enteritis, strangulating obstructions, and intraluminal obstructions.3,11,15,23,27 The transabdominal appearance of the GI viscera in healthy camelids has been described using a 5-megahertz (MHz) linear transducer.10 Little free abdominal fluid is observed in normal camelids. The small intestines are best viewed from either the right paralumbar fossa or the paramedian areas and demonstrate progressive motility with transient segments of fluid.10 Peritonitis is associated with increased free abdominal fluid.3,11,16 Enteritis is associated with increased wall thickness, whereas focal thinning is associated with ulcerative gastroenteritis.3 Interpretation of focal thinning associated with C3 ulceration is difficult because of the variability between camelids in wall thickness of the caudal lateral portion of C3.10 However, higher-resolution equipment may improve the sensitivity of this modality.10,28 Transabdominal ultrasonography has been a useful tool to identify abnormal bowel in camelids, including duodenal trichophytobezoar obstruction, especially when the camelid is too small for rectal palpation.3,11,12,15
Surgical Approaches and Techniques
Potential problems during anesthesia of patients with GI disease may include inadequate ventilation, hypotension, regurgitation, hypothermia, hypoproteinemia, postextubation respiratory obstruction associated with nasal edema, and hepatic necrosis.29 In camelids having severe distension of the C1, decompression of the C1 is advisable before induction of anesthesia. We have witnessed sudden severe regurgitation in these patients, especially when preceded by anorexia, during anesthesia induction. This caused profuse aspiration of C1 content and subsequent death. Decompression of the C1 may be possible by oral intubation of the C1 if the fiber (forage) content of the C1 is low. In cases where decompression of the C1 is not possible, a C1 stoma may be created to directly access and decompress C1 content. C1 fistulas are created in the left flank region under sedation and local anesthesia. After decompression of the C1, induction of anesthesia may be initiated more safely. Camelids suffering acute abdominal disease may require perioperative intravenous fluids, antibiotics,3,7–9,12,16,26,30 nonsteroidal antiinflammatories, and gastric acid production inhibitor.3,7–9,12,16,26,30 The anticipated lesion location determines the approach.
In camelids, access to the abdomen may be gained through the right lateral abdominal wall, right paracostal, left lateral abdominal wall, or ventral midline approach. The surgeon’s selection of these various methods is based on the expected location of the lesion. In general, right lateral abdominal wall laparotomy provides access to the liver, C3, right kidney, small and large intestines, gravid uterus, and right ovary (Figure 57-1). Right paracostal laparotomy is the approach of choice for duodenal obstructions because the duodenum is located ventral to the transverse processes of the lumbar vertebrae close to the last rib (Figure 57-2). Ventral midline incisions provide access to the small and large intestines, uterus, ovaries, and bladder. Left lateral abdominal wall laparotomy provides access to the C1, spleen, left kidney, left ovary, and gravid uterus and limited access to the intestines.
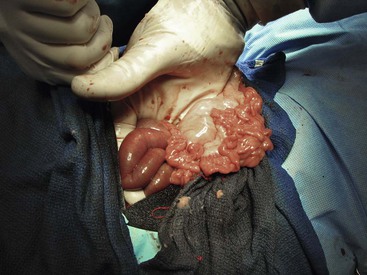
Figure 57-1 Right flank (lateral) approach to the abdomen of an adult alpaca. The small intestine is partially exteriorized. An intraluminal obstruction is recognized as the junction of distended (orad) and nondistended (aborad) intestine.
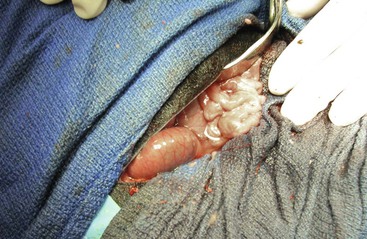
Figure 57-2 Right paracostal incision laparotomy in an adult alpaca. The duodenum is most easily accessed through this surgical approach.
Forestomach lesions are approached through the left lateral abdominal wall in cases of C1 involvement and either the right lateral abdominal wall or the ventral midline for C2 lesions, C3 lesions, or both.12 Surgical approaches to small intestinal lesions include the ventral midline and the right lateral abdominal wall approaches.3,12 Large intestinal lesions can be approached via the ventral midline or the right lateral abdominal wall.3,7,9,11,26 The proximal duodenum is best approached using the right paracostal approach rather than a ventral midline approach.3,12 The proximal duodenum can not be exteriorized via ventral midline. Right paracostal approach allows sufficient access so as to isolate and pack-off the duodenum from the rest of the abdomen. Splenectomy has been performed via ventral midline in an alpaca that was found to have a splenic torsion during an exploratory laparotomy.16 If splenic disease is diagnosed prior to surgery, the left lateral abdominal wall approach provides superior access to the spleen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


