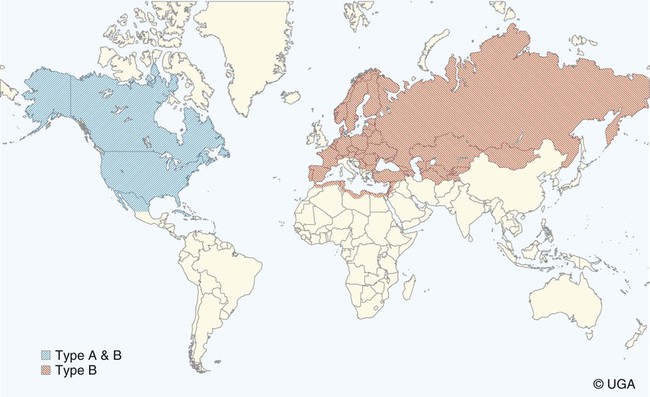
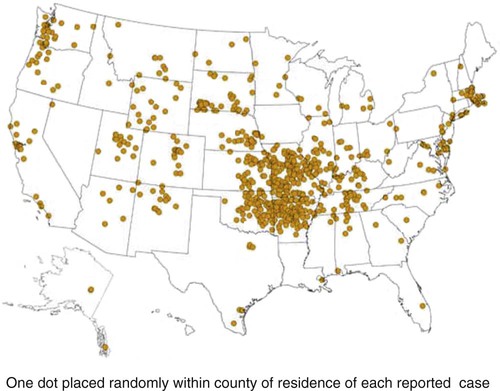
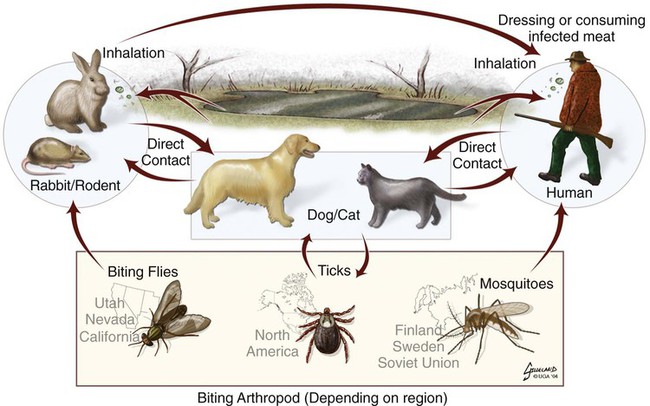
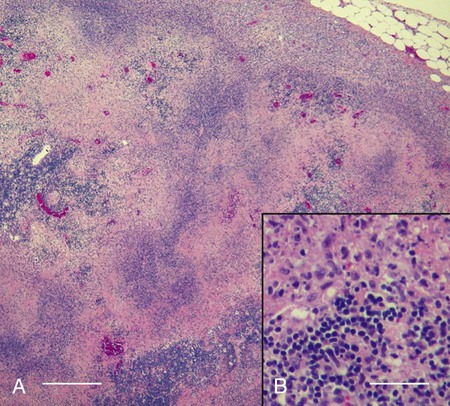
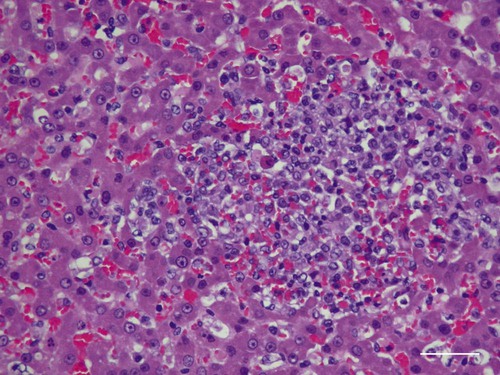
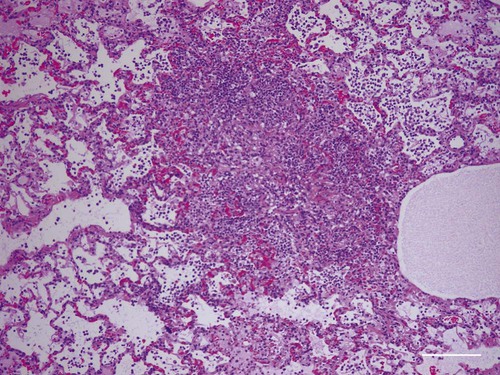
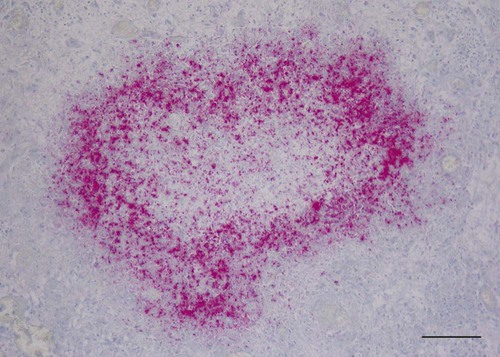
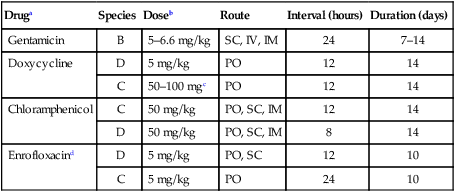
Tularemia is an acute bacterial infection of many avian and mammalian species, including dogs and cats and occasionally people. Terrestrial and aquatic mammals serve as reservoirs for the disease. It occurs throughout the temperate region of the Northern Hemisphere, predominantly between 20 and 70 degrees latitude.34,60 The etiologic agent, Francisella tularensis, is a small (0.2 µm × 0.2 to 0.7 µm), pleomorphic, facultative intracellular gram-negative, non–spore-forming bacillus. The other member of the genus is Francisella philomiragia, which is discussed in the next section. This genus is a member of the order Thiotrichales, in the gamma subdivision of Proteobacteria, which also includes Coxiella burnetii, the cause of Q fever (see later). The tularemia bacillus has two main biovars: type A (ssp. tularensis; previously nearctica) and type B (ssp. holarctica; previously palearctica). Type A strains ferment glycerol and are highly virulent for laboratory rabbits (Oryctolagus cuniculus), and type B strains do not ferment glycerol and are avirulent for rabbits. Type A strains are associated with a terrestrial tick-rabbit cycle of infection and occur only in North America. They are responsible for clinical illness in rabbits, cats and people. Type B strains have a more complex aquatic epidemiology—involving rodents, ticks, mosquitoes, mud, muskrats, beavers, and water—and occur throughout the Northern Hemisphere. Protozoa such as Acanthamoeba castellanii (see Chapter 78) have been shown to harbor the organism and may assist its survival in the aquatic environment.1 Both type A and B strains have been isolated from cats in the United States.6,22,22 Human and feline illness is generally more severe with type A strain infections.33,65 Two less frequently isolated biotypes include subspecies mediasiatica, found in Kazakhstan and Uzbekistan, and novicida, primarily from North America. Subspecies novicida has also been isolated from Australia and is of low virulence. Within the given subspecies, subpopulations of strains exist that correlate with differences in geographic distribution, in specific reservoir hosts and replication foci, and in virulence for mammalian hosts.59 Tularemia is endemic in the Northern Hemisphere between the latitudes of 30 degrees N and 71 degrees N (Fig. 46-1). This area includes much of Eurasia to countries on the Mediterranean coast of Africa. In North America, this region ranges from the Arctic Circle as far south as Guadalajara, Mexico. Tularemia is a rural disease in people and in their pets as a result of contact with wildlife reservoirs, their vectors, or the contaminated environment. It can be transmitted by ticks, biting flies, penetrating injuries, water exposure, food, and aerosols.25 In the United States, four tick species constitute the primary vectors for dogs and cats: the wood tick (Dermacentor andersoni) found in the Rocky Mountain region; the American dog tick (Dermacentor variabilis) found in the eastern two thirds of the country, as well as in the Pacific coastal states; the Pacific Coast tick (Dermacentor occidentalis) found in California and Oregon; and the Lone Star tick (Amblyomma americanum) found in the southern central and southeastern states. The incidence of infection for people in the United States is presented in Fig. 46-2. Because the epidemiology of tularemia is complex, and its host species so diverse,34 only the aspects that are important in its transmission to dogs and cats are discussed here (Fig. 46-3). Various tick species, which are true biologic vectors for the disease, serve as both long-term reservoirs and vectors of tularemia.27 The organism leaves the midgut of the infected tick and migrates to the salivary glands, where it is inoculated into the host during feeding. Additional contamination of the feeding site may occur via tick feces deposited during feeding.25 Infection can pass transovarially in ticks, and infection persists for life. Amplification of the infection rate in a tick population occurs when uninfected ticks feed on bacteremic animals. Although ticks are capable of transmitting tularemia at all three stages of their development, the adult and, less commonly, nymphal stages are most important in transmission to dogs, cats, and humans. Results of genetic analysis of strains Type A tularemia, indicate that small foci of infection involving ticks and reservoir hosts may occur in nature.26a Epizootic foci may occur during times of heightened evolved virulence of the organism resulting in amplified infection rate and severity in reservoir hosts and more likelihood of exposure to humans. However, transmission of infection to humans by vectors is thought to be uncommon compared to other means.25 Results of serologic studies on client-owned cats in Connecticut and New York State were a 12% to 24% exposure rate, depending on the immunoassay used.47 Outdoor cats are more likely to be exposed to infected ticks, rodents, and rabbits contributing to the relatively high exposure rate. The clinical presentation of human tularemia varies by route of infection and initial sites of localization. Typically, a localized infection occurs at the primary inoculation site and is associated with prominent regional lymphadenomegaly. In a highly susceptible host, fewer than 50 organisms can cause infection by parenteral inoculation, whereas at least 108 are needed for oral infection. Subsequent bacteremia and multiorgan involvement are common. The lungs, spleen, liver, lymph nodes, and skin are the sites of embolic spread (Fig. 46-4). Early embolic lesions, which begin as microabscesses, progress to granulomatous inflammation as the body attempts to wall off the infection. Bacteremic disease without antecedent localized infection is referred to as typhoidal tularemia. Cell-mediated immunity plays an important role in recovery. A similar disease pattern appears to occur in dogs and cats. The severity of illness in experimentally infected dogs varies with age; puppies are more susceptible than are young adults. Ingestion of infected tissues or intradermal challenge produced milder disease than intranasal challenge.21,36 When dogs were fed infected tissues, an acute 5-day illness began after a 48-hour incubation period.36 Fever (40.2° C [104.4° F]) and a mucopurulent discharge from the nose and eyes were present. Transient ulceroglandular tularemia follows intradermal challenge with concomitant development of fever, pustules at the inoculation site, and regional lymphadenomegaly. Cats have become ill after eating experimentally infected guinea pigs.68 Although some cats appear unaffected, younger cats succumb primarily to systemic (typhoidal) infection characterized by generalized lymphadenomegaly and miliary abscess formation involving the liver and spleen. After subcutaneous inoculation, similar findings have been apparent, and young kittens appear most susceptible.68 Some subcutaneously and intranasally inoculated cats have had areas of bronchopneumonia from which F. tularensis was isolated, as well as splenomegaly and multifocal hepatic necrosis. Despite the ability to produce experimental infections and high seroprevalence, indicating exposure in endemic areas, naturally acquired infections in dogs have been rare.* Dogs are considered relatively resistant to tularemia. Typically, a dog develops a brief episode of anorexia, listlessness, and low-grade fever. Sudden death of uncertain cause has occurred in a dog a few days after sniffing a dead infected rabbit. Fever (above 40.5° C [104.9° F]), mental depression, and mandibular lymphadenomegaly were observed in a dog 36 hours after it ate a rabbit.55 Another dog developed multifocal draining abscesses in the subcutaneous tissue and superficial lymph nodes in addition to fever, anorexia, myalgia, and shivering. Uveitis and conjunctivitis developed in the left eye, with subsequent transient conjunctivitis and corneal clouding in the right eye. Based on the large number of reports, cats are more susceptible to tularemia compared with dogs. The spectrum of illness associated with spontaneous feline tularemia has not been well described. In five typhoidal cases, pertinent clinical signs included fever; marked depression; pharyngeal, cervical, mesenteric, regional, or generalized (or any combination) lymphadenomegaly; palpable splenomegaly and hepatomegaly; acute shallow oral or lingual ulcers (or both); icterus; and panleukopenia with severe toxic changes of neutrophils.6,6a The oral and lingual ulcers were compatible with infection resulting from physical injury associated with ingestion of infected rodents or rabbits. In one cat, a chronically draining subcutaneous abscess, similar to the ulceroglandular form described in people, was observed.75 Other organisms that can cause a similar lesion include Pasteurella, Yersinia, Nocardia, mycobacteria, and Cryptococcus. Other cases in cats have been briefly described in epidemiologic reports of human cases.† Implicated cats had frequently eaten or mouthed wild rabbits before the onset of clinical signs. Variable signs of fever, anorexia, listlessness, lymphadenomegaly, draining abscesses, and, occasionally, icterus and death were reported. Some cats had no signs of illness.55,75 Testing for serologic evidence of microscopic agglutinating (MA) antibody is the most commonly used diagnostic procedure. Both dogs and cats develop antibodies, but titers tend to be lower than those observed in people. Titers from 140 to 160 are typical of recent infections in dogs.36,65 Using an MA method in cats, serum antibody titers to F. tularensis have been above 20.79 Negative MA screening titer results were found using sera from a dog, collected after 48 hours of exposure to an infected rabbit.55 Antibody increases may not be apparent until up to 3 weeks after exposure.23 Therefore, false-negative results may be observed with a single titer measurement early in the course of illness. Enzyme-linked immunosorbent assay techniques have been developed to detect antibodies to specific F. tularensis antigens in infected people7 and cats,47 but their usefulness for testing canine sera is uncertain. Indirect fluorescent antibody methods have also been used for serotesting. A fourfold increase in antibody titer is required to confirm active disease; however, a single titer of at least 160 suggests active infection.80 Organisms are not usually visible on Gram-stained smears. Direct fluorescent antibody methods can be used to detect the coccobacillary organisms in exudates and tissues, even with paraffin-embedded tissues. Polymerase chain reaction (PCR) has also been used to confirm infection. Lymph node aspirates have been of a mixed lymphoid cell population characteristic for hyperplasia. The isolation of F. tularensis from exudates or tissue specimens is the definitive method of diagnosis. In some cases, it has been more sensitive than immunochemical staining methods of tissues.75 The organism is fastidious and must be isolated on special media, such as supplemented chocolate agar, or by initial inoculation of a susceptible laboratory animal with subsequent culture of the liver and spleen. Blood-glucose-cysteine agar or brain-heart infusion agar supplemented with blood is needed for isolation at 37° C. Characteristic dewdrop colonies are isolated. Confirmation of the identity of the organism can be achieved with immunochemical or molecular methods. Biosafety level 3 measures must be used to handle infected tissue. Refer to Web Appendix 5 for a listing of laboratories that perform this isolation. Laboratories should always be notified in advance that the presence of this organism is suspected. The infectious dose for humans is less than 100 organisms, inhaled as an aerosol, accidentally inoculated, or splashed in the conjunctival sac. Cultures of F. tularensis and necropsies of animals with suspected tularemia should therefore be performed only in laboratories with adequate biosafety equipment. Testing of aspirated pus with PCR has been used for definitive diagnosis of this infection in people.17 Gross necropsy findings in affected dogs and cats are similar. Most of this information has been obtained in experimentally infected animals. Lymph nodes are often markedly enlarged in a regional or more generalized pattern, and they often contain multiple foci of necrosis. Draining sinus tracts may arise from the nodes. Hepatomegaly or splenomegaly, or both, may be present. Multiple small, grayish foci representing necrosis are commonly found in the spleen, liver, lung, and, occasionally, heart (Figs. 46-4 and 46-5). Segmental to diffuse hemorrhage of the small and large intestines has been reported in cats.6 These same cats had prominent ulceration in Peyer’s patches and colonic lymphoid follicles. Fibrinosuppurative to granulomatous inflammation is observed in many parenchymal organs (Figs. 46-6, 46-7, and 46-8). The causative bacteria are not easily demonstrated in the lesions except by specific immunohistochemical stains (Fig. 46-9).16 Where small numbers of organisms are present in lesions, culture may be more sensitive than immunohistochemistry for diagnosis.75 No substantial reports have been made on antimicrobial therapy of canine or feline tularemia. In one cat, surgical removal of a localized subcutaneous mass followed by treatment with amoxicillin-clavulanate was curative.75 In people, the aminoglycosides (streptomycin and gentamicin) are currently considered the drugs of first choice, but streptomycin has limited availability (see the Drug Formulary in the Appendix).20,58 Therefore, parenteral gentamicin treatment is the drug of choice (Table 46-1). Precautions should always be taken to monitor renal function during the treatment interval. Tetracycline and chloramphenicol are potential alternative drugs, but relapses occur frequently with their use. Doxycycline would be the preferred tetracycline because of its superior penetration. Historically, both streptomycin and tetracycline have been given successfully in controlled trials of antibacterial prophylaxis in human tularemia.64 Quinolones, such as ciprofloxacin and norfloxacin, may be effective67,71; however, treatment failures have been described.13 A significant number of these drugs are marketed for animal use. Consult the Drug Formulary in the Appendix and Chapter 30, for dosages and further information on each drug. Because of the potential risk of handling an infected dog or cat, maximal precautions should be used for handling contaminated secretions with gloves and wearing face and eye protection during wound care of hospitalized animals. TABLE 46-1
Francisella and Coxiella Infections
Tularemia
Etiology
Epidemiology
Pathogenesis
Clinical Findings
Dogs
Cats
Diagnosis
Pathologic Findings
Therapy
Druga
Species
Doseb
Route
Interval (hours)
Duration (days)
Gentamicin
B
5–6.6 mg/kg
SC, IV, IM
24
7–14
Doxycycline
D
5 mg/kg
PO
12
14
C
50–100 mgc
PO
12
14
Chloramphenicol
C
50 mg/kg
PO, SC, IM
12
14
D
50 mg/kg
PO, SC, IM
8
14
Enrofloxacind
D
5 mg/kg
PO, SC
12
10
C
5 mg/kg
PO
24
10
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Francisella and Coxiella Infections