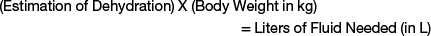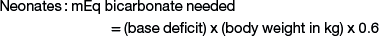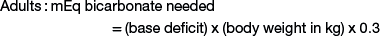Chapter 3 Fluid Therapy and Nutritional Support
Physiology of Body Fluids
In order to administer fluids and electrolytes properly, a general understanding of body fluid composition for the patient’s species, and of how this fluid is lost during disease states, is necessary. Total body water makes up approximately 60% of a sheep’s or goat’s body weight.1 This percentage can vary with age, body composition, and breed. In general, neonatal lambs or kids have relatively more body water than adults, and total body water in neonates may approach 75% to 80% of body weight. The larger total body water percentage is due primarily to a large extracellular fluid volume. By the age of 6 months, values for total body water and extracellular fluid volume are similar to those in adults. The larger total body water and extracellular fluid volume in the neonate do not provide a reservoir of fluid for the sick animal in these species.2 Overweight sheep and goats have decreased total body water content compared with that in lean animals, because adipose tissue contains very little water. Estimations of total body water for fattened sheep are approximately 50% of body weight.1
Total body water is distributed within two major compartments, extracellular fluid and intracellular fluid. Approximately two thirds of total body water is intracellular fluid (40% of body weight), and one third is extracellular fluid (20% of body weight). The extracellular fluid compartment can be further subdivided into the intravascular fluid or plasma volume (5% of body weight) and the interstitial fluid (15% of body weight). The interstitial fluid compartment consists of fluid surrounding cells, cerebrospinal fluid, connective tissue, and most importantly, the contents of the reticulorumen and the rest of the gastrointestinal tract. The reticulorumen is an important reservoir of fluid for adult animals during periods of water restriction, and the gastrointestinal tract also can be a site for water deposition during disease processes such as grain overload or endotoxemia. Although the intracellular and extracellular fluid compartments differ in electrolyte composition, they are in osmotic equilibrium and water can freely diffuse between them. The movement of water and electrolytes between compartments is governed by hydrostatic and oncotic forces. Sodium is the most important cation within the extracellular fluid compartment, accounting for about 95% of the total cation pool. Potassium is the major intracellular cation. The concentrations of sodium and potassium are maintained within and outside of cells by the Na+,K+-ATPase pump. Chloride and bicarbonate are the major anions within the extracellular fluid space, whereas phosphates, proteins, and other anions maintain electroneutrality with the potassium cation in the intracellular fluid compartment. When fluids are administered to dehydrated sheep or goats, fluid losses are replaced within the extracellular fluid compartment; therefore the fluids being administered should contain concentrations of ions similar to those found in the extracellular fluid compartment. Assessment of blood electrolyte concentrations and determination of acid-base status are performed using techniques that reflect conditions in the extracellular fluid compartment.
Dehydration is a common feature of many diseases. Dehydration results from inadequate fluid intake in the presence of increased fluid losses. With dehydration, all fluid compartments are affected. Dehydration initially results in reduction of the intravascular fluid volume, followed by contraction of the interstitial and intravascular fluid compartments. Most disease processes result in concurrent losses of fluids and electrolytes—resulting in a pathophysiologic condition referred to as isotonic or isoosmolar dehydration. In such cases, providing both fluid and electrolytes (mainly sodium) is important. Hypertonic dehydration, or a relative water deficit, occurs when water losses exceed losses of electrolytes; water deprivation is an example. By contrast, hypotonic dehydration, or relative water excess, occurs when electrolyte losses exceed water losses. Hypotonic dehydration also occurs with diarrhea, when losses of electrolytes and water occur concurrently (isotonic dehydration) but the water deficit is replaced by water consumption or administration of 5% dextrose solutions. Another example of hypotonic dehydration occurs in goats or sheep with obstructive urolithiasis, when sodium depletion exceeds water loss as sodium moves into the peritoneal cavity. In cases of urolithiasis with urinary bladder rupture, administration of fluids containing sodium is important (see Chapter 12).
Patient Assessment
Physical examination of hypovolemic or dehydrated sheep and goats is very important to ensure that the correct fluid type is administered at an appropriate rate, as well as for identifying the underlying disease process. Intercurrent disease processes, hypothermia, and perinatal asphyxia can lead to difficulty in accommodating intravenous fluids. Fluid therapy can have adverse effects such as volume overload and edema, so particular attention should be given to the cardiovascular and renal systems. Cardiovascular diseases may result in an inability to cope with an acute fluid load, and oliguric renal failure results in an impaired ability to excrete excess fluid (see Chapter 17).
Dehydration is most accurately assessed by changes in body weight before and after a disease event. Because this information usually is not available to the clinician, clinical assessment is used to assess degree of dehydration. The packed cell volume and total plasma protein can be used as tools to assess hydration status, but these measurements cannot be used to replace an estimation of hydration status from physical examination. The reference ranges for packed cell volume in healthy sheep and goats are 27% to 45% and 22% to 38%, respectively3; these ranges are too wide to be useful in estimation of hydration status. Total plasma protein concentration is dependent on colostral intake in neonates and may be elevated with chronic inflammation. In addition, a sheep or goat with anemia and hypoproteinemia in conjunction with dehydration, as occurs with intestinal parasitism, can nevertheless have a normal packed cell volume and total plasma protein concentration. Packed cell volume and total plasma protein concentrations are most useful in monitoring the progress of fluid therapy to prevent overhydration.
Although no standard method is available for assessing dehydration in sheep and goats, percent dehydration can be estimated by assessing heart rate; eyeball recession; mucous membranes for tackiness, color, and capillary refill time; and skin elasticity or turgor (Table 3-1). Degree of enophthalmos has been used to determine hydration status in calves4 and can be used to assess hydration in sheep and goats as well. The percent dehydration can be estimated by measuring the eyeball recession in mm and multiplying by 2. For example, a sheep or goat with eyeball recession of 4 mm is estimated to be 8% dehydrated. The duration of skin tenting also can be used to estimate hydration status. The percent dehydration is estimated by measuring the skin tent (in seconds), multiplying by 2, and then subtracting 4. For example, a sheep or goat with a skin tent duration of 6 seconds is estimated to be 8% dehydrated (6 × 2 = 12 − 4 = 8%). Some caveats to this clinical assessment of dehydration exist, and it is important to recognize that the poor skin elasticity and enophthalmos of dehydration also are seen in emaciated small ruminants.
TABLE 3-1 Estimation of Percent Dehydration from Physical Examination Findings
| Percent Dehydration | Physical Findings |
|---|---|
| <5% | History of fluid loss but no other abnormalities |
| 5% | Minimal depression, normal to mildly tacky mucous membranes, minimal enophthalmos, normal heart rate, normal capillary refill time (<2 seconds) |
| 8% | Depression, mild to moderate decrease in skin turgor (skin tent duration 2-4 seconds), obvious enophthalmos, slight tachycardia (heart rate >90 beats/minute), increased capillary refill time (3-4 seconds) |
| ≥ 10% | Severe depression, weakness, moderate to marked degree of decreased skin turgor (skin tent duration >5 seconds), dry and dark mucous membranes, tachycardia (>120 beats/minute), increased capillary refill time (>5 seconds), cold extremities |
Acid-base and electrolyte abnormalities are more difficult to assess without the aid of laboratory testing. Serum biochemistry and blood gas analyses can provide information on serum electrolyte (sodium, potassium, chloride, bicarbonate, calcium, magnesium, and phosphorus) abnormalities, acid-base disorders, or glucose abnormalities that require correction. In general, most cases of diarrhea in neonates and grain engorgement in adults will be characterized by a metabolic acidosis, whereas intestinal obstructions and renal disease will be associated with a metabolic alkalosis. Although the degree of acidosis can be estimated in calves with diarrhea using a clinical scoring system,5 no similar clinical scoring system has been created for use in neonatal sheep or goats with diarrhea (see Appendix 11).
Quantity and Rate of Fluid Administration
The fluid therapy plan is designed to replace deficits while supplying maintenance fluid needs and accounting for ongoing loss of fluids associated with the disease process (Table 3-2). The first priority for treating dehydration in a sheep or goat is to restore the extracellular fluid volume to normal. The following simple formula can be used to calculate the recommended amount of fluid for restoration of the animal to a normal hydrated state:
TABLE 3-2 Components and Formulas for a Fluid Therapy Plan
| Category | Amount/Formula |
|---|---|
| Deficit | % dehydration × body weight in kg |
| Maintenance | |
| Ongoing losses | Up to 5% of body weight in kg |
| Bicarbonate deficit | |
| Shock rate | 90 mL/kg/hour |
Intravenous fluid therapy is recommended in cases in which the estimation of dehydration is 8% or greater, because oral fluid therapy will be ineffective.6 For example, a 50-kg sheep that is 8% dehydrated will need 4 L of fluid to replace the deficit (0.08 × 50 = 4.00). A 5-kg kid with diarrhea that is 10% dehydrated will need 500 mL of fluid to replace the deficit. Because methods for precise measurement of the degree of dehydration are not available, it is important for the clinician to recognize that replacing the exact fluid deficit is not of chief concern. Rather, the clinician should replace a fluid deficit to restore tissue perfusion and improve mental capacity so oral fluids can be utilized. A general rule is to replace half of the fluid deficit over 4 to 6 hours, with the balance given over 12 to 24 hours. More often, the fluid deficit is replaced more rapidly (6 hours); however, care should be taken in the hypothermic neonate or in cases of sepsis, because generalized edema may result. Specifically, too-rapid delivery of intravenous fluid therapy can result in pulmonary and cerebral edema. Alternatively, the rate of fluid administration can be set at 50 mL/kg/hour, which is less than the shock therapy rate of 90 mL/kg/hour.
Calculation of maintenance fluids is based on species- and age-specific physiologic requirements. The normal, adult sheep or goat requires approximately 50 mL/kg/day to provide enough fluids for digestion and to replace losses through urine and defecation (sensible water loss) and sweat and respiration (insensible water loss). As stated previously, neonates have higher total body water than that in adults and therefore have higher maintenance fluid requirements. The maintenance fluid needs for lambs and kids can be up to 80 mL/kg/day. In developing the fluid therapy plan, both replacement of the deficit and inclusion of maintenance fluid requirements should be addressed. The maintenance fluid needs of sheep and goats, in 50 mL/kg/day, can be simply converted to 1 mL/lb/hour or 2 to 4 mL/kg/hour. An important consideration is that this maintenance fluid requirement can change with ambient temperature and feed intake, because diets may vary in moisture content. Careful monitoring is warranted for patients that are on continuous intravenous fluid therapy or receiving large volumes of fluids over a short period of time, because they can become hypoproteinemic and develop edema. Serial measurements of packed cell volume and total plasma protein are needed to prevent overhydration.
Fluid Type
Balanced crystalloid fluids vary in their content of electrolytes, but in general, balanced crystalloid solutions contain electrolyte concentrations similar to those in plasma. Ringer’s solution is an acidifying solution and is fairly similar to normal saline except for its slightly lower levels of sodium, higher levels of chloride, and additional potassium and calcium. Lactated Ringer’s solution is considered an alkalizing solution, but the lactate present requires hepatic metabolism to produce bicarbonate, and only the l-isomer of lactate is metabolized efficiently to produce bicarbonate. The balanced crystalloids Normosol-R and Plasma-Lyte A also are considered alkalizing fluids because they contain sodium acetate and sodium gluconate. Acetate and gluconate are bicarbonate precursors. Unlike lactate, which is metabolized by the liver, acetate is metabolized by muscle tissue. Gluconate has been shown to be ineffective as an alkalizing agent in calves when administered intravenously but is effective when given orally.7 Although some of these balanced crystalloid solutions are considered to be alkalizing, they are regarded as inferior in alkalizing ability to 1.3%, 5%, or 8.4% sodium bicarbonate.2
Sodium bicarbonate is the crystalloid fluid of choice for metabolic acidosis and can either be given as isotonic sodium bicarbonate (1.3%), or hypertonic sodium bicarbonate (5% or 8.4%), or added to other crystalloid solutions. Common causes of metabolic acidosis include absorption of d-lactate from the gastrointestinal tract (e.g., with grain engorgement or enterocolitis) and sodium loss with secretory diarrhea. Sepsis or other causes of systemic shock also can lead to metabolic acidosis as a result of l-lactate accumulation from poor tissue perfusion. To correct metabolic acidosis, a total carbon dioxide (CO2) measurement from a serum biochemistry panel or blood gas analysis is needed. The base deficit is calculated by subtracting the measured total CO2 from the normal total CO2 (approximate normal total CO2 is 25 mEq/mL). The amount of bicarbonate to administer can be calculated as follows:
Neonates have a larger bicarbonate space than that in adult animals and thus have greater bicarbonate replacement needs when losses occur.6 For example, a 5-kg kid with a base deficit of 15 will need 45 mEq of bicarbonate to correct the metabolic acidosis: 15 (base deficit) × 5 kg (body weight) × 0.6 = 45 mEq. If the 5-kg kid is 10% dehydrated, the fluid deficit is 500 mL. None of the crystalloid fluids previously discussed can provide enough base in 500 mL to correct the acidosis in this specific example, emphasizing the need for bicarbonate therapy in cases of metabolic acidosis in ruminants. The bicarbonate needed to correct metabolic acidosis can be given to sheep or goats as a 1.3% isotonic solution; alternatively, the deficit can be added to normal saline and administered intravenously. As a general rule, half of the calculated base deficit should be corrected if the metabolic acidosis results from dehydration only. The entire deficit can be corrected if the dehydration is due to neonatal diarrhea or grain engorgement. Sodium bicarbonate is available commercially as hypertonic solutions of either 5% (0.6 mEq/mL) or 8.4% (1 mEq/mL). Solutions of 5% sodium bicarbonate can be given intravenously without dilution so long as the dehydration is corrected at the same time. The administration rate for 5% sodium bicarbonate should not exceed 2 mL/kg/minute.8
Dextrose-containing intravenous crystalloid solutions such as 5% dextrose in water (D5W) should not be used routinely in small ruminant practice for stand-alone therapy, because once the dextrose is metabolized, the fluid contains no active solute. Infusion of 5% dextrose can lead to dilution of serum electrolytes along with the development of edema. However, glucose supplementation is important for hypoglycemic, hypothermic neonatal small ruminants and in ewes with pregnancy toxemia. Blood glucose can easily be measured with commercially available hand-held glucometers. To treat hypoglycemia, dextrose can be administered intravenously as a 50% solution (at a dose of 0.2 mL/kg of body weight) or as a 5% to 10% solution. Dextrose can be added to other crystalloid fluids to make a 1% or 2% solution (20 mL of 50% dextrose/L for each 1% of dextrose needed). For ewes with pregnancy toxemia, 5 to 7 g of glucose given intravenously six to eight times daily has been recommended.9
Colloids are high-molecular-weight compounds that, unlike crystalloids, do not readily leave the intravascular space. Examples of colloids are plasma, human serum albumin and synthetic compounds such as hetastarch, dextrans, and modified gelatin solutions. Plasma is used primarily in cases of failure of passive transfer and hypoproteinemia and is administered at a dosage rate of 20 to 40 mL/kg of body weight.10 The use of plasma for colloidal effects is relatively ineffective, because 50 to 100 mL/kg of body weight is required to raise serum albumin concentration by 1 g/dL. Sheep and goat plasma preparations are available commercially (Midwest Animal Blood Services, Inc., Stockbridge, Michigan). The use of colloids for expanding intravascular volume in ill small ruminants has not been evaluated.
Whole blood transfusions are indicated primarily when the red blood cell mass is inadequate to carry oxygen to the peripheral tissues. Whole blood transfusions are recommended in animals with clinical signs suggestive of tissue hypoxia, such as elevated heart and respiratory rates, weakness, and lethargy, or in which the packed cell volume drops below 15% to 20% in acute anemia and below 10% to 15% in chronic anemia. Whole blood can be administered at a dose of 10 to 15 mL/kg of body weight; however, this will result in an increase in packed cell volume of the recipient by only 3% to 4%. For hemorrhagic shock, at least half of the estimated blood loss should be replaced by whole blood.10 Whole blood transfusions also can be used as a source of plasma and can be given at a dose of 40 to 80 mL/kg of body weight. Monitoring of the transfusion is important, and the whole blood transfusion should be started at a slow rate (0.1 mL/kg/hour), with vital signs evaluated every 5 minutes. Clinical signs of anaphylactic reactions to whole-blood transfusions include fever, dyspnea, hiccoughing, muscle tremors, salivation, and lacrimation. If a transfusion reaction is noted, blood administration is ceased and epinephrine (1:1000) can be administered at a dose of 0.01 to 0.02 mL/kg of body weight intravenously.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree