Chapter 14 Diseases of the Eye
Ocular Anatomy
An understanding of the normal anatomy of the eye is essential for clinical recognition and accurate identification of ocular abnormalities likely to be encountered in veterinary practices that provide care for small ruminants. In these species eyes have retained essentially the same basic components and embryologic development over the course of evolution. Variations are additive to the basic design and have emerged largely because of ecologic factors such as light intensity and duration and feeding habits.1 Goats and sheep are arrhythmic ruminants—they are equally active diurnally and nocturnally.
Adnexa
Orbit
Sheep and goats have an enclosed orbit, typical of most grazing animals. In both species, the bony fossa of the orbit comprises lacrimal, zygomatic, frontal, sphenoid, and palatine bones. In addition, sheep have a maxillary bone and goats have an ethmoid bone that forms part of the orbit. The size, shape, and position of the orbit are closely associated with visual activity and feeding behavior.1 In general, prey species such as sheep and goats have eyes that are located more laterally on the skull and have mostly monocular vision.1 The orbit contains the globe, extraocular muscles, the orbital lacrimal gland, and fat. The globe is surrounded by three fascial layers of the orbit: the periorbita, which is the most external layer; the superficial muscular fascial layer, which encloses the lacrimal gland and the levator palpebrae superioris muscle; and the deep muscular fascia that sheaths the extraocular muscles and optic nerve.2 The orbital fat fills the orbital dead space and provides a cushion that protects the globe and extraocular muscles.
Extraocular Muscles
Seven extraocular muscles suspend the globe and move the eye. The four rectus muscles are the dorsal, ventral, medial, and lateral recti; they move the globe in the direction indicated by their names. The dorsal oblique muscle inserts on the dorsolateral aspect of the globe and rotates the dorsal aspect of the globe medially and ventrally. The ventral oblique muscle inserts on the ventrolateral aspect of the globe and rotates the globe medially and dorsally. The retractor bulbi muscle inserts posterior to the equator of the globe, forming an almost complete cone around the optic nerve. This muscle retracts the globe for additional protection. The oculomotor nerve (cranial nerve III) innervates the dorsal, ventral, and medial recti. The dorsal oblique is innervated by the trochlear nerve (cranial nerve IV), and the lateral rectus and retractor bulbi muscles are innervated by the abducens nerve (cranial nerve VI) (see Chapter 13).
Eyelids and Conjunctiva
The superior and inferior palpebrae—the eyelids—are two musculofibrous folds of thin skin continuous with the facial skin. The superior eyelid is more mobile than the inferior eyelid. The opening formed by the free edges of the eyelids is the palpebral fissure.1–3 The medial angle of the palpebral fissure is the medial canthus; the lateral angle is the lateral canthus. Histologic analysis shows that the eyelids have four tissue layers: the skin, the orbicularis oculi muscle, the tarsus and stromal layer, and the palpebral conjunctiva. The palpebral skin, containing small tubular and sebaceous glands, is thin and elastic and is covered by a dense coat of short hairs. The superior palpebrae has a row of cilia, and vibrissae are present a short distance from the superior and inferior palpebral margins in goats and sheep. The superior palpebral skin receives sensory innervation from the ophthalmic branch of the trigeminal nerve (cranial nerve V), and the inferior palpebral skin is innervated by the maxillary branch of the trigeminal nerve. The orbicularis oculi muscle encircles the entire palpebral fissure and functions to close this opening. It receives motor innervation by the palpebral branch of the facial nerve (cranial nerve VII). The superior eyelid is elevated by the levator palpebrae superioris muscle, which receives motor innervation from the oculomotor nerve (cranial nerve III). At the margins of both eyelids are the tarsal gland openings. The tarsal glands are sebaceous glands that produce the lipid component of the precorneal tear film. These glands open onto the edge of both eyelids through small openings arranged longitudinally. The palpebral conjunctiva is the mucous membrane that lines the inner aspect of the eyelids. The conjunctival epithelium has numerous goblet cells that contribute to the mucin layer of the precorneal tear film. The palpebral conjunctiva continues onto the globe as the bulbar conjunctiva, where it meets and is continuous with the corneal epithelium. The palpebral, bulbar, and nictitans conjunctivae are named according to their anatomic locations, but they are continuous.
The nictitating membrane (nictitans)—the third eyelid—is located ventromedially between the inferior eyelid and the globe. It is completely lined by conjunctiva and contains a T-shaped cartilaginous plate with a gland (the gland of the third eyelid) at its base. The horizontal part of the T lies at the free edge of the nictitating membrane. The gland surrounds the stem of the cartilage on the membrane’s posterior aspect. The nictitating membrane moves passively over the eye in a dorsolateral direction when the globe is retracted by contraction of the retractor bulbi muscle with displacement of the orbital fat.1
Lacrimal and Nasolacrimal Systems
The lacrimal system consists of the orbital lacrimal gland, the gland of the third eyelid, the accessory glands of Krause and Wolfring, the glands of Zeis, the tarsal glands, and the nasolacrimal duct system.1–3 The lacrimal gland lies in the dorsolateral wall of the orbit. The lacrimal gland is innervated by the lacrimal nerve (branch of the ophthalmic branch of the trigeminal nerve) and parasympathetic fibers from the facial nerve nucleus and, to a lesser extent, by sympathetic nerve fibers.4 Two large and four to five small excretory ducts originate from the central surface of the lacrimal gland in both sheep and goats.5 The lacrimal fluid drains into the dorsal fornix of the conjunctival sac and mixes with the secretions of the accessory glands.3 The tarsal glands and, to a small extent, the glands of Zeis produce the outer lipid layer of the precorneal tear film. The orbital lacrimal gland and the gland of the third eyelid produce more than 90% of the middle aqueous component of the precorneal tear film, with a minor contribution from the accessory glands of Krause and Wolfring. The inner mucin layer is produced by the conjunctival goblet cells.1 The three layers of the precorneal tear film are continuously spread across the eye’s surface by the eyelids and nictitating membrane during blinking. Unlike cattle, sheep and goats have lysozyme, an antibacterial enzyme, in their tears.6 Excess tear film pools in the lacrimal lake at the medial canthus. Mechanical pumping action draws the tear fluid into the superior and inferior lacrimal puncta located on the palpebral conjunctiva, just inside the edge of the eyelid and medial to the last tarsal gland.1 The superior and inferior lacrimal puncta continue as the superior and inferior canaliculi. The canaliculi coalesce at the nasolacrimal sac located in the lacrimal fossa of the lacrimal bone.1 The lacrimal sac empties into the nasolacrimal duct, which initially continues rostrally through the osseous lacrimal canal and the osseous lacrimal groove of the maxilla. It then parallels the mucous membrane of the middle meatus and opens on the nasal mucous membrane at the junction of pigmented and nonpigmented skin.5
Globe
The globe (bulbus oculi) is nearly spherical in shape. The average anterior-to-posterior axis of the globe in sheep is 26.85 mm1 and in goats is 24.24 mm.7 The globe is composed of three tunics, or coats: the fibrous, vascular, and nervous tunics. The external fibrous tunic is made up of dense collagenous connective tissue that resists the eye’s internal pressure and gives the globe its round shape. The fibrous tunic is composed of the cornea and sclera, which coalesce at the corneoscleral junction or limbus. The middle vascular tunic is composed of the uvea, which includes the iris, ciliary body, and choroid. The inner nervous tunic includes the retina and optic nerve. The three tunics surround the clear intraocular media: the aqueous humor, lens, and vitreous humor.
Fibrous Tunic
Cornea
The cornea is the most powerful refractive surface of the eye. In sheep and goats, the shape of the cornea is elliptical, with its horizontal diameter greater than its vertical diameter. In sheep, the average width of the cornea has been reported as 22.4 mm8 and 27 mm4; and the average height as 15.4 mm8 and 19 mm.4 The sheep cornea is thickest at its center (0.8 to 2.0 mm) and thinnest at its edge (0.3 to 0.5 mm), as determined on postmortem measurements.1 The cornea is innervated by the long ciliary nerves, which derive from the ophthalmic branch of the trigeminal nerve.
In domestic animals the cornea has four layers: the epithelium, stroma, Descemet’s membrane, and endothelium. The epithelium covers the outermost corneal surface and is continuous with the conjunctival epithelium. The stromal layer makes up 90% of the cornea and is composed of a lamellar arrangement of collagen fibrils with scattered keratocytes. After a deep corneal injury, keratocytes can differentiate into fibroblasts and contribute to scar formation.1 Descemet’s membrane is a clear, acellular membrane on the posterior aspect of the stroma. On clinical probing, it is the deepest layer visible before corneal perforation occurs. The endothelium is a single layer of cells lining Descemet’s membrane. The endothelium has an active pump mechanism responsible for corneal deturgescence.9 Endothelial cell loss or injury results in corneal edema from imbibing of aqueous by the stroma.
Sclera
The sclera makes up the posterior 80% of the fibrous tunic and serves as the primary tissue for support and protection of the intraocular structures. The sclera also is composed of collagen fibrils; however, they are irregularly arranged, and the scleral epithelium is thicker than the corneal epithelium. Scleral thickness at the entry point of the optic nerve in the sheep is 1.0 to 1.2 mm. The sclera thins at the equator to 0.25 to 0.30 mm and thickens at the corneoscleral junction to 0.4 to 0.5 mm.1
Vascular Tunic (Tunica Vasculosa Oculi)
Iris
The iris is the smallest component of the uvea. It is a muscular diaphragm suspended between the cornea and the lens. It is attached to the sclera at its periphery by the pectinate ligaments and to the ciliary body. The iris divides the space between the cornea and the lens into the anterior and posterior chambers of the anterior segment. Its central aspect has an aperture, the pupil, that changes in size to adjust the amount of light entering the eye and reaching the retina. The iris sphincter muscle lies concentrically near the pupillary margin, receives parasympathetic innervation, and functions to cause constriction of the pupil (miosis). The iris dilator muscle has fibers arranged radially from the sphincter to the ciliary border, receives sympathetic innervation, and functions to cause dilation of the pupil (mydriasis). The pupil is oval in a horizontal plane in sheep and goats and has several round, variably sized black masses at the superior and inferior aspects of the pupillary border called granula iridica. The granula iridica are extensions of the posterior pigmented epithelium of the iris. They enhance the effect of pupillary constriction. Iris color depends on the density of the pigmentation (melanin) in the iris stroma.1
Ciliary Body
The ciliary body is the middle portion of the uvea that joins the choroid (posterior uvea) to the peripheral iris (anterior uvea). It consists of an anterior section (pars plicata) and a posterior section (pars plana). The pars plicata consists of radial folds called ciliary processes that are thick with shallow valleys between them in herbivores.10 Zonular fibers insert on the ciliary processes and lens equator to hold the lens in place. The ciliary processes also have well-developed capillary beds and specialized epithelium with an active transport mechanism that produces the major portion of the aqueous humor.1,9 Aside from the ciliary processes, the ciliary muscles make up the main part of the ciliary body. This musculature is poorly developed in ungulates, accounting for their very limited accommodative ability. Evolution has allowed herbivores to develop large corneas, horizontally oval-shaped pupils, and large anterior chambers for better night vision and good motion detection. However, these evolutionary changes also have led to the loss of ciliary musculature development.
Neural Tunic
The neural tunic includes the retina and optic nerve, both derivatives of the forebrain. The retina and optic nerve are the only portions of the brain that can be seen on a physical examination, so observations of these structures can provide clinical information about the animal’s physical status. The retinal blood vessels and, to a small extent, the vitreous provide nutrition for the inner layers of the retina. The inner layer of the choroid (choriocapillaris) provides the outer layers of the retina with nutrients. The retinal metabolic rate is one of the highest in the body, and therefore if either the retinal or choroidal vasculature is even marginally compromised, the retina can become ischemic.1
The retina has 10 layers, the outermost of which is the retinal pigmented epithelium (RPE), and nine inner histologic layers, known as the sensory retina, which include the clinically important photoreceptors and ganglion cells. The RPE is a single layer of cells between the sensory retina and the choriocapillaris. It is nonpigmented in the superior half of the fundus, allowing exposure of the tapetum to light. The RPE has tight interepithelial junctions that form the major portion of the blood-retina barrier, and it removes photoreceptor metabolic waste products.
The photoreceptors include the rods and cones. Rods function in dim light vision. Cones function in bright light and are involved with color recognition and visual acuity. The retina in all ungulates is primarily composed of rods. The rod-to-cone ratio for sheep is 30:1 to 40:1.11 No rod-to-cone ratio has been reported for goats. Both sheep and goats have two types of cones, allowing these animals dichromatic color vision.1 When stimulated by light, the photoreceptors initiate the electrical impulse through the other layers of the sensory retina eventually reaching the ganglion cells. The axons of the ganglion cells make up the optic nerve (cranial nerve II), which then carries the impulse to the optic tracts, optic radiation, and finally the visual cortex of the brain. The area centralis of the retina is the area of maximal cone density, and the visual streak is the area of maximal ganglion cell density. The central retina of sheep is similar to that of other mammals, with an area centralis and a single visual streak. Goats have an area centralis and two visual streaks—a horizontal streak and a vertical streak.12 Sheep and goat retinas have a holangiotic vascular pattern with very prominent vessels, as is typical of ruminants. The term holangiotic means that all quadrants of the retina are vascularized with vessels extending from the optic nerve to the periphery. Sheep retinas have three or four pairs of vessels (artery and vein) in the dorsal, ventral, ventronasal, and ventrotemporal quadrants; and an additional five to eight arterioles and venules radiating from the nasal and temporal portion of the optic disk. Paired vessels may wrap around each other, especially the larger, dorsal vessels. Occasionally the superior arteriole and venule wrap around each other. Goat retinas have three to six arteries (one to three dorsal and two or three ventral) and two or three retinal veins. The dorsal (tapetal) fundus appears more vascularized than the nontapetal fundus owing to the more numerous vessels branching across the tapetum.13
The tapetal fundus is triangular, can be yellow to bluish-purple, and is stippled with the stars of Winslow (end-on choroidal capillaries). The dorsomedial tapetal fundus has more pigment than the other sections. The nontapetal fundus is pigmented owing to presence of pigmentation in the RPE cells in this region. In sheep, the optic disk is located within the nontapetal fundus just ventral to the tapetal-nontapetal junction; in goats, it usually is located in the tapetal fundus just above the tapetal-nontapetal junction. Sheep have a kidney bean–shaped optic disk; goats have a rounder optic disk surrounded by a ring of pigment.14 The small dark depression in the center of the optic disc is the physiologic cup or pit.
Clear Intraocular Media
Aqueous
Aqueous humor is the optically clear fluid within the anterior chamber (between the cornea and the iris) and posterior chamber (between the iris and the lens). Aqueous is produced by the nonpigmented epithelium of the ciliary processes and flows into the posterior chamber, through the pupil, and into the anterior chamber. From the anterior chamber, aqueous can exit the globe by either the conventional or unconventional pathway into the scleral venous circulation. The major portion of aqueous follows the conventional pathway through the iridocorneal trabecular meshwork to the scleral veins. In the unconventional (uveoscleral) pathway, the aqueous diffuses across the iris and ciliary body into the suprachoroidal space (between the choroid and the sclera) and into the scleral veins. The percentage of outflow through the uveoscleral pathway has been determined for many species but not for cattle, sheep, or goats. The continuous production and outflow of aqueous maintain the normal intraocular pressure of the globe. The average normal intraocular pressure in goats has been reported at 7.9 to 11.8 mm Hg in Pygmy goats15 and 13.9 mm Hg in Angora goats.16 The aqueous humor provides glucose, oxygen, amino acids, and electrolytes for nutrition of the avascular cornea and lens and also removes their metabolic waste products.
Lens
The lens further focuses light entering the eye to allow for sharp focus of visualized images. The lens is a transparent, biconvex, almost spherical structure. It is held in position by the zonular ligaments that arise from the ciliary body processes. The lens rests against the iris anteriorly and in the patellar fossa of the vitreous posteriorly. Herbivorous animals have a marginally functional accommodative mechanism and therefore have poor near vision.17
The lens is transparent and avascular and receives the major part of its nutrients from the aqueous humor. The lens grows throughout life at a slow, regulated rate by means of continued division and differentiation of the lens epithelial cells into lens fiber cells. The newest fiber cells are located peripherally and the oldest become the most centralized and compressed lens fibers. As the animal ages, these centrally compressed fibers become a distinct area of nuclear sclerosis that is always bilaterally symmetrical and homogeneous; these changes do not affect vision. The average diameter of the sheep lens is between 14.5 and 15.53 mm; it weighs approximately 2.3 g.1
1. Samuelson D.A. Ophthalmic anatomy. In Gelatt K.N., editor: Veterinary ophthalmology, ed 4, Ames, Iowa: Blackwell Publishing, 2007.
2. Sisson S., Grossman J.D. The sense organs and common integument. In Grossman J.D., editor: The anatomy of domestic animals, ed 4, Philadelphia: WB Saunders, 1953.
3. Dyce K.M., Sack W.O., Wensing C.J.G. The sense organs. In Dyce K.M., Sack W.O., Wensing C.J.G., editors: Textbook of veterinary anatomy, ed 2, Philadelphia: WB Saunders, 1996.
4. Prince J.H., Diesem C.D., Eglitis I., Ruskell G.L. Anatomy and histology of the eye and orbit in domestic animals. Springfield: Charles C Thomas; 1960.
5. Sinha R.D., Calhoun M.L. A gross, histologic, and histochemical study of the lacrimal apparatus of sheep and goats. Am J Vet Res. 1996;27(121):1633.
6. Brightman A.H., Wachsstock R.S., Erskine R. Lysozyme concentrations in the tears of cattle, goats and sheep. Am J Vet Res. 1991;52(1):9.
7. Ribeiro A.P., Miguel L.S., Juliana P.R., et al. Ocular biometry in a colony of Saanen goats with different ages. Proceedings 39th Annu Meet Am Coll Vet Ophthalmol 45. 2008. Boston, MA
8. Martin C.L., Anderson B.G. Ocular anatomy. In Gelatt K.N., editor: Veterinary ophthalmology, ed 1, Philadelphia: Lea & Febiger, 1991.
9. Gum G.G., Gelatt K.N., Esson D.W. Physiology of the eye. In Gelatt K.N., editor: Veterinary ophthalmology, ed 4, Ames, Iowa: Blackwell Publishing, 2007.
10. Duke-Elder S. The eyes of mammals in the eye of evolution. St Louis: Mosby; 1958.
11. Braekevelt C.R. Retinal photoreceptor fine structure in the domestic sheep. Acta Anat. 1983;116(3):265.
12. Gonzalez-Soriano J., et al. A quantitative study of ganglion cells in the goat retina. Anat Histol Embryol. 1997;26(1):39.
13. Galan A., Martín-Suárez E.M., Granados M.M., et al. Comparative fluorescein angiography of the normal sheep and goat ocular fundi. Vet Ophthalmol. 2006;9(1):7-15.
14. Whittaker C.J.G., Gelatt K.N., Wilkie D.A. Food animal ophthalmology. In Gelatt K.N., editor: Veterinary ophthalmology, ed 3, Philadelphia: Williams & Wilkins, 1999.
15. Broadwater J.J., Schorling J.J., Herring I.P., et al. Ophthalmic examination findings in adult pygmy goats (Capra hicus). Vet Ophthalmol. 2007;10(5):269.
16. Whelan N.C., Thompson D. Normal ophthalmic diagnostic test values in Angora goats. Procedings 39th Annu Meet Am Coll Vet Ophthalmol 44. 2008. Boston, MA
17. Ofri R. Optics and physiology of vision. In Gelatt K.N., editor: Veterinary ophthalmology, ed 4, Ames, Iowa: Blackwell Publishing, 2007.
Ophthalmic Examination Techniques for Small Ruminants
Preliminary Considerations
Although special instrumentation and proper restraint are necessary, routine eye examination is not excessively time-consuming and promotes not only familiarity with variations of normal eye appearance but also an appreciation of the variety of ocular lesions encountered in different small ruminant species and breeds. An ophthalmic examination is strongly indicated for all sheep and goats exhibiting obvious or primary ocular or periorbital symptoms. It also should be included as a key component of a routine physical examination and whenever systemic disease is present,1 because the overall appearance of the eyes may reflect the general condition of the animal. Information obtained from an eye examination can be helpful in differentiating among many systemic diseases, leading to a more precise diagnosis and institution of a tailored treatment regimen.
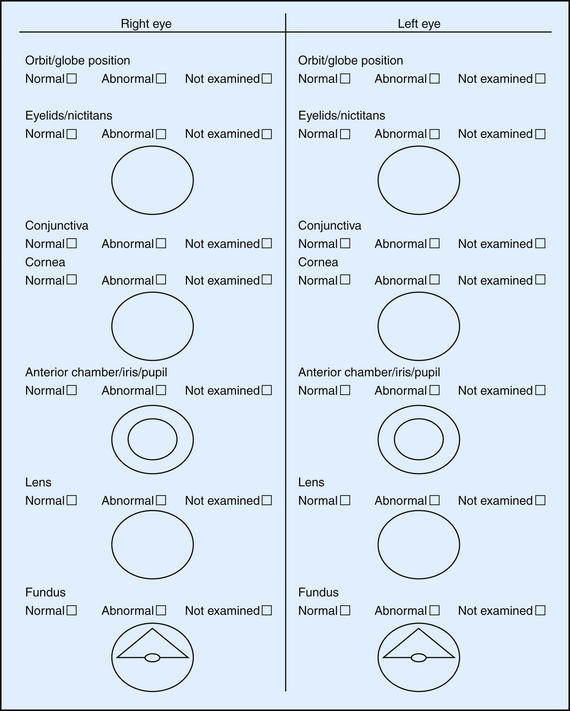
It is imperative to document historical findings, clinical signs and interpretation, final diagnosis, treatment administered, and progression of the condition. Record keeping is invaluable, especially in the management of complicated eye diseases or with patients unresponsive to treatment. Unfortunately, such scenarios are not always anticipated, so every case should be well documented from the very beginning.2 Special forms are helpful in organizing the examination process, allowing efficient recording of all observations in a user-friendly manner (Figure 14-1). Drawing and labeling a diagram of the lesions will facilitate objective evaluation of progress over time.
Getting the History
A thorough ophthalmic history begins with ascertaining the animal’s signalment including age, breed, gender, pregnancy status, and presenting ophthalmic problem. Owners and caretakers should be interviewed about initial signs and symptoms such as presence of ocular pain (blepharospasm, apparent photophobia or epiphora); nature of ocular discharge, if present; change in color or size of the eye(s); visual status in bright versus dim light; and behavioral changes (e.g., isolation from the rest of the flock). Other factors to identify include the duration of the ocular condition, its progression (improving, same, or worsening), and whether it is worse on one side or the same on both sides. Investigate if related (in case of hereditary conditions) and unrelated herd mates also are affected with similar clinical signs. Inquire information about treatment administered and whether it changed the appearance, pain or vision status of the affected eye(s). Next, ask about the environment in which the sheep or goats are housed (e.g., indoor (bedding type, air quality), outdoor (pasture, dry lot, stocking density), exposure to temperature extremes or potential hazards, present and previous diet, vaccination and deworming status including products used and dates, recent or past diseases diagnosed in the flock, existence of previous eye problems, medical therapy administered, response to treatment, and intended use of the animal. Ocular changes can be clues to an animal’s health status, so questions concerning the animal’s ophthalmic problems should be accompanied by inquiries about the animal’s physical condition. The examiner should avoid asking so-called leading questions that may induce the owner to overinterpret observed clinical signs.
Initial Vision Assessment
After taking the history, the examiner should observe the animal’s movements in a small area before beginning the ophthalmic examination. The animal should be encouraged to maneuver around obstacles in bright and dim light. Because sheep and goats have laterally placed eyes, unilateral blindness is less likely to be compensated for by the contralateral eye. An animal often turns its head in an attempt to see in front of it when visual acuity is compromised on one side. If the examiner still harbors doubts concerning vision, each eye can be covered individually for better assessment.
During initial inspection and before restraint, the head carriage, appearance and symmetry of the face, the eyes and periocular region should be first examined from a distance in normal ambient light for obvious gross abnormalities. More specifically, periocular swelling or alopecia, palpebral fissure size, ocular or nasal discharge or dryness, redness or other color changes, corneal clarity and moistness, and size and position (enophthalmia, exophthalmia, strabismus) of the globes in their respective orbit should be noted.1,3 In most cases, enophthalmia is associated with moderate to severe dehydration, but sunken-appearing eyeballs also may reflect loss of periorbital fat in animals with overall poor body condition. During this initial assessment, the animal’s temperament and requisite methods of restraint also are determined.1
Instruments and Supplies for Ophthalmic Examination
Ophthalmologic instruments and supplies should be placed in a portable box or carry-on tote for easy access on field calls. In a hospital setting, the ophthalmic box should be located in a designated area of the examination room, readily available for use. Instruments and supplies recommended for small ruminant ophthalmic examination are listed in Box 14-1.
BOX 14-1 Recommended Instruments and Supplies for Ophthalmologic Examination
Instruments
Direct ophthalmoscope with cobalt blue filter (Welch-Allyn) with Finnoff transilluminator head attachment
20-, 28-, or 30-diopter indirect ophthalmoscopy lens
Dressing forceps with serrated tips, 6-inch, or Graefe fixation forceps (nonlocking)
Lacrimal cannula, 22- or 23-gauge
Kimura spatula or cytobrush or microbrush for preparing cytology specimens
Restraint for Eye Examination
The ophthalmic examination ideally is conducted in dim ambient light, preferably in a darkened room or stall. If this is not feasible, a blanket or dark cloth can be used to cover the animal’s head during examination to evaluate the ocular condition.3 In most small ruminants, this evaluation can be performed using manual restraint of the animal in a standing posture; in sheep, “sitting” the animal on its rump is an alternative means of restraint. Depending on the species, size, and temperament of the animal, placing it in a chute, stand, or crate and application of halter can contribute to optimal immobilization. Use of a simple rope halter helps limit head movement and allows for safe and expedient completion of the task. If the eye is painful, topical proparacaine 0.5% can be used to eliminate superficial (corneal and conjunctival) pain, thereby facilitating the examination. Swabbing for subsequent culture and Schirmer tear testing, if indicated, should be performed before instillation of a topical anesthetic. An auriculopalpebral nerve block may be required for examination of patients exhibiting severe blepharospasm not alleviated by topical anesthetic.
Assessment of Neurophthalmic Reflexes
Before touching the head, the examiner should assess the eyes for symmetry in size and position, note the presence of abnormal ocular discharge, observe the eyelids as they pass over the ocular surface, and record any rubbing, blepharospasm, or other abnormalities. The menace response can be used to evaluate the optic nerve (cranial nerve II) and facial nerve (cranial nerve VII) for presence of vision and ability to blink, respectively. This is an acquired response and therefore may not occur in normal lambs and goat kids younger than 2 weeks of age. In this age group, vision can be better evaluated by observing the animal’s ability to maneuver around obstacles in an enclosed area. After checking for the menace response, the palpebral reflex should be tested to confirm the presence of the ability to blink and to assess the completeness of the blink. The palpebral reflex test is performed by touching the skin around the eye. This test assesses the trigeminal nerve (cranial nerve V) and facial nerve (cranial nerve VII).
Detailed Ophthalmic Evaluation
The conjunctiva should not be hyperemic, thickened, or edematous (indicating chemosis). Examination for hemorrhage, foreign bodies (especially beneath the nictitating membrane), and lymphoid follicle hyperplasia is indicated. Samples from the conjunctiva for culture and sensitivity testing, cytologic analysis, immunofluorescent antibody (IFA) testing, polymerase chain reaction (PCR) assay, and biopsy can be obtained in physically restrained animals after the application of topical anesthetic solution. Fluorescein dye should not be applied before sample collection for IFA testing because it may cause a false-positive result.4
The slit beam of a direct ophthalmoscope can be used to assess the depth of a corneal ulcer by how deeply the beam is projected on the ulcer. If the ulcer is deep and fluorescein dye uptake is not evident, a descemetocele is likely. With a perforated corneal ulcer, aqueous humor may be seen draining from the perforation, or the iris or fibrin may occlude the perforation. Such ulcers should not be manipulated, and minimal diagnostic testing should be performed, because surgical intervention is the treatment of choice.
The anterior chamber is evaluated for clarity and depth. Damage to the blood-aqueous barrier allows protein and cells into the aqueous humor, creating turbidity or the Tyndall effect (aqueous flare). The slit beam or the smallest circle on a direct ophthalmoscope can be used to identify aqueous flare. The beam of light is focused directly on the cornea, which is then observed at 90 degrees to the direction of the beam as it passes through the anterior chamber. The light should not be visible passing through the anterior chamber. Aqueous flare is seen when protein and cells absorb light and the light beam is visible passing through the aqueous humor. A shallow anterior chamber can be caused by a perforating corneal injury, anterior lens subluxation, intumescent cataract, iris mass, or iris bombé, characterized by a 360-degree posterior synechia (resulting in complete adherence of the pupil to the lens), with the peripheral iris bowing forward. A deep anterior chamber can be caused by buphthalmia (enlargement of the globe from glaucoma), posterior lens luxation, or hypermature resorbing cataract.5
Intraocular pressure (IOP) in most species is between 15 and 25 mm Hg. The average IOP reported in Corriedale sheep was 10.61 ± 1.4 mm Hg (range, 9 to 13 mm Hg) as measured using a Perkins applanation tonometer.6 The average intraocular pressure in caprine species has been reported at 7.9 to 11.8 mm Hg (range, 6 to 14 mm Hg) using a Tonovet rebound tonometer and 10.8 mm Hg (range, 8 to 14 mm Hg) using a Tonopen applanation tonometer in Pygmy goats.7 In Angora goats, mean IOP was reported at 13.9 mm Hg (range, 8 to 20 mm Hg) using a Tonopen applanation tonometer.8 The most common tonometers used in veterinary medicine are the Tonopen applanation tonometer and the Tonovet rebound tonometer. Both are easy to use in sheep and goats. With the Tonopen, topical anesthesia is required before the examiner gently touches the cornea several times to obtain a computer-averaged pressure reading. The Tonovet does not require topical anesthesia but has a more precise requirement for head position so that the tonometer probe is parallel to the ground. The probe assesses IOP at the corneal surface several times for a computer-averaged pressure reading, but the examiner does not have to touch the cornea directly. Proper technique is critical for accurate IOP readings. The examiner must avoid any pressure on the globe by placing a finger on the dorsal orbital rim and on the ventral orbital rim to stretch the eyelids open. The person restraining the animal must avoid holding the neck, because any pressure on the jugular veins will increase IOP. A high reading is consistent with glaucoma. The lens, vitreous, and fundus are best evaluated through a dilated pupil. The pupil should be dilated with a short-acting topical parasympatholytic such as 1% tropicamide. Time to effect for tropicamide is 10 to 20 minutes, and the effect lasts between 4 and 8 hours.5 The lens should be evaluated for position and clarity. Nuclear sclerosis is a normal aging change that does not preclude evaluation of the fundus but must be differentiated from cataract. It will appear as a bilaterally symmetric, homogeneous, slight grayness to the center (nucleus) of the lens.
A fundic examination can be performed by either direct or indirect ophthalmoscopy. Direct ophthalmoscopy is performed at a distance of 2 to 3 cm from the patient’s eye. The large circle is used when the pupil is dilated, and the smaller circles are used when the pupil is not dilated. The instrument is set at either 0 or the red (negative diopter) 2 to begin the examination. The numbers are sequentially changed to bring a lesion into focus depending on its location. If a lesion is posterior to the plane of the fundus, it will be in focus at a more negative diopter setting; a lesion anterior to the plane of the fundus will be in focus at a more positive (green) diopter setting. The disadvantages of direct ophthalmoscopy include the small field of view (approximately 2% of the entire fundus), difficulty in examining the peripheral fundus, and limited ability to see through any opacification of the clear media. The advantages of direct ophthalmoscopy include greater magnification and the ability to alter the dioptric strength of the ophthalmoscope.5
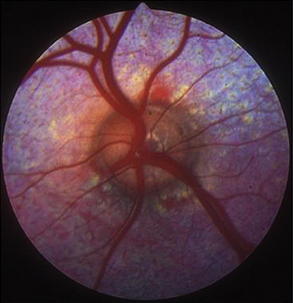
Indirect ophthalmoscopy requires use of a focal light source, such as the Finnoff transilluminator, to be held adjacent to one of the examiner’s eyes and an indirect lens (held at arm’s length) positioned 2 to 4 cm in front of the patient’s eye after the tapetal reflex has been identified. A relatively inexpensive, 20- to 30-diopter, ophthalmic indirect lens is effective. The image seen is virtual but is inverted and reversed. The advantages of indirect ophthalmoscopy include a larger field of view (approximately 40% of the entire fundus depending on the strength of the lens), which allows the peripheral fundus to be examined more completely; stereopsis (use of both of the examiner’s eyes) for depth perception; and the ability to see through mild to moderate opacification of the clear ocular media. Disadvantages include the need for a relatively dilated pupil. Examination in a darkened room along with use of a dimmed light source often allows fundic evaluation without dilation in herbivores through their horizontally oval pupils. Another limitation of indirect ophthalmoscopy is that it requires practice for the examiner to become proficient with the technique. In examining the fundus by indirect ophthalmoscopy, movement of the examiner’s head should follow in the direction that is to be visualized within the fundus. The examiner should have a pattern for examining the fundus beginning with the optic nerve, dividing the fundus into quadrants, and examining each one by evaluating the vessels and color of the tapetal and nontapetal fundi (Figures 14-2 and 14-3).
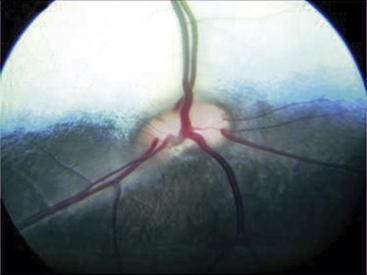
Figure 14-2 Normal fundus of a sheep.
(Courtesy Dr. Paige Evans, Leesburg, Virginia/Annapolis, Maryland.)
Auriculopalpebral Nerve Block
The auriculopalpebral nerve is a branch of the facial nerve and provides motor function to the eyelids. It can be palpated along the dorsal margin of the zygomatic arch. Local anesthesia of the auriculopalpebral nerve results in flaccid eyelids, which facilitates manipulations of the lid and examination of the cornea and conjunctiva, especially in a painful eye. When combined with topical anesthesia (0.5% proparacaine), local anesthesia helps with removal of foreign bodies, cytology, flushing the lacrimal puncta, and subconjunctival injections. The auriculopalpebral nerve block is performed by first palpating the nerve over the zygomatic arch and then tenting the skin over the nerve to insert a 22- or 25-gauge needle to the hub. A 3-mL syringe with 2% lidocaine is then attached, and 2 to 3 mL of lidocaine is infiltrated subcutaneously around the nerve.9 Local anesthesia of the eyelids may be accomplished with up to 4 to 5 mL of anesthetic infiltrated into each eyelid quadrant. In small ruminants, the amount of lidocaine 2% used for local anesthetic purposes should remain below the toxic dose (5 mg/kg).
Special Diagnostic Procedures
Corneoconjunctival Bacterial Culture
Reports describing the normal conjunctival flora of sheep and goats are scarce. In clinically normal sheep, 60% of eye swabs were negative for bacterial growth.10 In sheep, the most commonly isolated bacteria were similar to Branhamella ovis (previously Neisseria ovis) and were recovered in low numbers. Other frequently isolated organisms were Micrococcus and Streptococcus species. Less common isolates included Corynebacterium, Acinetobacter, Bacillus, Staphylococcus, Pseudomonas, Moraxella, Escherichia coli, and Pasteurella.1,10,11 Moraxella bovis is not a cause of infectious keratoconjunctivitis in goats.11 However, conflicting reports indicate the possible pathogenicity of Moraxella in ovine keratoconjunctivitis.12,13
Technique
After gentle retraction of the upper and lower eyelids, the affected conjunctiva or cornea selected area is swabbed using a standard tip or micro tip bacterial culturette (e.g., Copan Transystem swab or BBL CultureSwab, respectively, both made by Copan Diagnostics, Inc., Corona, California). Care should be taken to avoid contamination from the palpebral margins. The sample should immediately be placed in the transport tube or plated onto an appropriate growth medium. Corneoconjunctival swabbing ideally should be performed before application of topical anesthetic or fluorescein stain to the eyes. Topical anesthetic solutions have been reported to have antibacterial effects.1 Once collected, the sample is submitted to a veterinary microbiology laboratory, with care taken to specify appropriate culture conditions (especially with Mycoplasma spp.) for the presumptive clinical diagnosis. Anaerobic bacteria and fungi are rare ocular pathogens in sheep and goats.11 Fungal cultures usually can be obtained using standard culturettes; however, scraping the tissue with a sterile Kimura cytology spatula and placing the sample directly onto fungal culture media may provide even better results.11
Corneoconjunctival Cytology
Conjunctival cytologic evaluation is valuable for the diagnosis of infectious keratoconjunctivitis. Corneal cytologic examination is useful in characterizing cellular infiltrates such as for bacteria, fungi, and type of inflammatory cells. Cell samples obtained from conjunctival scraping also can be submitted for PCR testing. Before the procedure is undertaken, the cornea and conjunctiva should be anesthetized with topical 0.5% proparacaine; an auriculopalpebral nerve block may be beneficial. If conjunctival follicles are present, these areas should be avoided to obtain a more representative sample. Cytologic samples are obtained either by gently scraping the tissue with a spatula or by using a cytobrush (e.g., Care Express Products, Inc., Cary, Illinois) or regular-size Microbrush (Microbrush International, www.microbrush.com), which is simply rolled over the affected tissue. The cytobrush is inexpensive and very easy to use and creates slides with evenly distributed cells with little crush artifact. Cytologic samples should be very gently rolled or smeared onto a slide and allowed to dry somewhat for 1 or 2 minutes before staining with Diff-Quick or Wright’s or Gram’s stain.11,13,14 At least one slide should always be left unstained for submission to a veterinary clinical pathologist if required. Viral, mycoplasmal, or chlamydial organisms are poorly identified by Gram staining.11
Healthy conjunctiva is characterized cytologically by numerous epithelial cells, occasional lymphocytes, and rare neutrophils. Intracytoplasmic melanin granules may be observed in dark-faced breeds and can be mistaken for bacteria or chlamydial elementary bodies. Goblet cells are more common in animals with subacute to chronic keratoconjunctivitis but can be a normal finding in corneal or conjunctival cytologic preparations. Neutrophils are the predominant cell type seen in acute conjunctivitis, especially of bacterial or viral origin. A few mononuclear cells, multiple bacteria, and degenerating epithelial cells also should be present. Lymphocytes and plasma cells are more typical to chronic infection. Presence of small numbers of eosinophils in otherwise normal sheep probably indicates a local reaction to environmental irritants.15
Nasolacrimal Flushing
Signs of nasolacrimal duct obstruction may include moderate to severe epiphora (wet face appearance) that persists for days to weeks or accumulation of mucus or purulent material, in the absence of obvious ocular lesions or mild conjunctivitis, especially medially. Presumptive diagnosis is made on the basis of delayed or absent passage of fluorescein stain from the lacrimal puncta to the nasal punctum. For normograde flushing of the nasolacrimal duct, after application of topical anesthetic (0.5% proparacaine or 2% lidocaine) a 5-mL syringe is filled with buffered saline or eyewash solution, and a nasolacrimal canula or blunted, smooth 20- or 22-gauge stainless steel hypodermic needle is used to canulate the dorsal and ventral lacrimal puncta, near the medial canthus.1 Fluorescein stain can be added to the irrigating solution to aid visualization of the fluid passage. In larger patients, a small canine urinary catheter or 3.5F flexible polypropylene catheter (e.g., Tom Cat Catheter, Sherwood Medical Industries, Inc., St. Louis, Missouri) may be used for normograde and retrograde flushing. In ruminants, the nasal orifice of the nasolacrimal duct is located caudolateral to the alar fold.
Imperforate lacrimal puncta, congenital atresia of the nasal puncta, or agenesis of the distal nasolacrimal duct should be suspected in young animals presented with bilateral nasolacrimal duct obstruction. Surgical intervention and management should follow published guidelines for small animals.3
Imaging Techniques
Radiography
Plain radiographs of the skull in dorsoventral, lateral, anterior-posterior, and oblique views may reveal disease processes in and around the orbit, paranasal sinuses, tympanic bulla, and maxillary teeth.3 Radiographic examination of the small ruminant bony orbit can be technically difficult and may or may not yield diagnostic images. However, it can be helpful to identify fracture, osteomyelitis with or without bony sequestrum, soft tissue swelling, and radiopaque foreign bodies in the ocular or periocular area.16
Contrast radiography can be performed after plain radiographs have been obtained. This technique is indicated mainly for performance of dacryocystorhinography, in which a contrast agent is injected into the nasolacrimal system to achieve radiographic visualization in a patient that is heavily sedated or, ideally, under general anesthesia. This study may be useful for evaluation of patients experiencing chronic or recurrent dacryocystitis, and for localization of foreign body (typically a grass awn), soft tissue mass, or stricture within the nasolacrimal system. Two to eight mL of contrast medium should be slowly infused through the lacrimal puncta. Lateral and dorsoventral radiographs are sufficient for evaluation of the condition. If a partial obstruction is present, the ipsilateral nasal punctum should be occluded during the infusion, with release of occlusion just before the radiographs are exposed.
Ultrasound Examination
Ultrasonography is particularly useful to examine intraocular structures that are not visible on the ophthalmic examination and to view the retrobulbar orbital space. Intraocular tumors, some foreign bodies, retinal detachment, and retrobulbar masses or abscesses can be detected using this modality. Ultrasound guidance also can be used for fine needle aspiration of orbital and ocular lesions.3 A small, 7.5- or 10-MHz probe in B-mode is recommended because it provides a two-dimensional cross section of the eye.3
Treatment Techniques
Cleaning the Eyes and Periocular Tissues
Proper cleaning of the eyes often is done inadequately, usually owing to a lack of a suitable eyewash bottle.2 A 6- or 8-ounce plastic goosenecked bottle that produces a sizable stream of buffered saline to the eye is an inexpensive choice for this purpose. Commercially available eyewash squirt-type bottles also can be used. In all cases, the examiner must avoid aiming the stream at the cornea and holding the bottle tip too close to the eye surface, because either may lead to damage of the cornea if the animal moves suddenly. Likewise, the tip should not touch any ocular or periocular tissues, tears, mucus, exudate, or blood owing to the risk of aspirating contaminated fluids back into the eyewash bottle. Mucoid or purulent ocular discharge can be wiped off from the conjunctiva or lid margins with a dry gauze pad or lightly moistened cotton ball. Care must be taken to avoid abrasion of the cornea during this process.
Topical Medications
Administration of topical eye medications in small ruminants is relatively easy to accomplish when proper restraint has been obtained but can be challenging in a noncooperative patient. In most cases, ophthalmic ointments and solutions can be applied to the eye surface directly from the tube or bottle. An alternative method of application is by use of a clean gloved fingertip to smear a ½- to 1-inch strip of ointment across the medial canthus or one of the lid margins.2 Use of ophthalmic ointments should be avoided if a deep corneal ulcer or corneal perforation is suspected, because such preparations are very irritating to intraocular structures. Liquid medications are administered using one hand below the mandible to direct the patient’s nose upward while the thumb holds the lower lid open. The upper eyelid is retracted with the back of the second hand as it rests on the patient’s head while holding the medication at a distance of 2 to 4 cm from the eye surface. A single drop is then instilled. The purpose of placing the hand on the animal’s head is to allow for simultaneous movement with the animal’s head and decrease the likelihood of contamination or iatrogenically induced ocular trauma from contact of the bottle with the patient’s eye.
Subpalpebral Ocular Lavage System
For safe and reliable delivery of ophthalmic solutions, a single-hole subpalpebral ocular lavage system (SPL) can be a valuable means of providing topical ocular therapy, especially in a patient with severe ocular disease requiring medication as often as every hour,2 or with a very painful eye in which direct manipulation and treatment of the eye can be expected to be difficult. The lavage apparatus is commercially available (Eye Lavage Kit, Mila International, Inc., Erlanger, Kentucky) or can be easily fabricated from polyethylene tubing. Homemade fabrication of an SPL entails cutting a 90-cm-long piece of #190 polyethylene tubing and creating a footplate flange by warming the tube extremity over a match flame until it is softened and then pressing it gently against the flat surface of a scalpel blade.17
The patient can be sedated before placement of an SPL, if necessary. An auriculopalpebral nerve block as previously described will ease the procedure. The skin at the exit point of the needle tip, in the area of the dorsal to dorsolateral orbital rim, is aseptically prepared and locally anesthetized by infiltrating 2 mL of 2% lidocaine subcutaneously at the site the needle will exit. Using a 22-gauge needle hub (obtained by breaking off the needle while leaving the hub attached), 2 mL of 2% lidocaine is sprayed into and across the dorsal conjunctival fornix. The lavage tubing is secured into a hubless needle. The needle-tubing unit is held in one sterilely gloved hand; the needle tip securely positioned along the veterinarian’s index finger. Before insertion in the palpebral fissure, the veterinarian’s hand is turned such that the index fingernail lies between the cornea and the needle.17 The operator’s opposite hand should lift the upper eyelid away from the cornea. The finger and needle are then pushed dorsolaterally through the palpebral conjunctiva of the upper eyelid until the needle is touching the inside of the orbital rim. Once properly positioned, the needle is fully inserted through the upper eyelid, just rostral to the orbital rim, and the lid is released. The tubing is gently pulled through until the footplate reaches its destination in the dorsal conjunctival fornix. An alternative placement in the lower, medial conjunctival fornix between the eyelid and nictitans has been described in the horse.18
Medications should be given individually as combining some drugs cause precipitates in the tubing19 and risks diluting the effectiveness of medications. Minor lid swelling can be expected within 48 hours after SPL placement. The swelling must be differentiated with migration of the footplate subconjunctivally which occurs more commonly with small home-made footplate. Tearing in the lavage tubing as well as loss of the injection cap also can occur. A tubular stockinette can be placed and fitted around the head of the patient to protect the tubing from getting caught on objects and subsequently torn. Improper placement of the lavage system or loosening of the butterfly sutures can result in corneal ulceration as a consequence of rubbing of the footplate on the cornea. It is therefore essential to check for and correct such technical problems: loose tubing should be tightened and an improperly placed SPL must be removed promptly.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree