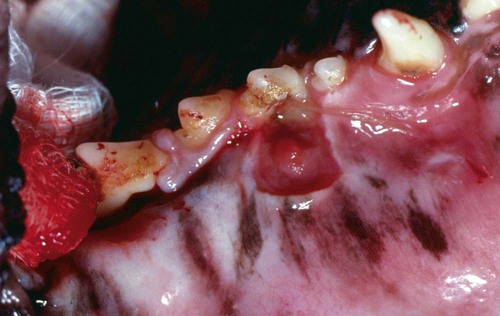
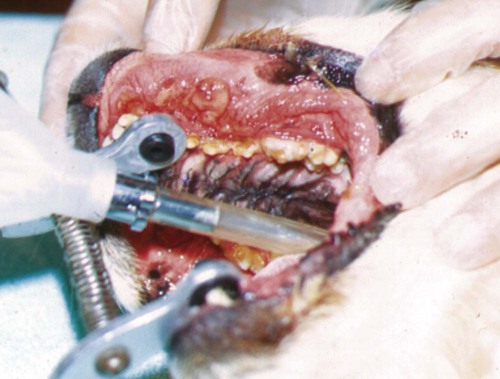
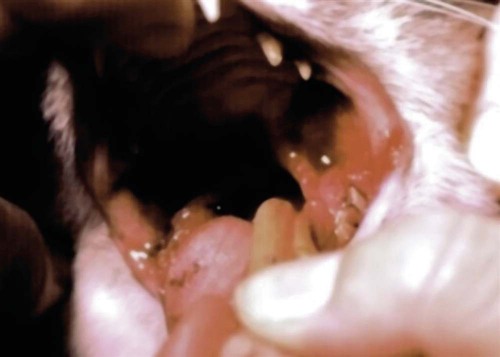
The resident microbial flora of the canine and feline oral cavity are composed of a wide variety of aerobic bacteria and facultative and obligate anaerobic bacteria, mycoplasmas and yeasts (Tables 88-1 and 88-2). In canine saliva, Actinomyces, Streptococcus, and Granulicatella (previously nutritionally variant streptococci), and in dental plaque, Porphyromonas, Actinomyces, and Neisseria have been the most prevalent nonobligate anaerobes.102 The frequency with which individual species are isolated depends on culture methods, sampling sites, and breed and individual differences. From a clinically significant standpoint, bacterial culture of specimens from the oropharyngeal region is meaningless because of the diversity of commensal organisms and the lack of accurate quantitative methods to determine imbalances. Furthermore, only a fraction of the GI microbes can be cultured, and genetic analysis may offer more accuracy in detecting elusive pathogens. The new science of metagenomics involves simultaneously analyzing the functional and sequence-based diversity of nucleic acid from all organisms in a given specimen. Checkerboard DNA-DNA hybridization using probes from human oral bacterial species has been used to determine the relative proportions of bacterial species at various sites in the oral cavity of clinically healthy beagle dogs.386 Samples of supragingival and subgingival dental plaque and the oral soft tissue surfaces of the tongue, cheek, and tonsil were evaluated. Although a similar diversity of organisms existed on all surfaces, the relative proportions were different, especially between the soft tissue and dental surfaces. Further quantitative and canine organism-specific analysis will be needed in healthy and diseased animals to establish the reference limits of the oral microflora using genetic analysis and determine changes with various health conditions. TABLE 88-1 Microflora Most Commonly Isolated from the Oral Cavities of Clinically Healthy Dogs B, Tonsillar and nontonsillar or salivary; N, nontonsillar or salivary; S, supragingival (plaque) or subgingival scrapings; T, tonsillar; U, unspecified sites. TABLE 88-2 Facultative and Obligate Anaerobic Flora of Feline Gingival Margins NC, No attempted culture; NR, not reported. aIncludes Micrococcus spp., Streptococcus sanguis, Streptococcus mutans, and Streptococcus mitis. bActinomyces viscosus, Actinomyces hordeovulneris, and Actinomyces denticolens. In one other study, Lactobacillus fermentum and Veillonella parvula were also isolated.110 cIncludes Streptococcus epidermidis, Alcaligenes spp., Actinobacter spp., Achromobacter spp., and group G β-hemolytic streptococci. dBacteroides tectum, Bacteroides fragilis, Bacteroides heparinolyticus, Bacteroides salivosus, Porphyromonas (Bacteroides) gingivalis, and other Bacteroides pigmented group members, including Prevotella spp., Tannerella forsythia (Bacteroides forsythus), and Bacteroides gracilis. eIncludes Bacteroides asaccharolyticus, Bacteroides melaninogenicus, Bacteroides oralis, Bacteroides fragilis, and Bacteroides unspeciated. fFusobacterium alocis, Fusobacterium nucleatum, Fusobacterium russii, and Fusobacterium unspeciated. gClostridium villosum and Clostridium novyi. Antimicrobial agents used in treating oral infections such as gingivitis and stomatitis should be chosen with the composition of the resident microflora in mind. Organisms found in bite-inflicted and soft-tissue wound infections (see Chapter 51), pleural (see Chapter 87), and peritoneal infections (see Intra-Abdominal Infections, later) reflect the composition of the oral microflora. Inflammation and recession of perialveolar gum margins are common findings in dogs and cats.291 These lesions are initially caused by excessive accumulation of dental plaque resulting from deposition of by-products from breakdown of food and saliva by normal resident microflora. Plaque is an organic matrix of salivary glycoproteins and polysaccharides adhering to the tooth surface that provides sites for oral bacteria to proliferate. Microflora involved with supragingival and subgingival plaque in healthy mucosal sites in dogs are resident streptococci and Actinomyces species.171,172 At first, these gram-positive, nonmotile aerobic cocci and bacilli predominate. These bacteria produce a glycopolysaccharide referred to as a glycocalyx, which facilitates the attachment of other bacteria. This biofilm is further consolidated into a film, or plaque. Plaque accumulation results in inflammation of the gums. Plaque can become mineralized, resulting in calculus (tartar) (Fig. 88-1). As periodontal inflammation progresses with associated calculus formation, gram-negative motile, obligate anaerobic rods and spirochetes proliferate.310,515 Pups have very few Porphyromonas species in their dental plaque; however, bacterial numbers increase as the pup matures and inevitably periodontal disease develops.199 Supragingival plaque is visible as is its associated calculus formation. However, subgingival plaque incites an inflammatory response in the gingival tissue that can lead to periodontitis and chronic periodontal disease with eventual loss of teeth. Early bacterial damage occurs in the subgingival epithelial tissues with penetration of the interdental rete pegs. Proliferating spirochetes may disrupt the intercellular junctions, creating a portal of entry for other bacteria.310 Many of these bacteria possess proteolytic enzymes that allow them to colonize and invade periodontal tissues. Periodontal pathogens destroy the integrity of the dentition and oral mucosae and can also cause bacteremia or distant tissue embolization. Unfortunately, periodontal disease is irreversible when pockets form and teeth loosen, and damaged gum tissue cannot be restored to health. In humans, periodontitis, which results from bacterial proliferation in the gingival sulcus, has been associated with an increased population of Porphyromonas (Bacteroides) gingivalis.139 In comparison, microflora from anaerobic plaque and gingival crevices of dogs with periodontal disease are Porphyromonas denticanis–Porphyromonas gulae and Porphyromonas salivosa154; Prevotella and Wolinella species that are pigmented, gram-negative rods; Actinobacillus actinomycetemcomitans; and Clostridium and Fusobacterium species. Reduced numbers of streptococci, enterococci, and staphylococci are found compared with resident flora (Table 88-3).198,199 To reverse this imbalance, periodontal application of selected beneficial bacteria (Streptococcus sanguinis, Streptococcus salivarius, and Streptococcus mitis) after removal of dental plaque and scale in dogs inhibits pathogen recolonization of periodontal pockets,475 presumably through competitive or immunologic means. In cats, differences in the microflora between clinically healthy animals and those with gingivitis have not been appreciated; however, they have not been as extensively studied (see Table 88-2). P. gingivalis is the most likely periodontal pathogen associated with progressive periodontal disease in cats.331–334 Salivary microflora, which differs from plaque microflora, remain relatively constant in the presence of periodontal disease. TABLE 88-3 Percentage Distribution of Gingival Microflora from Dogs with Healthy or Diseased Gingival Tissues171,172,239,504 ND, Not determined. Referring to relative prevalence and frequency of isolating these organisms: +, low; ++, intermediate; +++, high. aSupragingival and subgingival collection sites. Values are percentages of isolates. bEsculin-positive, streptococci (S. faecalis, group D streptococci, enterococci). cEsculin-negative, streptococci (S. mitis; includes facultative and anaerobic spp.). dIncludes aerobic and facultative spp. eNonpigmented B. fragilis group. fPigmented group containing Porphyromonas, including P. asaccharolyticus, P. gingivalis, P. endodontalis, P. canoris, P. denticanis, P. gulae, P. salivosa, and P. circumdentaria; Odoribacter, including Odoribacter splanchnicus, and Odoribacter denticanis; Prevotella, including P. intermedia, P. loescheii, P. melaninogenica, and P. denticola; Bacteroides nodosus (Dichelebacter nodosus); and Wolinella (Campylobacter) curva and Wolinella (Campylobacter) rectus. gF. necrophorum (ssp. funduliforme and necrophorum); F. pseudonecrophorum; F. nucleatum (ssp. animalis, fusiforme, nucleatum, polymorphum, and vincentii). Periodontitis can also develop whenever tooth damage occurs, exposing the dental pulp. Acute periapical periodontitis begins within 20 days of pulp exposure in dogs and progresses with time.242 Fractures of teeth usually result in pulp infection before revascularization of the canal by vital tissue can occur. Although facultative bacteria predominate at first in root canal infections, anaerobes displace them with time.22 Filling the canal with a triple antibacterial paste (ciprofloxacin, minocycline, and metronidazole) in a resorbable matrix base early after injury has been shown to avert the problem of endodontic infection and promote revascularization of the pulp canal.530 Calculus is mineralized dental plaque that adheres to tooth surfaces, facilitating additional plaque formation and periodontal inflammation. Complete removal of all supragingival and subgingival calculi is essential in control of periodontal disease. Long-term intermittent periodontal care for dogs is highly recommended because it slows the progression of gingival recession and depth of periodontal crevices that develop in dogs not receiving dental hygiene.193 Gingival hypertrophy and alveolar abscess formation are sequelae of calculus formation.324,327 The type of microflora present, the diet, and the animal’s chewing habits may be important in the formation of calculus.265,503 Advancing age is a predisposing factor in dogs.180 Leukopenia and bacteremia occasionally develop as secondary effects.270 Surgical or mechanical debridement of diseased teeth or surrounding tissues may be required. Although bacterial pathogens are important in periodontitis, antimicrobial therapy alone does not eliminate progression of disease. Proinflammatory cytokines, such as tumor necrosis factor (TNF) and interleukin (IL)-1, have been important in the progression of periodontal disease in experimental animals.84,131 Cats with more severe periodontal disease have greater serum antibody titers to P. salivosa and P. gingivalis than those with less severe periodontal disease.274,330,334,335 Inhibition of IL-1 and TNF with specific blockers has been beneficial in inhibiting inflammation and preserving bone in the periodontal tissues. Oral IFN-α4, administered to dogs with naturally occurring gingivitis, reduced both periodontal bacterial counts and associated gingival inflammation.200 Other anti-inflammatory drugs, such as metalloproteinase matrix (e.g., tetracyclines), adelmidrol (an aliamide), and prostaglandin inhibitors, such as nonsteroidal anti-inflammatory drugs (NSAIDs), have been beneficial in this regard. These findings support the concept that periodontal tissue loss is caused by an exaggerated host response to certain species of bacteria with the ability to grow on the tooth surface and in periodontal structures and the triggering of an inflammatory cascade. Adjunctive management methods such as providing hard diets, regular tooth brushing, and oral hygiene chews have been shown to reduce the incidence of calculus accumulation.* Diets or supplements with Omega-3 fatty acids reduce the local gingival inflammatory response in P. gingivalis infection and may provide some benefit.234 Treatment includes the extraction of severely affected teeth, debriding necrotic or proliferative gum margins, root planing with curettes, and scaling calculi from the remaining involved supragingival and subgingival dental surfaces by manual or ultrasonic means. More aggressive surgical debridement is needed for advanced periodontal disease.419 Systemic antimicrobials such as tetracycline, metronidazole, and tinidazole (Table 88-4) and topical chlorhexidine have been evaluated for treatment of periodontitis in experimentally affected dogs.367,377 Clindamycin treatment for 5 days after dental prophylaxis reduced subsequent oral odor and dental plaque formation when dogs were monitored for up to 70 days.501 Treatment of clinically healthy beagle dogs with clindamycin for 14 days reduced the amount of gingival crevicular fluid, dental plaque, and gingivitis.538 Tetracycline and metronidazole have been beneficial in reducing dental calculus or preventing its reformation when they have been given with or without mechanical cleansing. After 1 year of tetracycline therapy, dogs with periodontal disease had less alveolar bone resorption than untreated controls.528 Metronidazole therapy has had similar beneficial effects.336 Tinidazole had good efficacy against P. gingivalis–like bacteria.404 The use and efficacy of systemic antimicrobial therapy for this purpose has been questioned as an alternative for indicated dental procedures178 and may lead to antimicrobial-resistant strains. Systemic antimicrobial therapy is not indicated as a long-term solution to periodontal infections. For example, in dogs with periodontitis and periodontal bone defects, reestablishment of the oral microflora with beneficial species of microorganisms, after dental scaling and root planing, facilitated the healing process in the perialveolar bone and controlled progression of periodontal infection.318 TABLE 88-4 Systemic Therapy for Oral Infections B, Both dog and cat; C, cat; D, dog; IV, intravenous; PO, by mouth; SC, subcutaneous. In severe pyorrhea, prescribe a drug effective against gram-negative facultative anaerobes by combining whatever drug is chosen with the aminoglycoside (gentamicin) or quinolone (enrofloxacin). aFor additional information, see the Drug Formulary in the Appendix. bDose per administration at specified interval. cMetronidazole and spiramycin are available in combination (Stomorgyl) outside the United States for treatment of oral infections. dOnly given once just before or during anesthesia for dental procedures. In general, the desired spectrum is used to treat anaerobic bacteria. Perioceutics are pharmaceutical formulations that are placed into periodontal pockets to allow for sustained antimicrobial activity against periodontal pathogens. They are used as an adjunct to scaling and cleaning the dental surfaces. These perioceutics, consisting of tetracyclines and having antibacterial and anticollagenase activity, are delivered into the periodontal pocket via a blunt cannula. Once there, the biodegradable polymer gels coagulate, allowing for sustained release of the drug for up to 13 weeks.63,233 Dogs with periodontal disease that had dental cleaning followed by doxycycline polymer applied to their gingival crevices had less periodontal disease than untreated animals after 12 weeks of monitoring.165,175,175 In addition to a reduction in the depth of alveolar pockets, a reduction in aerobic microaerophilic and anaerobic bacterial microflora and proteolytic enzymes, such as collagenase, have been observed.539 Topically applied polymers containing doxycycline (Heska, Fort Collins, CO; Pharmacia Animal Health, Piscataway, NJ) or minocycline (Sunstar Inc., Takatsuki, Japan) are available for treatment and control of periodontal disease in dogs. Flushing dental surfaces once daily with 0.1% to 0.2% chlorhexidine149 or alternate-day brushing410 may delay accumulation of calculi. Unfortunately, chlorhexidine may stain teeth light blue, but a commercial product formulated especially for dental purposes (Nolvadent, Fort Dodge Labs, Fort Dodge, IA) does not stain. Monoperoxyphthalic acid applied before chlorhexidine was used resulted in less dental staining.59 Daily application of chlorhexidine gel was effective in reducing recurrence of dental plaque in dogs.170 Twice-daily applications of nisin, an antimicrobial peptide, were as effective as chlorhexidine in preventing calculus in dogs.188 Numerous cleansers and brushes have been developed for daily brushing of dogs’ teeth, and they warrant consideration when a dog has a tendency to form calculi. Special diets are also available that may help to reduce calculus formation. Daily feeding of a commercial dental food significantly reduced plaque and gingivitis by 39% and 36%, respectively, compared with daily feeding of a control dry-food diet.266 The oral administration of probiotics may also benefit oral health by preventing the growth of pathogenic bacteria or by modulating mucosal immunity in the oral cavity. A small number of in vitro and in vivo studies have been performed on the role and effects of probiotics on oral health. There are several important modifications for “oral probiotics” that should be considered when compared to probiotics used for promoting intestinal health. For example, oral probiotic bacteria should adhere to and colonize on dental tissue and should be part of the biofilm. They should not ferment sugars, which subsequently lowers the pH and is detrimental to dental health.308 Consumption of products containing probiotic lactobacilli was shown to successfully reduce the risk of caries and the number of mutans streptococci in the oral cavity.49 A few studies also revealed that probiotic lactobacilli strains were useful in reducing gingival inflammation and the number of black-pigmented rods including P. gingivalis in the saliva and subgingival plaque.196,243 A split-mouth, double-blind randomized pilot study, involving eight beagle dogs with moderate experimentally induced periodontitis, documented the impact of multiple subgingival applications of pellets containing S. sanguinis, S. salivarius, and S. mitis.318 The bone density within periodontal pockets treated with the beneficial bacteria improved significantly after 12 weeks, whereas there was a nonsignificant change in the control group that received a single root planing at baseline. In addition, there was a significant increase in the alveolar bone level at the end of the study based on repeat radiographs compared to the control group. These studies clearly illustrate that the probiotic route clearly has potential for improving oral health, but much more needs to be known about the oral microbiota in health and which organisms are capable of restoring and maintaining health when delivered in a probiotic format. A P. denticanis-gulae-salivosa bacterin that was commercially available as an aid in preventing periodonal disease in no longer licensed155 (see Immunoprophylaxis for specific diseases, Chapter 100 and Web Appendix 3). In addition, recombinant protein vaccines targeting extracellular proteinases and adhesins of Porphyromonas spp. have been effective in attenuating Porphyromonas infection in mice.116 In cats with naturally occurring periodontal disease, clindamycin, doxycycline, or spiramycin-metronidazole, but not amoxicillin-clavulanate, reduced the numbers of P. gingivalis.333 Furthermore, improvement was observed in the degree of periodontal inflammation. This occurred despite the fact that all isolated organisms showed susceptibility to all the tested drugs. These drugs should probably be selected for adjunctive treatment along with mechanical debridement in cats. The use of special diets supplemented with dental chews may be effective in reducing calculus formation on cat teeth.191,194 Cats were fed either a dry diet only or a dry diet supplemented with dental chews.194 A two-period, crossover design was used, and the test phase lasted 4 weeks. Results indicated that the daily addition of dental chews to a dry diet was effective in reducing plaque and calculus accumulation and the severity of gingivitis. In other studies, cats fed large kibbles with mechanical cleaning qualities had less gingivitis and tartar than cats on small kibble diets with or without daily brushing.494 Dental brushing has also been helpful in the prevention of periodontal disease in cats.191,194 Daily application of zinc ascorbate gel has also been effective in decreasing bacterial growth, plaque formation, and gingivitis in cats when it was used after dental prophylaxis.62 A syndrome of gingival hypertrophy and associated plaque formation has been observed in Abyssinian and Persian kittens around the time of eruption of their adult teeth.524 It may be caused by inborn immunodeficiency with secondary proliferation of gingival and plaque-forming bacteria. Treatment involves gingival tissue debridement, plaque removal, and frequent dental prophylaxis. Systemic antibacterial therapy may temporarily halt the progression, but it cannot be used indefinitely. Fortunately, most cats have a reduction in the severity of proliferation as they mature. Ophthalmic manifestations can occur from dental disease because of the close proximity of the caudal maxillary teeth and the orbit (Box 88-1). Systemic complications of periodontal disease, including cerebral and myocardial infarction, systemic hypertension, and early mortality, have been linked in people.89,358 A large body of evidence in humans suggests that patients with diabetes, in particular when inadequately controlled, are at an increased risk of developing periodontal disease305 and show accelerated alveolar bone loss.472 From the opposite viewpoint, the presence of periodontal disease itself in diabetic patients may influence their glycemic control.471 There is evidence that resolution of periodontal inflammation can improve metabolic control of blood glucose in diabetics,241 and the mechanism underlying the effect is likely due to resolution of inflammation from the periodontal tissues. Similar relationships of periodontal inflammation to heart, liver, and kidney disease have been made in dogs.79 However, controversy has been addressed in veterinary publications as to the role of oral cavity infections and systemic disease.161 In some retrospective studies, no statistical association was found between bacterial endocarditis in dogs and either dental or oral surgical procedures or oral infection.352,460,460 Unfortunately, detailed prospective screening and grading of oral cavity changes were not undertaken in these studies. In contrast, a statistical association was found between periodontal disease severity in dogs and degenerative changes in the left atrioventricular valves and pathologic changes in the liver and kidney.351 Other studies involving whole-mouth periodontal scoring show a correlation of the severity of oral disease with microscopic lesions in distant organs79 and increased mediators of inflammation in serum.375 Further focused prospective studies with suitable control animals will be needed to make conclusive assessments. Bacteremia associated with dental manipulations may be clinically asymptomatic, cause acute septicemia, or subsequently result in bacterial endocarditis or localized embolic tissue infections (see Chapter 86).378 The severity of bacteremia frequently correlates with the degree of periodontitis present. In people, use of antibacterial prophylaxis during dental procedures in patients predisposed to infective endocarditis is accepted, but also debated.146 In cats with periodontitis undergoing dental scaling and extraction, 36% had positive blood culture results at the time of the dental procedures.153 Bacteria most commonly isolated were Propionibacterium acnes, Pasteurella multocida, and Staphylococcus epidermidis. None of the cats became ill after the dental procedures. Septic pericarditis in a cat was attributed to presumed bacteremia from a prior dental procedure.263 Positive blood culture results of resident microflora have also been found in up to 40% of dogs with periodontal disease undergoing dental scaling and tooth extraction, and none showed clinical evidence of bacteremia.152 Dental procedures should not be performed at the same time as other surgical procedures, because the release of bacteria from the dental procedure can infect surgical wounds. Oral microflora may establish themselves in tissue injured or devitalized by routine surgical procedures, sutures, or foreign implants. When dentistry is performed on immunocompetent animals with clinical oral cavity infections, prophylactic administration of antimicrobial drugs has been recommended (see Table 88-4). Routine use of antimicrobials for dental procedures should be mandatory in immunocompromised animals. Amoxicillin is the drug of choice for humans undergoing dental cleaning where prophylactic administration is indicated. Intravenous administration of aqueous penicillin during ultrasonic dentistry in dogs did not alter the prevalence of bacteremia before or after dental manipulation.36 In dogs and cats, pretreatment with clindamycin at 5.5 mg/kg for 5 days decreased plaque and reduced aerosolization of bacteria during ultrasonic teeth cleaning.541 Ampicillin (amoxicillin) has been recommended empirically beginning 1 hour before dental procedures.182 On the basis of in vitro susceptibility testing of periodontal disease–associated bacteria, chloramphenicol, cephalosporin, erythromycin, or gentamicin have also been recommended. With the exception of erythromycin, all can be given parenterally while the animal is anesthetized. Gentamicin should never be administered alone because it is relatively ineffective against anaerobic bacteria. For these reasons, ampicillin (amoxicillin), chloramphenicol, clindamycin, metronidazole, or cephalosporin with or without gentamicin or a quinolone (although rarely used) are recommended for dental prophylaxis. Prerinsing the oral cavity with chlorhexidine solutions can also be performed just before the sealing and cleaning procedure. The Drug Formulary in the Appendix and Table 88-4 should be consulted for drug information and appropriate dosages. Therapy should begin no earlier than 4 hours before the dental procedure and should preferably be continued throughout the procedure by intravenous infusion. The most critical period is during the procedure. Drug administration should be terminated no longer than 12 hours after the dental procedure because no additional benefits are gained, and the risk increases for antibacterial-resistant bacterial infections. Because of the large numbers of bacteria in the oral cavity and especially the gingival crevices, public health considerations are important. Face masks and ocular shields should always be worn by humans who clean animals’ teeth. In addition, biofilms that form on the inside surface of water lines of ultrasonic scalers may harbor Legionella species, which might produce acute pneumonia in susceptible individuals.527 Inflammation of the tongue from infection is common among lingual lesions.87 Primary viral causes in dogs include canine distemper virus, canine calicivirus,104 canine parvovirus, and feline calicivirus (FCV) (see Chapters 3, 8, and 14). Bacterial, fungal, and protozoal organisms can also involve the tongue, producing suppurative or granulomatous lesions.357 Primary physical injury can occur via trauma, bite wounds, burns, caustic substances, and foreign bodies. Metabolic conditions such as diabetes mellitus, hyperadrenocorticism, uremia, and niacin deficiency can induce lingual ulcerations. In addition, autoimmune diseases such as pemphigus vulgaris, bullous pemphigoid, and systemic lupus erythematosus are associated with ulceration of the tongue. Secondary infections develop in these ulcers with opportunistic organisms such as Candida spp. or oral bacterial microflora. Organisms are not often found on histologic examination; however, suppuration and overlying ulceration are usually apparent. Foreign material such as hair, bone, or plant matter can be present in lingual wound infections. In humans, necrotizing ulcerative gingivostomatitis (NUG), or “trench mouth,” is a syndrome with multiple causes and is characterized by oral ulcerations and secondary infections. NUG may be a manifestation of systemic or immunosuppressive illness. Opportunistic overgrowth of oral microflora occurs in many diseases when immune defenses are impaired or oral ulcerations develop.391,470 Similar to periodontitis, motile (predominantly anaerobic) bacteria, including spirochetes, often proliferate and may invade tissues before the development of necrotic lesions.385 NUG may also be observed in dogs or cats and has been well documented in Maltese terriers162 (Fig. 88-2). It is caused by a similar proliferation of anaerobic and spirochetal bacteria (Borrelia and Treponema).329 The histopathologic infiltration in the oral lesions of immunocompromised animals consists primarily of leukocytes, in contrast to the lymphoplasmacytic infiltrates seen in immune hyperresponsive disorders279 (see Feline Lymphocytic Plasmacytic Ulceroproliferative Gingivostomatitis, later). As in humans, the causes of NUG in dogs and cats are equally diverse and include many acquired immunosuppressive conditions, such as diabetes mellitus, persistent neutropenia, feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) infections, and canine Cushing’s disease. Exogenous glucocorticoid administration in dogs also increases susceptibility to NUG.310 As in humans, psychologic and physical stresses may be involved in some animals. Bristles of plant awns can induce a similar syndrome when they become embedded in gingival tissues.303 Fungal stomatitis can result from infection with Candida species. Candidiasis can be associated with diffuse oral inflammation, especially on the tongue and at mucocutaneous junctions. See Chapter 63 for additional information concerning its diagnosis and treatment. The pattern of oral ulceration may be helpful in determining its underlying cause (Table 88-5). Regardless of its cause, oral ulceration has a particular clinical syndrome characterized by reluctance to eat, hypersalivation, halitosis, and evidence of pain on opening the mouth. Hemorrhage may occur spontaneously or after oral manipulation. Although rare, systemic signs may include fever, lymphadenomegaly, and lethargy. Ulcers may be distributed throughout the oral cavity but are usually concentrated on the dental, labial, and gingival surfaces. Ulcers may be covered by a pseudomembranous exudate. White pseudomembranous plaques can be found in cases of candidal stomatitis. In many neutropenic cats infected with FeLV, the ulcers characteristically have minimal exudation; some can progress rapidly, with sloughing of large portions of the caudal oropharynx or larynx. TABLE 88-5 Comparison of Ulcerative Lesions in Oral Cavities of Dogs and Cats B, Both dog and cat; C, cat; D, dog. aEosinophilic infiltration on biopsy. bSee Chapters 8 and 9. fLymphocytic-plasmacytic infiltration on biopsy and polyclonal hyperglobulinemia are typical. Feline immunodeficiency virus co-infection can exacerbate. gSee Chapters 11 and 12. hMay be observed as an idiopathic syndrome in young cats. See text discussion of gingivitis in kittens. Retroviral infections are occasionally associated with a rapidly spreading gingival necrosis. Underlying disease processes and other causes of NUG should be eliminated or treated when they are encountered. Plant awns or foreign material embedded in the gums can be scraped away with a scalpel when the gum margins are debrided. Symptomatic therapy of stomatitis includes changing the diet to soft or bland foods to encourage eating and lessen the mechanical irritation of ulcers. With chronic, nonhealing ulcers, it is often beneficial to remove dental tartar and normal or diseased teeth in affected regions of the mouth.27 Some lesions that arise adjacent to or later involve the tonsillar crypt may originate as or later develop into squamous cell carcinomas. Systemic therapies for stomatitis are summarized in Tables 88-4 and 88-6. Topical or systemic antimicrobial therapy appears to hasten the resolution of NUG significantly, perhaps by inhibiting the overgrowth of anaerobic and spirochetal bacteria that colonize and impair healing of ulcerated lesions in the oral cavity. First-choice drugs include amoxicillin (with or without clavulanate), clindamycin, doxycycline, or metronidazole. Oral administration of lactoferrin, an iron-chelating agent, was effective in treating intractable stomatitis in cats with and without FIV infections.408 For dosages and other information, see Table 88-6 and the Drug Formulary in the Appendix. Improvement was presumably a result of its antibacterial effects. Candidal stomatitis is best treated by topical application of antifungal drugs, such as clotrimazole and nystatin, or with systemic ketoconazole. Historically, tetracycline, chloramphenicol, ampicillin, and penicillin solutions have been applied topically for bacterial stomatitis, although the first two drugs may cause anorexia in cats. Systemic antibacterial therapy directed primarily against anaerobic bacteria appears to be more efficacious in treating stomatitis. Relapses may occur in some cases after termination of antimicrobial therapy, and a repeated course of therapy may be required. TABLE 88-6 Adjunctive Drug Therapy for Feline Ulcerative Gingivostomatitis IM, Intramuscular; IL, intralesional; PO, by mouth; prn, as needed. aSee the Drug Formulary in the Appendix for additional information. bDose per administration at specified interval. cInjection under anesthesia as a last resort. dGive every 7 days until improvement, then once every 14 to 35 days as needed. eDose reduced to every other day after this time and than gradually discontinued. Not available in the United States and some other countries. fAfter this time and clinical resolution, therapy should be tapered to the lowest dose and frequency that is effective in maintaining remission. Frequently this involves every other day for 1 to 2 months or until remission. A longer term maintenance regimen may consist of twice-weekly dosing on a continual basis. gDrug given for 4 consecutive days of each week after this time.1 Chronic faucitis in cats is a multifactorial disease thought to be caused by a variety of persistent microbial organisms in the oral cavities of cats with immunologic hyperresponsiveness. Although a variety of infectious agents have been associated with this disorder, direct causation has not been proven.365 FeLV, FIV, FCV, FHV-1, and Bartonella species can be present subclinically, making correlations difficult. FCV can be detected in many cats with this condition; however, the disease has not been experimentally reproduced.24,91,91 The presence of a persistent infectious agent such as FCV and an aberrant host immune response, with or without a co-infecting agent such as FIV,236,477,477 is likely responsible for disease manifestations. Hard, dry cat food also appears to have a role in exacerbating palatine ulceration in cats with acute FCV infection.222 The prevalence of FeLV infection in affected cats with stomatitis is lower than that of FIV. Faucitis is characterized by vesicular, ulcerative, and later, proliferative lesions in the mucosa, usually accompanied by mucosal ulceration and lymphoplasmacytic or eosinophilic infiltrations in the tissue of the caudal pharynx at the glossopharyngeal arch (fauces).218,477,477 Faucitis frequently appears among a number of cats within a household and tends to be recurrent after the initial treatment or is unresponsive to treatment. In chronic stages, proliferation of granulation tissue forms large masses in the caudal pharynx (Fig. 88-3). FCV has been isolated in a high percentage of cats with this syndrome compared with clinically healthy cats or those with oral ulcers at other locations. Cessation of FCV shedding was associated with resolution of chronic gingivostomatitis in one cat.1 It is likely a hypersensitivity phenomenon to persistent FCV infection. Co-infections with FIV or FeLV result in the most severe lesions. CD8+ T cells, representing cytotoxic and suppressor T cells, have been in the high reference range for all cats. In one study, cats with chronic gingivostomatitis were more likely to shed FCV and FHV-1 together than either virus alone.269 Compared with nondiseased cats, affected cats showed a significant increase in the relative messenger RNA (mRNA) expression of IL-2, IL-4, IL-6, IL-10, IL-12 (p35 and p40), and interferon (IFN)-γ. These results suggest that the normal feline oral mucosa is biased toward a predominantly (Th) type 1 profile of cytokine expression and that during the development of lesions seen in feline chronic gingivostomatitis, a shift in the cytokine profile from a type 1 to a mixed type 1 and type 2 response occurs.158
Gastrointestinal and Intra-Abdominal Infections
Oral Cavity
Oral Microflora
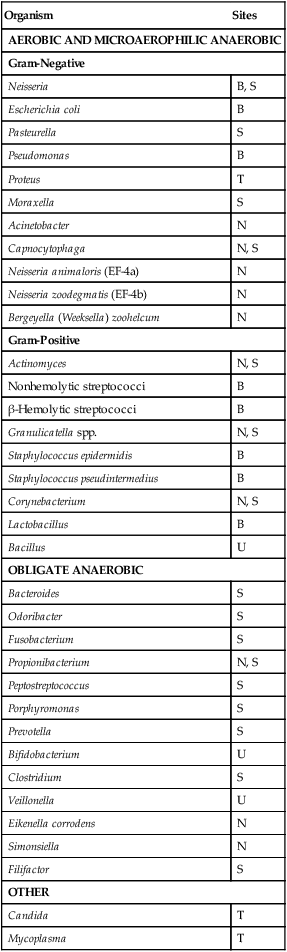
Organism
Sites
AEROBIC AND MICROAEROPHILIC ANAEROBIC
Gram-Negative
Neisseria
B, S
Escherichia coli
B
Pasteurella
S
Pseudomonas
B
Proteus
T
Moraxella
S
Acinetobacter
N
Capnocytophaga
N, S
Neisseria animaloris (EF-4a)
N
Neisseria zoodegmatis (EF-4b)
N
Bergeyella (Weeksella) zoohelcum
N
Gram-Positive
Actinomyces
N, S
Nonhemolytic streptococci
B
β-Hemolytic streptococci
B
Granulicatella spp.
N, S
Staphylococcus epidermidis
B
Staphylococcus pseudintermedius
B
Corynebacterium
N, S
Lactobacillus
B
Bacillus
U
OBLIGATE ANAEROBIC
Bacteroides
S
Odoribacter
S
Fusobacterium
S
Propionibacterium
N, S
Peptostreptococcus
S
Porphyromonas
S
Prevotella
S
Bifidobacterium
U
Clostridium
S
Veillonella
U
Eikenella corrodens
N
Simonsiella
N
Filifactor
S
OTHER
Candida
T
Mycoplasma
T
Organism
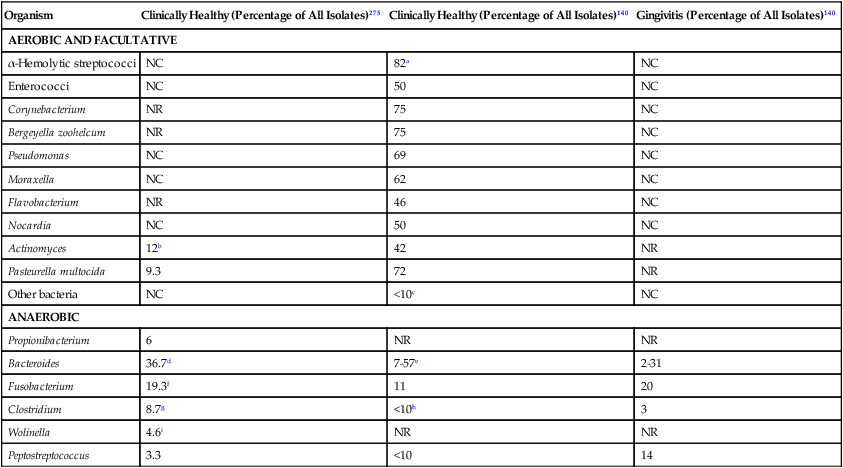
Clinically Healthy (Percentage of All Isolates)275
Clinically Healthy (Percentage of All Isolates)140
Gingivitis (Percentage of All Isolates)140
AEROBIC AND FACULTATIVE
α-Hemolytic streptococci
NC
82a
NC
Enterococci
NC
50
NC
Corynebacterium
NR
75
NC
Bergeyella zoohelcum
NR
75
NC
Pseudomonas
NC
69
NC
Moraxella
NC
62
NC
Flavobacterium
NR
46
NC
Nocardia
NC
50
NC
Actinomyces
12b
42
NR
Pasteurella multocida
9.3
72
NR
Other bacteria
NC
<10c
NC
ANAEROBIC
Propionibacterium
6
NR
NR
Bacteroides
36.7d
7-57e
2-31
Fusobacterium
19.3f
11
20
Clostridium
8.7g
<10h
3
Wolinella
4.6i
NR
NR
Peptostreptococcus
3.3
<10
14
Gingivitis and Periodontitis
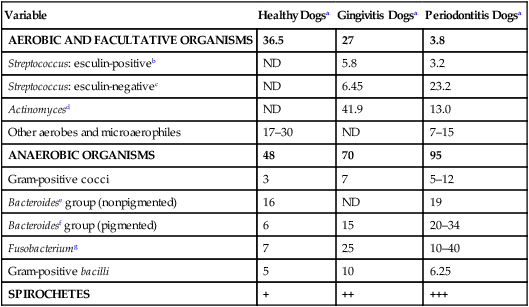
Variable
Healthy Dogsa
Gingivitis Dogsa
Periodontitis Dogsa
AEROBIC AND FACULTATIVE ORGANISMS
36.5
27
3.8
Streptococcus: esculin-positiveb
ND
5.8
3.2
Streptococcus: esculin-negativec
ND
6.45
23.2
Actinomycesd
ND
41.9
13.0
Other aerobes and microaerophiles
17–30
ND
7–15
ANAEROBIC ORGANISMS
48
70
95
Gram-positive cocci
3
7
5–12
Bacteroidese group (nonpigmented)
16
ND
19
Bacteroidesf group (pigmented)
6
15
20–34
Fusobacteriumg
7
25
10–40
Gram-positive bacilli
5
10
6.25
SPIROCHETES
+
++
+++
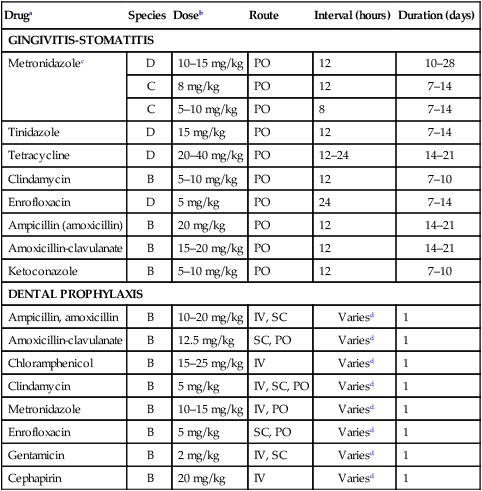
Druga
Species
Doseb
Route
Interval (hours)
Duration (days)
GINGIVITIS-STOMATITIS
Metronidazolec
D
10–15 mg/kg
PO
12
10–28
C
8 mg/kg
PO
12
7–14
C
5–10 mg/kg
PO
8
7–14
Tinidazole
D
15 mg/kg
PO
12
7–14
Tetracycline
D
20–40 mg/kg
PO
12–24
14–21
Clindamycin
B
5–10 mg/kg
PO
12
7–10
Enrofloxacin
D
5 mg/kg
PO
24
7–14
Ampicillin (amoxicillin)
B
20 mg/kg
PO
12
14–21
Amoxicillin-clavulanate
B
15–20 mg/kg
PO
12
14–21
Ketoconazole
B
5–10 mg/kg
PO
12
7–10
DENTAL PROPHYLAXIS
Ampicillin, amoxicillin
B
10–20 mg/kg
IV, SC
Variesd
1
Amoxicillin-clavulanate
B
12.5 mg/kg
SC, PO
Variesd
1
Chloramphenicol
B
15–25 mg/kg
IV
Variesd
1
Clindamycin
B
5 mg/kg
IV, SC, PO
Variesd
1
Metronidazole
B
10–15 mg/kg
IV, PO
Variesd
1
Enrofloxacin
B
5 mg/kg
SC, PO
Variesd
1
Gentamicin
B
2 mg/kg
IV, SC
Variesd
1
Cephapirin
B
20 mg/kg
IV
Variesd
1
Gingivitis in Kittens
Systemic Effects of Oral or Periodontal Infections
Glossitis
Gingivostomatitis and Pharyngitis
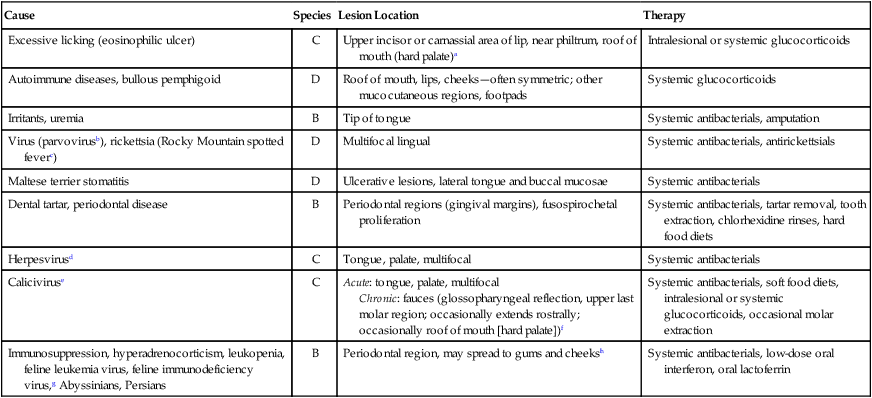
Cause
Species
Lesion Location
Therapy
Excessive licking (eosinophilic ulcer)
C
Upper incisor or carnassial area of lip, near philtrum, roof of mouth (hard palate)a
Intralesional or systemic glucocorticoids
Autoimmune diseases, bullous pemphigoid
D
Roof of mouth, lips, cheeks—often symmetric; other mucocutaneous regions, footpads
Systemic glucocorticoids
Irritants, uremia
B
Tip of tongue
Systemic antibacterials, amputation
Virus (parvovirusb), rickettsia (Rocky Mountain spotted feverc)
D
Multifocal lingual
Systemic antibacterials, antirickettsials
Maltese terrier stomatitis
D
Ulcerative lesions, lateral tongue and buccal mucosae
Systemic antibacterials
Dental tartar, periodontal disease
B
Periodontal regions (gingival margins), fusospirochetal proliferation
Systemic antibacterials, tartar removal, tooth extraction, chlorhexidine rinses, hard food diets
Herpesvirusd
C
Tongue, palate, multifocal
Systemic antibacterials
Caliciviruse
C
Acute: tongue, palate, multifocal
Chronic: fauces (glossopharyngeal reflection, upper last molar region; occasionally extends rostrally; occasionally roof of mouth [hard palate])f
Systemic antibacterials, soft food diets, intralesional or systemic glucocorticoids, occasional molar extraction
Immunosuppression, hyperadrenocorticism, leukopenia, feline leukemia virus, feline immunodeficiency virus,g Abyssinians, Persians
B
Periodontal region, may spread to gums and cheeksh
Systemic antibacterials, low-dose oral interferon, oral lactoferrin
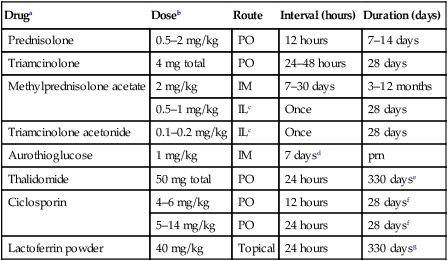
Diagnosis
Therapy
Druga
Doseb
Route
Interval (hours)
Duration (days)
Prednisolone
0.5–2 mg/kg
PO
12 hours
7–14 days
Triamcinolone
4 mg total
PO
24–48 hours
28 days
Methylprednisolone acetate
2 mg/kg
IM
7–30 days
3–12 months
0.5–1 mg/kg
ILc
Once
28 days
Triamcinolone acetonide
0.1–0.2 mg/kg
ILc
Once
28 days
Aurothioglucose
1 mg/kg
IM
7 daysd
prn
Thalidomide
50 mg total
PO
24 hours
330 dayse
Ciclosporin
4–6 mg/kg
PO
12 hours
28 daysf
5–14 mg/kg
PO
24 hours
28 daysf
Lactoferrin powder
40 mg/kg
Topical
24 hours
330 daysg
Feline Lymphocytic Plasmacytic Ulceroproliferative Gingivostomatitis (Faucitis)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Gastrointestinal and Intra-Abdominal Infections