Chapter 3 Gastrointestinal Immunology
Introduction
Gastrointestinal Immunoanatomy: Inductive and Effector Sites
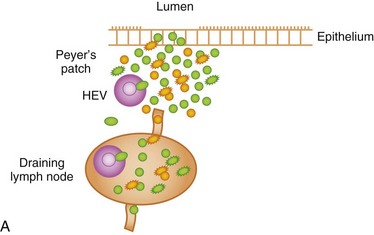
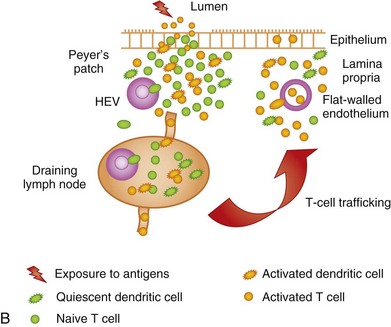
The GI immune system is compartmentalized into (a) afferent or inductive sites, where antigen-presenting cells (APCs) prime naive T and B cells to initiate the immune response, either by processing and presenting local antigens or by migration from the lamina propria (LP), an important site of antigen sampling, and (b) efferent or effector sites, where antibody and T-cell–mediated responses are mounted after extravasation, retention, and further differentiation of the lymphocytes. The afferent arm of the GI immune system comprises Peyer patches (PPs), isolated lymphoid follicles (ILFs), and mesenteric lymph nodes (MLNs), while the efferent arm comprises lymphocytes located in the LP and epithelium; cryptopatches, loosely organized clusters of approximately 1000 cells located at the base of the intestinal crypts, have also been described in the mouse. Cryptopatches were originally thought to be sites of extrathymic intraepithelial lymphocyte (IEL) development, but more recent studies suggest that they are precursors of ILFs. Additional species-specific accumulations of lymphocytes include the rare T-cell–dominated “lymphocyte-filled villi” of rats and man, colonic “lymphoglandular complexes” of pigs, and the “continuous ileal PP” of ruminants, pigs, and dogs, which appears to be a primary lymphoid organ responsible for B-cell development.1–3 Located throughout the small intestine, PPs comprise at least three aggregated lymphoid follicles with an overlying follicle-associated epithelium (FAE) containing microfold (M) cells, specialized epithelial cells that transport antigens from the lumen to the underlying lymphoid tissue. This process, called transcytosis, is mediated by mechanisms that include endocytosis of clathrin-coated vesicles, actin-dependent phagocytosis, and fluid-phase pinocytosis or macropinocytosis. M cells express a receptor for secretory (s) immunoglobulin (Ig) A on their apical surface, which facilitates the transcytosis of IgA-coated bacteria to the underlying APC-rich dome region of the PP. M cells lack a rigid internal cytoskeleton and are easily deformed by lymphoid cells migrating into the epithelium; this flexibility allows the creation of a pocket beneath the M cells, from which lymphoid cells can enter and leave without breaching the M-cell membrane. These pockets are thought to represent specialized extensions of the germinal centers of the underlying follicles, and contain equal proportions of memory T and B cells. The subepithelial dome region is rich in CD11b− and CD11b+ CD8α− dendritic cells (DCs), potent APCs that capture antigens transported across the M cells. The FAE expresses the chemokines CCL20, CXCL16, and CCL9, creating a micromilieu that attracts the underlying DCs. Following antigen uptake, the DCs either migrate directly to the adjacent interfollicular T-cell zones of the PPs, or pass to the MLNs where they may interact with naive T cells (Fig. 3-1).
What Is Known in Dogs and Cats?
PPs are recognized in both the dog and cat. There are approximately 20 PPs in the dog, spanning the whole of the small intestine and ranging in size from a few millimeters to 4 cm.4 The single ileal PP in the dog differs from those of the duodenum and jejunum, with large follicles, a small dome and very little interfollicular tissue.2,3 The ileal PP has fewer T cells than the proximal PPs, consistent with its putative role in B-cell ontogeny.2,3 Pinpoint-sized collections of lymphoid follicles resembling PPs are occasionally present in the stomach, colon, and rectum of the dog.4 Lymphocytes in the paracortical and interfollicular areas of PPs are predominantly CD4+, while B cells in the dome region of canine PPs express IgA or IgG.2,3 Lymphoid follicles are present throughout the intestine and are the predominant type of aggregated lymphoid tissue in the canine colon and rectum. They comprise isolated follicles of 0.5 to 3 mm that occupy the LP and submucosa, and serve a similar function to the follicles within PPs.4
The intestinal LP of the dog contains plasma cells, T cells, and putative DCs.4–6 Plasma cells are predominantly IgA+ and are concentrated within the crypt regions in the small intestine and deep LP in the colon; fewer of them are found within the villi and superficial LP of the colon and there is a progressive decline in the density of IgA+ plasma cells from the duodenum to the ileum.6–9 In contrast, CD4+ and CD8+ T cells present within the canine LP are concentrated toward the tips of the villi, with no apparent differences in proximal to distal small intestinal distribution.6,10 The ratio of CD4+:CD8+ T cells is approximately 60 : 40 in the LP and 15 : 85 in the epithelium.10 Subtractive analysis suggests that a population of CD3+CD4−CD8− cells exists in the canine villus epithelium, thought to represent γδ T cells.10 Immunohistochemical studies have suggested that αβ and γδ IELs are present in approximately equal numbers in the canine duodenum, jejunum, and ileum.11 Up to 20 IELs per 100 enterocytes have been counted in each region of the small intestine.6,11 A more recent flow cytometric study has suggested that αβ IELs outnumber γδ by up to 2 : 1 in the proximal small intestine and by up to 5 : 1 in the colon, perhaps reflecting the greater sensitivity of this technique.12 The distribution of T cells is similar in the feline LP13,14; however, IELs in cats are more numerous, with more in the villus than crypt epithelium and a rising count from the duodenum (50 IELs/100 enterocytes) to ileum (80 IELs/100 enterocytes).14 The majority of feline IELs are CD8+ (up to 50%), with fewer CD4+ cells (up to 10% to 15%) and a sizable population of CD3+CD4−CD8− cells (thought to be γδ+).13,14 IgA+ plasma cells predominate in the small intestine, with increasing numbers around the crypts; however, in contrast to the dog, there is a trend for the number of IgA+ cells to increase from the duodenum to ileum.14 IgG+ plasma cells are more numerous in the feline colon, with fewer IgA+ and IgM+ cells.15 A small number of IgM+ IELs may be observed in cats,14 but these are not documented in other species and their significance remains unclear.
A significant number of lymphoid cells within the canine LP are CD45R+, raising speculation that a proportion of the T cells found within this compartment are naive, in contrast with the predominantly effector/memory T-cell phenotype observed in the murine and human LP.6 A recent study demonstrated that the LP of random source cats has more CD4+ T cells expressing the activation marker CD25 (the interleukin [IL]-2 receptor α chain) than that of specific pathogen-free cats, suggesting that exposure to luminal antigens increases the number of activated/memory T cells within this compartment, in common with other species.16 Examination of expression of class II molecules of the major histocompatibility complex (MHC) in the canine small intestine reveals a web of subepithelial dendritic-like cells in the villus LP.10,17 However, the strongest MHC class II expression occurs within the PPs and some expression of this antigen is also apparent within the epithelium, particularly within the ileum.10,17 A similar pattern of LP MHC class II expression has been documented in the cat, although epithelial labeling was negative in one study13 and weak and restricted to PPs in another.14
Innate and Adaptive Arms of the Gastrointestinal Immune System
Overview
Box 3-1 summarizes the defense mechanisms of the GI tract, including both nonimmune and immune-mediated components.
Box 3-1
Defense Mechanisms of the Gastrointestinal Tract
Notes:
1 See reference 18 for further information.
2 Members of the trefoil family are characterized by the possession of at least one copy of the trefoil motif, a 40-amino-acid domain that contains three conserved disulfides.19 Trefoil peptides are synthesized by mucin-secreting GI epithelial cells, including goblet cells, and function to stabilize the mucus barrier. Upregulated at sites of mucosal injury, they also promote angiogenesis and stimulate epithelial cells to migrate into the denuded area of mucosa. Deficient expression of trefoil peptides is thought to predispose to intestinal inflammation and malignancy.
3 Defensins are antimicrobial peptides of two structurally different families (α and β) expressed by Paneth cells in the small intestine (α-defensins) and epithelial and plasma cells in the colon (β-defensins; Paneth cells are rare in the colon). They form micropores in the phospholipid bilayer of bacterial membranes, thus causing loss of structural integrity. Defective synthesis of defensins is thought to contribute to the pathogenesis of Crohn’s disease.
4 See next section for information on this important family of pattern recognition receptors.
5 NOD2 is a prototypical NOD-like receptor (NLR) expressed by monocytes, macrophages, dendritic cells, and Paneth cells; it acts as an intracellular microbial sensor whose ligand is muramyl dipeptide, a peptidoglycan motif common to both Gram-positive and Gram-negative bacteria. NOD2 polymorphisms are associated with Crohn’s disease, thought to be caused by a decrease in the negative regulation of TLR responses occurring in the normal GI tract, and thus a pathologic increase in responses to the normal flora.
6 “Autophagy” (more specifically: macroautophagy) is a process characterized by the formation of double-membrane cytosolic vesicles (autophagosomes) that deliver their cargo to the lysosome for degradation. The autophagosome is formed by a dynamic process of membrane expansion, rather than budding off from preexisting organelles. Autophagy is important in the maintenance of cellular homeostasis, complementing the ubiquitin-proteasome system in the clearance of proteins; it may also eliminate damaged or surplus organelles. By sequestering invasive microbes, autophagy is thought to play a role in intestinal defense, and abnormalities in autophagy have been associated with Crohn’s disease.
Pattern Recognition Receptors and the Epithelium
To date, TLRs 2 to 5, 7, and 9 have been cloned in the dog, and peripheral blood mononuclear cells are known to express TLRs 2 and 4.20–22 The complete sequences of TLRs 4 and 9 have been cloned in the cat,20 but only partial sequences have been cloned for TLRs 1 to 3 and 5 to 8.23 Real-time polymerase chain reaction (PCR) assays have been developed for detection of messenger RNA (mRNA) encoding TLRs 1 to 9, and these have shown mRNA encoding TLRs 2 to 5 and 7 to 9 within feline T and B cells.23 Immunohistochemistry and immunogold electron microscopy have provided evidence for the expression of TLR-4 (the receptor for lipopolysaccharide [LPS]) by macrophages in the canine lung, small intestine, liver, and spleen; epithelial cells within the lung, small intestine, cornea, and renal tubules were also labeled by this cross-reactive antibody.24 Primary colonic epithelial cells in the dog express mRNA encoding the nucleotide-binding oligomerization domain 2 (NOD2) and TLRs 2 and 4, and IL-7 and IL-8 responses can be elicited by exposure of these cells to the respective ligands, peptidoglycan (TLR-2) and LPS.25 A recent study has also suggested that TLRs 2, 4, and 9 (all responsive to bacterial products) are upregulated in canine IBD; furthermore, respective mRNA transcript abundance showed no change with therapy despite clinical improvement, raising the intriguing possibility of a primary defect representing a genetic predisposition, as described in human IBD.26 This important group of PRRs is likely to remain the focus of continuing research.
Lymphocyte Trafficking, Activation, and Gastrointestinal Imprinting
Naive T cells continuously migrate from the bloodstream into the inductive sites of the GALT, traversing the high-endothelial venules (HEVs) in a multistep extravasation cascade involving (a) tethering and rolling mediated by sialomucins and selectins (e.g., peripheral lymph node addressin [PNAd] expressed by HEVs interacting with L-selectin [CD62L] expressed by naive T cells); (b) activation, firm adhesion, and transmigration, mediated by chemokines, integrins, and Ig superfamily members (e.g., intercellular cell adhesion molecule-1 [ICAM-1] and mucosal addressin cell adhesion molecule-1 [MAdCAM-1] expressed by HEVs respectively interacting with leukocyte function antigen-1 [LFA-1] and the integrin α4β7 expressed by naive T cells); and (c) chemotaxis mediated by chemokines (e.g., CCL19 and CCL21 produced by stromal cells and presented on the luminal face of HEVs, interacting with CCR7 expressed by naive T cells). The lymphocytes then migrate through the tissue parenchyma in search of cognate antigen. If these antigens are not found, the T cells leave the lymphoid tissue via the efferent lymphatics, to be carried back to the bloodstream via the thoracic duct; from there, the cells can continue their journey to other secondary lymphoid organs. However, if cognate antigens are encountered, the naive lymphocytes are activated and are imprinted with a preference to home back to the tissues in which they were primed by the resident DCs, mediated in the case of the GI immune system by upregulation of α4β7 and CCR9 expression by the T cells. Thus, on returning to the systemic circulation via the efferent lymphatics, these T cells preferentially home back to the intestinal LP to execute their effector functions, this time gaining access to the tissue via the normal endothelium of postcapillary venules (see Fig. 3-1). To an extent, they may also re-enter the PPs and MLNs by means of α4β7−MAdCAM-1 interactions. The vitamin A metabolite, retinoic acid (RA), appears to be implicated in imprinting intestinal tropism (high-level α4β7 and CCR9 expression) onto activated CD4+ and CD8+ T cells. DCs from MLNs and PPs express the enzymes catalyzing the production of RA from retinol, thus equipping them with the molecular machinery required for imprinting. CCL25 expressed by the small intestinal epithelium and presented on the surface of HEVs is also important in mediating the chemotaxis of CCR9+ T cells into the LP and towards the epithelium. Naive B cells undergo recirculation in a similar manner; however, CXCL13 presented by HEVs adjacent to or within the lymphoid follicles interacts with CXCR5 expressed by naive B cells, which are, in turn, attracted to the mantle zone by CXCL13 deposited on the dendrites of follicular DCs. B cells undergo priming just outside the lymphoid follicles by interactions with cognate T cells and APCs, before reentering the follicles and migrating to the germinal centers. The B cells may then leave the follicles as memory/effector cells. During inflammation, the recirculation routes of lymphocytes may be broadened, thus helping to explain the extraintestinal manifestations of certain GI infections and IBD. In contrast to the conventional view that naive T cells migrate only to lymphoid tissues, failing to gain access to extralymphoid sites, recent studies show constitutive migration of naive CD4+ and CD8+ T cells into the small intestinal LP. However, the majority of cells in this compartment show an activated or memory phenotype.
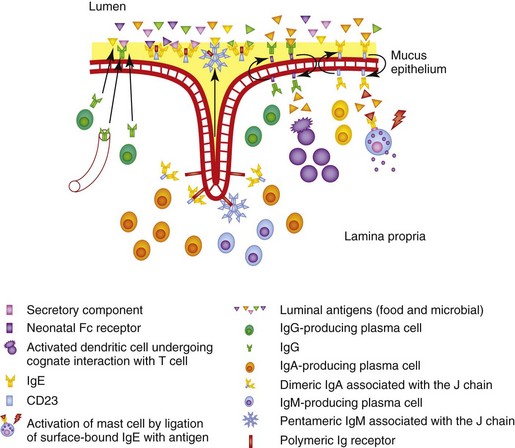
The polymeric Ig receptor (pIgR) is expressed on the basolateral surface of intestinal epithelial cells and mediates endocytosis and transcytosis of J chain–linked dimeric IgA or pentameric IgM (Fig. 3-2). The ectodomain of the pIgR, termed the secretory component (SC), is cleaved at the junction with the membrane-spanning region and binds covalently to one of the sIgA molecules in each dimer, affording protection against proteolysis; it associates with the IgM molecules in a noncovalent manner, remaining in dynamic equilibrium with free SC in the local microenvironment. There are two potential routes of transmission of locally produced and serum-derived IgG into the intestinal lumen. The first is passive, involving the paracellular diffusion of IgG, whereas the second involves the neonatal Fc receptor (FcRn), which is an MHC class I–related molecule that binds to the Fc domain of IgG. The FcRn is important in neonatal life as it mediates transfer of colostral IgG across the intestinal epithelium. Expression of FcRn is downregulated at the time of weaning in rodents, but continues into adulthood in man. Little is known about the FcRn expression in other species, although FcRn was recently characterized in piglets.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree