Chapter 13 Diseases of the Neurologic System
Examination of the Neurologic System
Complete Neurologic Examination
Assessment of Chief Complaint
The clinical history is an important step in the diagnosis of neurologic disease. Information related to onset, duration, and progression of the chief complaint can assist with an etiologic diagnosis (What is the cause?) after the anatomic diagnosis (Where is the lesion?) has been made. In collecting historical information, it is important to determine the nature of the first clinical signs but also to define the relationship between the severity of clinical signs with respect to time (as on a sign-time graph). Some neurologic diseases occur acutely, with all clinical signs apparent within hours. Traumatic, toxic, infectious, and metabolic diseases can manifest with this pattern, whereas degenerative, neoplastic, or some viral disorders may develop more slowly, requiring days to weeks before the full complement of clinical signs becomes apparent. In addition to specific information related to the chief complaint, information on diet, housing, gestational status, and vaccination and deworming regimens should be part of the information gathered from the client. In interviewing clients for historical information, ambiguous or leading questions should be carefully avoided, because the information thus obtained may be inaccurate.
Mentation
Evaluation of mentation can assist the clinician in differentiating intracranial from extracranial disease processes. As described previously, some systemic diseases will result in depression without nervous system pathology. During the period of initial observation, the animal’s mental status and behavior can be assessed, but this must be done when the animal is not stimulated. For animals to be alert and oriented, the cerebral cortex and the ascending reticular activating system must be functioning properly. The ascending reticular activating system makes up the major portion of the brain stem parenchyma and is responsible for arousal and sleep-wake transitions in animals. Consequently, disorders involving the ascending reticular activating system can cause somnolence. The ascending reticular activating system is composed of several neuronal circuits connecting the brain stem to the cortex. External stimuli, such as light, touch, sound, smell, and temperature, help to maintain consciousness. An animal should appear as sensitive to its environment as its herdmates. If removed from its usual environment, the normal animal will be alert and cautious of new situations and aware of the examiner. The animal should follow the examiner’s movement with its head, eyes, and ears. All animals should avoid painful stimuli. Abnormal mentation in ruminants can be placed into one of the following categories: (1) excitement, mania, or hyperesthesia; (2) seizures; (3) depression; (4) aimless circling, stupor, and coma; (5) abnormal vocalization; and (6) blindness.1 Stupor is characterized as a condition of unresponsiveness to environmental stimulation such as light and sound, with rentention of response to painful stimuli. By contrast, a comatose animal is nonresponsive to either environmental or painful stimulation.
Gait and Posture
Posture typically is evaluated with the animal at rest in a comfortable position and unrestrained. Head, neck, trunk, and limb posture should be assessed and abnormalities identified. Head tilt, rotation of the neck and thoracic, and wide-based stance are examples of abnormal head, neck, trunk, and limb posture, respectively. A “base-wide” stance can be caused by lesions within the vestibular system, cerebellum, or spinal cord. The inverse posture, or “base-narrow” stance, can result from muscle weakness due to peripheral nerve disease, abnormalities of neuromuscular junctions, or disorders of the skeletal muscles. Spasticity is a condition of increased tone of skeletal muscles producing abnormal limb posture. Abnormal distribution of weight to one side should be noted, because this finding can indicate either weakness of ipsilateral extensor muscles from a peripheral nerve disorder or increased tone of contralateral extensor muscles, which would indicate a UMN lesion.2
Assessment of Cranial Nerves
Cranial Nerve II (Optic Nerve): Menace Response
Vision is the function of CN II. The nerve transmits sensory information from electrochemical receptors in the retina to the visual cortex in the occipital lobe of the cerebrum. The visual pathway (Figure 13-1) is an afferent pathway and consists of an extraparenchymal portion (retinas, optic nerves, optic chiasm) and an intraparenchymal portion (optic tracts, lateral geniculate nucleus in the thalamus, optic radiations, visual cortices). In ruminants, 90% of optic nerve fibers cross at the chiasm to enter the contralateral optic tract; this arrangement has important implications in evaluating lesions of the visual pathways. During assessment of the chief complaint, the client may report that the animal appears blind, but an important consideration is that depressed or somnolent animals or animals with loss of balance due to cerebellar or vestibular disease can stumble and appear blind.
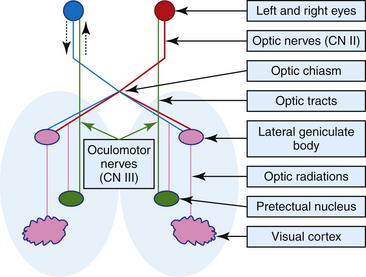
Figure 13-1 Schematic of the simplified pathways of vision and pupillary light reflex (see text for details).
The menace response evaluates the entire visual pathway, CN VII, and the cerebellum. This test assesses a response, rather than a reflex, because the response involves the cerebrum and thus is a learned response. The presence of a response or its magnitude parallels the maturity of the cerebellum—thus a reduced response is detected in kids and lambs less than a week of age. A diminished or absent menace response also can be observed in animals that are severely depressed or have cerebellar disease or CN VII lesions. The normal response is characterized by an eyelid blink, ocular retraction, and head aversion as the examiner rapidly moves a finger toward the eye from a rostral direction. It is important to limit air movement toward the eye and not to touch the eye or adnexa, because such maneuvers may result in a response in animals with intact facial sensation (CN V). Alternatively, the examiner can drop an object into the animal’s visual field from above, which should elicit the menace response from the animal. This method is considered imprecise, with a stimulus that is too slow, for adequate evaluation of this reflex in ruminants. When a visual deficit is observed during the menace response or obstacle test, pupillary light reflexes are tested to assist in localizing the lesion and in characterizing the blindness as central or peripheral.
Cranial Nerve III (Oculomotor Nerve): Pupillary Light Reflex
The oculomotor nerve contains parasympathetic fibers responsible for constriction of the pupils and motor fibers which influence movement of the eye. The sympathetic nervous system is responsible for pupil dilation, so as a result of such stimulation, stressed and frightened animals can have dilated pupils. The pupillary light reflex assesses CN II and CN III. The afferent pathway for pupillary constriction during light stimulation follows a similar pathway as the afferent pathway for vision (optic nerve, optic chiasm, and optic tract); however, before reaching the synapse in the lateral geniculate nucleus in the thalamus, nerve fibers associated with the pupillary light reflex diverge from those of the optic tract and synapse on the pretectal nucleus, which sends a majority of its neurons to the contralateral oculomotor nucleus (see Figure 13-1). This forms the basis for the direct pupillary light reflex. The pretectal nucleus also sends some of its neurons to the ipsilateral nucleus of CN III; this neuroanatomic arrangement forms the basis for the indirect or consensual pupillary light reflex. Before performing the pupillary light reflex test, the examiner should assess the pupils for size at rest and symmetry and check the eyes for the presence of primary ocular disease. The normal sheep or goat will often have large pupil diameters owing to sympathetic stimulation from fear. Ideally, animals are moved to a dimly lit location so that external light does not influence the examination. Pupils that are very small are considered miotic, and dilated pupils are mydriatic. Occasionally, inequality in pupil size may be observed, but if the size difference is not extremely pronounced, this may be a normal finding for the animal. Severe asymmetry is termed anisocoria. To assess for irregularity in pupil size, the clinician can move the animal from a dark to a bright area. Although a sympathetic lesion will prevent the affected pupil from dilating in the dark, lesions of CN III (parasympathetic nerve) will prevent the pupil from constricting in bright light. To assess the pupillary light reflex, a strong light source should be used to overcome sympathetic pupil dilation. The light beam is directed into one eye in a nasotemporal direction toward the temporal region of the retina. The direct response should be constriction of the examined pupil, and the opposite eye also should constrict as a result of the consensual pupillary reflex, although this is difficult to assess by a single examiner. If the intraparenchymal visual pathways are affected by a neurologic disorder (central or cortical blindness), the menace response will be absent on the side contralateral to the lesion, but the pupillary light reflexes will be intact. With involvement of the extraparenchymal visual pathway (retina, optic nerve, optic chiasm), blindness on the side of the lesion is characteristic, and the pupillary light reflexes will be abnormal.
Cranial Nerves III (Oculomotor Nerve), IV (Trochlear Nerve), and VI (Abducent Nerve): Movement of Eye
The clinician should first examine the eye position in relationship to the head at rest and note if strabismus (abnormal position of the eyeball) exists. Strabismus can be the result of damage to the nerves or the muscles they innervate. Further evaluation of the motor function of CNs III, IV, and VI can be performed by moving the animal’s head. Sheep and goats should drop the eyes as the head is lifted. When the nose is elevated, the eyes tend to maintain a horizontal axis, and ventral strabismus becomes apparent. Slow, lateral (horizontal) motion of the head should cause the animal’s eye to try to remain focused straight ahead, with the result that the eye moves slowly in the opposite direction of head movement. However, as the head continues to turn, vestibular influences will then move the eye quickly in the same direction. This movement pattern is referred to as physiologic nystagmus, or normal inducible vestibular nystagmus, and indicates normal function of extraocular muscles, the vestibular system, and CNs III, IV, and VI and their connections in the medial longitudinal fasciculus. Clinical assessment for lesions affecting CNs III, IV, and VI can be performed by moving the animal’s head and observing the ocular position. Cerebellar and vestibular diseases also produce nystagmus, but the strabismus changes whenever the head and neck are moved. With paralysis of CN III, IV, or VI, the strabismus should be present with all positions of the head. Lesions involving CN III can result in ipsilateral ventrolateral strabismus and mydriasis, usually without vision loss in either eye. In addition, CN III is responsible for innervation of the levator palpebrae muscle, but ptosis (eyelid droop) occurring as a result of CN III lesions is not commonly observed in sheep or goats, because the frontalis muscle can lift the upper eyelid. Lesions in CN IV can result in ipsilateral, contralateral, or bilateral dorsomedial strabismus. Bilateral dorsomedial strabismus occurs in several diffuse encephalopathies in sheep and goats such as polioencephalomalacia (PEM) and listeriosis, but whether this abnormality is the result of a true bilateral lesion involving the CN VI nucleus is unclear.2 Lesions involving CN VI result in ipsilateral medial strabismus with a more forward positioning of the eye. In addition, failure to retract the globe may be noted during assessment of the palpebral reflex. This is not entirely specific to CN VI, because eyeball retraction also may require function of all extraocular muscles, including those innervated by CN III and CN IV.
CN V (Trigeminal Nerve): Corneal and Palpebral Reflexes
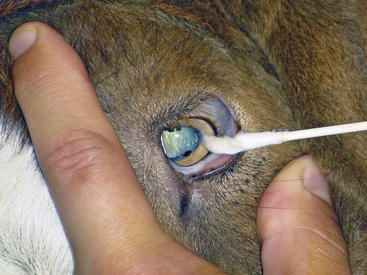
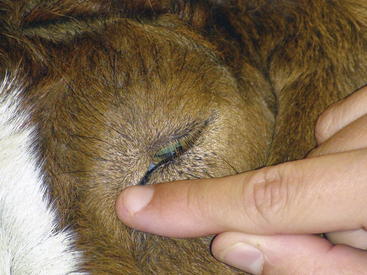
Function of the sensory branches is tested by corneal and palpebral reflexes (Figures 13-2 and 13-3) and assessing sensation across multiple areas of the face. In the palpebral and corneal reflexes, CN V is the afferent (sensory) portion, whereas CN VII is the efferent (motor) portion of the reflex. The corneal reflex is performed by slowly advancing a finger or cotton swab toward the animal’s eye and placing it directly on the cornea. The palpebral reflex is performed by touching a finger on periococular skin without the animal’s visualizing the finger. The corneal reflex and touching the medial canthus of the eye for the palpebral reflex assess the ophthalmic branch of CN V, which innervates the eye and surrounding skin and is responsible for the maintenance of corneal epithelium. The maxillary branch of CN V can be assessed by touching the lateral canthus of the eye during elicitation of the palpebral reflex. The normal reflex response with intact CN V, CN VI, and CN VII is closure of the lid, retraction of the eye, and aversion of the head, respectively. The mandibular branch of CN V can be assessed by touching the ear base and observing for closure of the lid. A deficient palpebral reflex with a normal menace response suggests a lesion in the trigeminal nerve or ganglion. Loss of CN V innervation to the corneal epithelium can result in neurotropic or exposure keratitis, because the affected animal cannot sense corneal dryness or the presence of ocular foreign bodies.
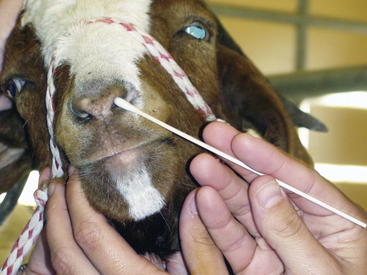
Damage to any branch of the trigeminal nerve results in sensory losses to the areas it innervates. Deficits in CN V function will manifest as the ipsilateral loss of sensation over the face and affected animals will not reflexly blink or twitch the face. This is a subcortical reflex and does not require conscious input. The consciously mediated, coordinated movement of the head away from the noxious stimuli is assessed by stimulating the nasal septum. The examiner applies stimulation using a finger or cotton swab to the inner (medial) surface of the nasal septum (Figure 13-4). The response in a normal animal is blinking and facial twitching; the head is pulled away in response to a painful stimulus. This response requires conscious recognition of the noxious stimulus by way of CN V maxillary nerve to the contralateral parietal cortex. This determination is important, because an animal with a CN V maxillary branch abnormality will have neither sensation nor conscious recognition of pain, whereas a sheep or goat with a contralateral cerebral cortical lesion will have normal sensation but no conscious recognition of the painful stimuli.
Cranial Nerve VII (Facial Nerve): Facial Expression, Other Brain Stem Function
The facial nerve (CN VII) is predominantly a motor nerve, providing innervations to muscles responsible for facial expression, but CN VII also contains parasympathetic nerve fibers that provide innervations to the lacrimal gland, and mandibular and submandibular salivary glands. The CN VII neurons supplying innervations to the muscles of facial expression are located within the brain stem. Assessment of CN VII motor function is performed through the menace response test and eliciting the corneal and palpebral reflexes as discussed previously. Symmetry and posture of the eyelids, ears, and lips are important to evaluate; abnormal findings can provide initial evidence of CN VII dysfunction. Goats and sheep of breeds with erect ears should hold them upright, whereas those with pendulous ears should be able to move the base of the ear canal to follow external stimuli. In goats and sheep with compromised CN VII motor function, eyelid droop (ptosis), lack of ear movement, ear droop, and deviation of the nasal filtrum can be observed. CN VII is most easily assessed by the menace response and palpebral reflex. Simultaneous loss of the menace response and the palpebral reflex, characterized by a failure to blink rapidly and completely, suggests a lesion in CN VII innervations to the orbicularis oculi muscle.3 The animal’s vision will be intact when lesions are limited to CN VII. CN VII dysfunction results in protrusion of the tongue on the affected side of the mouth, and the animal may drool. Feedstuff often is found packed into the cheek pouch on the affected side. Damage to CN VII can be localized according to the clinical signs.
CN VIII (Vestibulocochlear Nerve): Head Tilt and Other Reflections of Vestibular Function
The cranial nerve VIII has two main divisions: vestibular and cochlear. The vestibular division is responsible for maintaining the position of the head and other structures relative to gravity; the cochlear division functions in hearing.4 The objective assessment of hearing loss in large animals is difficult and requires the use of electrodiagnostic testing (brain stem auditory evoked response). Animals that have bilateral hearing losses may be easier to assess because they do not respond to loud environmental noises.
Cranial Nerves IX (Glossopharyngeal Nerve) and X (Vagus Nerve): Laryngeal and Pharyngeal Function
The glossopharyngeal nerve, or CN IX, carries motor and sensory fibers to and from the rostral pharynx, palate, larynx, and tongue. The glossopharyngeal nerve also contains a parasympathetic component that innervates the parotid and zygomatic salivary glands. The vagus nerve, or CN X, provides motor innervation to the pharynx, larynx, palate, and striated muscles of the esophagus by the recurrent laryngeal nerve. The parasympathetic branch of CN X arises from the vagal nucleus in the medulla and innervates the abdominal and thoracic viscera, with the exception of the pelvic viscera. Damage to CN IX and CN X results in clinical signs related to laryngeal and pharyngeal function. Affected animals have difficulty swallowing and may drool from an inability to swallow saliva. Choke also may be observed. The gag reflex can be used to assess normal function. In normal animals, placing a tongue depressor in the back of the mouth elicits the gag reflex, in which the caudal portion of the tongue pushes the tongue depressor forward. The clinician should always wear gloves when examining a small ruminant with suspected CN IX or X disease, because oropharyngeal paralysis is common in rabid animals. Inspiratory stertor may be heard as a result of unilateral or bilateral paresis of the pharynx and larynx. Animals with pharyngeal paralysis can regurgitate food through the nose. In sheep and goats, disease of CNs IX and X is rare.
Spinal Reflexes
Assessment of spinal reflexes tests the integrity of the lower motor neuron (LMN) but also can provide some information on influences of the upper motor neurons (UMNs) on the LMN (Table 13-1). The UMNs are a group of neurons that do not physically exit the nervous system and provide stimulatory or inhibitory influences to the LMN. The LMNs are composed of the peripheral nerves and the effector organs (primarily skeletal muscles). Several responses can be observed when spinal reflexes are tested. A normal response can be observed, which indicates normal sensory and motor components of the reflex arc. An exaggerated response often is observed with UMN pathway abnormalities. A diminished or absent response indicates LMN disease in either its sensory or motor components. In addition to diminished responses, animals with LMN disease exhibit muscle atrophy, hyporeflexia or areflexia, hypotonia or atonia, and paresis.
TABLE 13-1 Summary of Lower and Upper Motor Neuron Signs
| Parameter | Lower Motor Neuron Segmental Signs | Upper Motor Neuron Long Tract Signs |
|---|---|---|
| Motor function | Paralysis—loss of muscle power, flaccidity | Paresis to paralysis—loss of voluntary movements |
| Reflexes | Hyporeflexia to areflexia | Normal to hyperreflexia (especially myotatic reflexes) |
| Muscle atrophy | Early and severe: neurologic; contracture after several weeks | Late and mild: disuse |
| Muscle tone | Decreased | Normal to increased |
| Electromyographic changes | Abnormal potentials (fibrillation, positive sharp waves) after 5 to 7 days | No changes |
| Associated sensory signs | Anesthesia of innervated area, paresthesia or hyperesthesia of adjacent areas | Decreased proprioception; decreased perception of superficial and deep pain |
From Oliver JE Jr, Lorenz MD: Handbook of veterinary neurologic diagnosis, Philadelphia, 1983, WB Saunders.
Testing the patellar reflex evaluates motor and sensory components of the femoral nerve. The femoral nerve innervates the quadriceps muscles, which are responsible for extension of the stifle and weight-bearing in the hindlimb. The patellar reflex is a tendinous reflex and is elicited by lightly tapping the patellar tendon with a reflex hammer while observing an extension of the stifle. Patellar reflex testing is a subjective assessment, and clinicians should be as consistent as possible in technique. To begin, the limb should be in relaxed flexion with the patellar tendon just barely tightened. The tendon is palpated, and then, while the examiner’s fingers are kept on the tendon, the limb is flexed until the tendon feels tight. To raise tension in the tendon, the clinician can place a hand under the foot while extending the digits. The tapping on the tendon is done with a pendulum motion. The reflex cannot be elicited if the limb is tense, but by tapping the tendon rhythmically, the animal relaxes over time. The strength of the patellar reflex is proportional to the force applied to the tendon. The plexor (hammer) used for examination of large dogs is adequate for testing the reflex of small ruminants. The patellar reflex combined with the proprioceptive reaction is used to determine the integrity of the LMN. With LMN lesions, deficits exist in conscious proprioception and patellar reflexes, whereas deficits of conscious proprioception in animals with intact patellar reflexes indicate lesions in the UMN.
Pain
Whereas spinal reflexes test the LMN, assessments of conscious proprioception, voluntary motor functions, superficial pain sensation, and deep pain sensation are used to test UMNs. With compromise to the spinal cord, conscious proprioception is the first deficit observed, followed in order by voluntary motor function, superficial pain sensation, and deep pain sensation. Superficial pain sensation can be assessed by applying a noxious stimulus over a dermatome or cutaneous zone, which is an area of skin on the animal’s body surface that is innervated by a single nerve. A two-step pinch technique is recommended to test superficial pain sensation. First, a small area of skin is lightly tented using a hemostat. After a slight pause, a second, sharp skin pinch is applied. Intact superficial pain sensation is present if a reflex withdrawal occurs, and the UMN is intact if the animal demonstrates conscious recognition of the pain through an aversion response, vocalization, or both. Deep pain sensation is determined by placing a large hemostat or needle-holders across the digit just above the coronary band, and progressively pinching to stimulate the periosteum. As with the superficial pain sensation, a positive response is conscious recognition of the stimulus as evidenced by aversion, vocalization, or both. The assessment of deep pain sensation is important for prognosis for the recumbent small ruminant with neurologic disease, because deep pain is the last function to be lost with a severe spinal cord lesion.
Localization of Neurologic Lesions
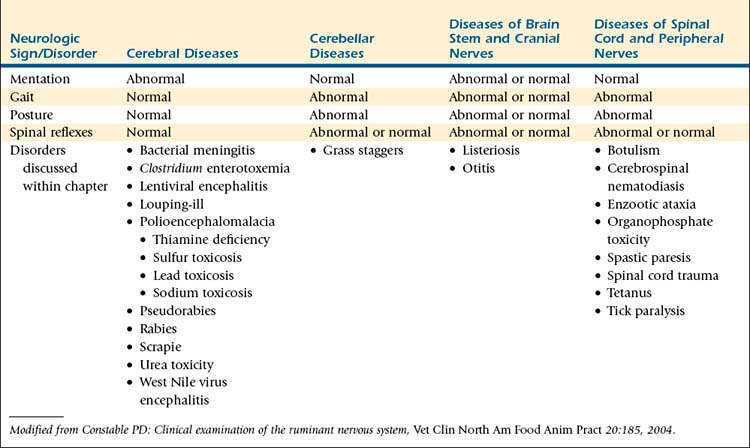
During the complete neurologic examination, abnormalities of nervous system function should be identified, characterized, and recorded. Some abnormalities in nervous system function can be readily ascribed to specific segments of the nervous system, whereas for others, the origin of dysfunction is more difficult to identify. Determining the neuroanatomic location of lesions or abnormalities within the nervous system is important with respect to management and prognosis for the sheep or goat with neurologic disease. For sheep and goats with suspected neurologic disease, clinical signs or specific pathologic processes should be ascribed to four functional areas of neuroanatomy: (1) the cerebrum, (2) cerebellum, (3) brain stem and cranial nerves, and (4) the spinal cord and peripheral nerves (Table 13-2). If the location of a lesion is not readily apparent after the complete neurologic examination, repeating all or specific portions of the neurologic examination can reveal subtle abnormalities missed earlier.
TABLE 13-2 Association of Neurologic Signs With Functional Deficits of Clinically Relevant Neuroanatomic Locations
Cerebral Disease
Nervous system disorders involving the cerebrum can be variable in severity and frequently are characterized by alterations in mental acuity, behavioral changes, seizures, and blindness. Diffuse or symmetric cerebral disease often does not affect the gait on flat surfaces, but gait can appear abnormal on ascending or descending slopes. Likewise, postural and proprioceptive reflexes are normal with diffuse cerebral disorders unless the affected animal is moved across slopes. In most animals with diffuse cerebral disease, spinal reflexes are normal. Of note, metabolic abnormalities are considered the most common cause of symmetric cerebral disease in ruminants.1 Dehydration and acid-base and electrolyte abnormalities often result in depression in small ruminants.
With unilateral lesions located within the cerebrum, a majority of clinical signs will be observed contralateral to the lesion, with the exception of circling and head turn, which usually are in the direction of the lesion. Specific examples of clinical signs associated with asymmetric cerebral disease are contralateral hemiparesis, circling with the head turned toward the side of the lesion, contralateral facial sensation deficits, and presence of a contralateral menace deficit with normal palpebral reflexes and normal pupillary light reflexes.
Spinal Cord and Peripheral Nerve Diseases
Sciatic (ischiadic) nerve paralysis can occur after pelvic or lumbosacral fractures. The nerve arises from spinal cord segments L6 to S2 and travels in the vertebral canal before its fibers exit. The sciatic nerve innervates the muscles that extend the hip and flex the stifle before dividing into the peroneal and tibial branches. The tibial nerve provides motor innervations to the gastrocnemius muscle, whereas the peroneal provides motor innervations to the extensors of the digits. The tibial and peroneal nerves also collect sensory input from the distal portion of the hindlimb. Whereas lumbosacral fractures usually cause bilateral hindlimb paresis or paralysis, damage to the proximal sciatic nerve, which can be a consequence of acetabular and femoral fractures, results in dysfunction of flexor muscles only. The extensor muscles of the stifle remain functional, allowing the animal to bear weight but not flex the stifle. The animal will exhibit a dropped hock, and the limb will be knuckled over. On testing, the animal’s flexor response is greatly inhibited, and with pinching of the medial claw, the animal flexes its hip without flexing the rest of the limb. This differential response occurs because the medial side of the limb still has intact sensory innervation through the saphenous branch of the femoral nerve. Many of these injuries resolve over time, but a poor prognosis is associated with the complete loss of deep pain.
Ancillary Tests
As noted previously, the objectives of the neurologic examination are to verify that the sheep or goat has disease of nervous system origin and to determine the anatomic location of the lesion within the nervous system. Once the neuroanatomic location has been identified, additional diagnostic testing can be performed to identify a specific causative disorder or contributing factor. A precise etiologic diagnosis is important because individual sheep and goats typically are members of herd or flock, and elucidation of the etiology will allow implementation of preventive strategies aimed at reducing the potential for disease in other at-risk animals. A number of diagnostic tests are available for the neruologic workup of a small ruminant, but owing to cost and availability of testing equipment, only a few tests can be routinely used in practice settings for the diagnosis of neurologic diseases in small ruminants. Complete blood count and serum biochemistry profile, cerebrospinal fluid (CSF) analysis, and routine imaging studies such as survey radiographs can be used during the diagnostic evaluation of sheep and goats with suspected nervous system disease.5,6
Complete Blood Count and Serum Biochemistry Panel
Because metabolic disorders are the most frequent cause of symmetric cerebral dysfunction, the complete blood count and serum biochemistry panel should be performed to evaluate for the presence of hypocalcemia, hypoglycemia, acid-base disorders, electrolyte abnormalities, and inflammatory conditions. Anomalies observed during routine blood workup may be primary or secondary to the nervous system disorder.6 (see Appendix 2)
Cerebrospinal Fluid Analysis
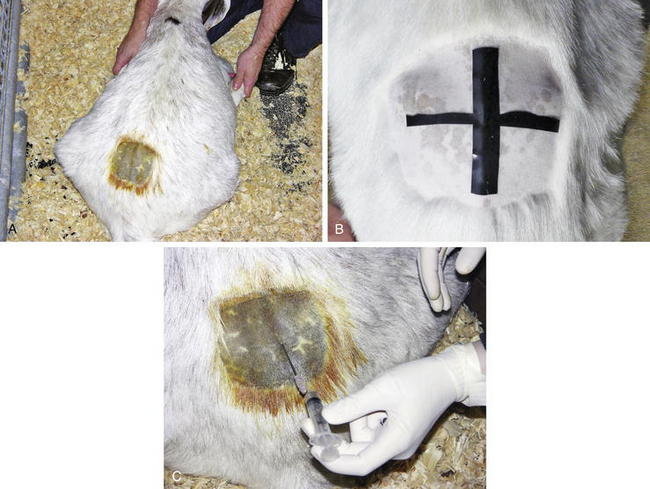
CSF is located within the subarachnoid space; therefore diseases involving the CNS can lead to alterations in the normal composition of the CSF. The CSF can be collected from the atlantooccipital space but is more easily obtained at the lumbosacral site. General anesthesia or heavy sedation is required for atlantooccipital CSF collection, and anesthesia for neurologically impaired animals often is contraindicated. Positioning and restraint are critically important for successful collection of CSF (Figure 13-5, A). Sampling can be performed in the standing sheep or goat provided that restraint is sufficient to prevent lateral motion, or the animal can be sedated. If recumbent, most sheep or goats can be manually restrained in sternal recumbency. Ideally, the animal is positioned such that the hips are flexed and the pelvic limbs extended alongside the abdomen, with the pelvis kept straight and level. The skin over the lumbosacral space should be clipped and aseptically prepared. A palpable indentation should be felt at the lumbosacral space, and this site should be infiltrated with 2% lidocaine (0.5 mL administered subcutaneously [SC]) (Figure 13-5, B). A final scrub should be applied, and the clinician should don sterile gloves.
For lambs and kids weighing less than 30 kg, a 20- or 21-gauge, 1-inch needle can be used; an 18- or 20-gauge, 1.5-inch needle can be used for adult sheep and goats. A disposable needle or a stylet-type spinal needle can be used. The needle should be inserted on midline halfway between the last palpable lumbar dorsal spinous process and the first palpable sacral dorsal spinous process. The needle should be placed perpendicular to the spine from the lateral view and straight up and down as viewed from the back of the animal. If bone is encountered, the needle should be redirected either cranially or caudally. The needle is advanced until a slight “pop” is felt as the needle passes through both the interarcuate ligament and the subarachnoid membrane. The animal may move or jump slightly when the needle punctures the dura mater, or the tail and anus may reflexively contract. The clinician can periodically remove the stylet to check for presence of CSF in the hub of the needle. Approximately 1 mL of fluid/5 kg of body weight can be safely removed, but only 1 to 2 mL is necessary for cytologic evaluation. Gently and slowly aspirating CSF or allowing it to flow freely from the needle prevents excessive movement and blood contamination (Figure 13-5, C). The CSF samples should be placed in ethylenediamine tetraacetic acid (EDTA) for cytologic analysis and in a serum separator tube for culture. For biochemical analysis, CSF should be placed in serum separator or lithium heparin tubes. Cytologic evaluation of CSF should be performed rapidly, ideally within 60 minutes of collection. If this is not possible, the CSF can be mixed with an equal volume of 40% ethanol to preserve the cells.
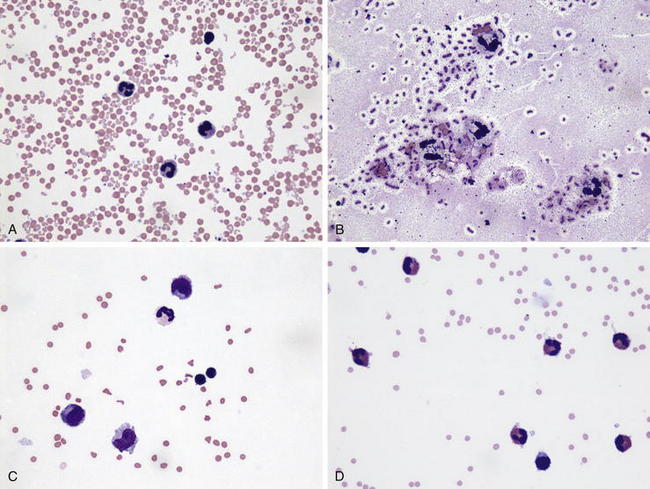
Once collected, CSF can be evaluated for gross appearance, cytology, protein concentration, biochemical composition, and presence of bacteria. Normal CSF is clear and colorless. Red discoloration indicates presence of blood in the CSF, and the hemorrhage may be iatrogenic (Figure 13-6, A) or the result of the collection or from previous hemorrhage within the CSF. In general, blood from a previous hemorrhage is evenly mixed with the CSF and often does not clot, as opposed to iatrogenic hemorrhage during collection, in which the red discoloration may lessen as additional fluid is collected and the CSF will clot. Xanthochromia is orange or yellow discoloration of the CSF, and this finding can be observed for up to 10 days after occurrence of bleeding within the CSF. Turbid CSF usually indicates a high white blood cell count, as can occur with bacterial meningitis. The total nucleated cell and differential counts should be performed to assist with an etiologic diagnosis. Normal CSF contains less than 10 nucleated cells/μL, with a majority of cells being mononuclear (see Appendix II , Table F). Bacterial infections of the nervous system usually are characterized by a neutrophilic pleocytosis (Figure 13-6, B), with the exception of listeriosis in small ruminants where mononuclear pleocytosis (Figure 13-6, C) is usually present. Mononuclear pleocytosis can also be observed with viral encephalitides and PEM. Cerebrospinal nematodiasis, secondary to aberrant migration of nematode parasites, often results in marked elevations of eosinophils, which may be the predominant CSF leukocyte in affected animals (Figure 13-6, D). Normal protein concentrations in CSF are considerably lower than in blood. CSF protein concentration of healthy sheep is less than 40 mg of protein/dL and that of healthy goats less than 15 mg of protein/dL. CSF glucose content generally is low compared with that in the peripheral blood. Glucose concentrations normally are 80% of the value in peripheral blood, and decreased concentrations are detected in animals with bacterial meningoencephalitis as a consequence of bacterial glucose consumption.
Medical Imaging
After a lesion has been localized within the CNS, plain survey films may be helpful to identify luxations of the vertebral column, osteomyelitis, or fractures of the pelvis. Survey radiographs of the skull can be used to diagnose fractures or assess involvement of the tympanic bulla in cases of otitis. Radiographic techniques used in medium to large dogs are applicable in sheep and goats. For UMN disease of the forebrain, brain stem, or cerebellum, several diagnostic imaging procedures can be performed. The structural integrity of the UMN anatomy can be evaluated by the use of computed tomography (CT) and magnetic resonance imaging (MRI). Myelography can be used to identify compressive or expansive lesions in the spinal cord. Electromyography also can be used to determine whether specific neurons are responsible for neuromuscular disease by assessing the electrical activity of the muscle after a neuron is stimulated. Electroencephalography can be used to assess the electrical activity in various parts of the brain. It is used primarily in cases of presumed neurologic disease manifested by seizures, narcolepsy, and encephalopathy.
1. Constable P.D. Clinical examination of the ruminant nervous system. Vet Clin North Am Food Anim Pract. 2004;20:185.
2. Mayhew I.G. Neurologic evaluation. In: Mayhew I.G., editor. Large animal neurology. Ames, Iowa: Blackwell Publishing, 2009.
3. Bagley R.S. Fundamentals of veterinary clinical neurology. Ames, Iowa: Blackwell Publishing Professional; 2005.
4. Parent J. Clinical examination. In: Anderson D.E., Rings D.M., editors. Current veterinary therapy: food animal practice. St Louis: Saunders Elsevier; 2009:274-278.
5. Francoz D. Ancillary tests. In: Anderson D.E., Rings D.M., editors. Current veterinary therapy: food animal practice. St Louis: Saunders Elsevier; 2009:279-283.
6. Scott P.R. Diagnostic techniques and clinicopathologic findings in ruminant neurologic disease. Vet Clin North Am Food Anim Pract. 2004;20:215.
Cerebral Diseases
Bacterial Meningitis and Encephalitis
Etiology and Pathophysiology
In meningitis, one or more of the three layers (dura mater, arachnoid, and pia mater) covering the CNS are inflamed, and involvement of adjacent structures (CNS or spinal cord) is common. Meningitis and meningoencephalitis may result from many different disorders but principally follow extension of local processes or hematogenous dissemination.1 Infections extending into meninges and nervous tissues may be caused by surgical procedures such as dehorning and tail docking, thermal osteonecrosis after cauterization for dehorning, sinusitis, otitis interna, and skull fractures. Bacteremia associated with pneumonia, omphalophlebitis, mastitis, endocarditis, or other septic processes also may cause meningoencephalitis. Hematogenous infection is especially common in neonatal septicemia arising with failure of passive transfer. Any of several bacterial pathogens may be involved in the disease. In neonatal meningoencephalitis, Escherichia coli, Pasteurella multocida, Streptococcus spp., Staphylococcus spp., and Arcanobacterium pyogenes have been reported and invasion of the CNS may depend on various virulence factors.1 Goat kids affected by polyarthritis and pleuropneumonitis caused by Mycoplasma mycoides ssp. mycoides, may develop meningoencephalitis.2 Pseudomonas aeruginosa may cause septicemia and meningitis in goats secondary to mastitis.3 In the CSF, host immune defense mechanisms provide only limited protection because antibody and complement concentrations of CSF are low.1,3 Bacterial and inflammatory insults may potentially lead to congestion and infarction of arachnoidal or subependymal veins, decreased CSF absorption, intraventricular hypertension, and necrosis of nerve cells.1,3
Clinical Signs
Affected animals often are severely lethargic and depressed but may be hyperexcitable. In cases associated with neonatal septicemia, diarrhea and dysthermia are common. Clinical examination reveals hyperesthesia, a stiff and extended neck, and pain induced by movement of the head and neck. Passive manipulation of the neck may result in sudden tonic extension and rigidity of the limbs.3 Loss of cranial nerve functions may be recognizable as nystagmus, strabismus, and facial palsy.1,3 Progression of the disease leads to decreased sensory functions, propulsive walking, seizures, and coma.
Diagnosis
Meningitis should be suspected on the basis of clinical signs, especially in neonates with indications of failure of passive transfer and sepsis. To differentiate the disease from metabolic abnormalities, serum electrolytes and glucose should be evaluated. Confirmation of meningitis is based on CSF analysis or postmortem examination. Marked increases in protein concentration, total leukocyte count, and proportion of neutrophils in CSF samples are characteristic of bacterial meningitis. CSF glucose concentration is below that in serum, reflecting bacterial consumption of glucose in the CSF.1 Xanthochromia and free or intracellular bacteria also may be present. Bacteriologic culture and sensitivity testing should be attempted if therapy is intended.
Treatment
Case-fatality rates in farm animals with bacterial meningitis are high, owing in part to late recognition of the disease. Therapy is based on aggressive and prolonged administration of antibiotics, supplemented with antiinflammatory drugs, and anticonvulsive therapy as needed. The choice of antibiotic should be guided by an initial Gram stain of CSF or by culture and sensitivity testing. Preferably, antibiotic therapy should be administered by the intravenous route to attain maximum peak blood and CSF concentrations.3 The use of a third-generation cephalosporin (e.g., ceftiofur, 1 to 5 mg/kg given intravenously [IV] one to three times daily) has been recommended, and a combination of antibiotics may be used (e.g., a β-lactam plus a trimethoprim-sulfonamide agent).1 These recommended regimens are empirical, and other intravenous antibiotics may be used. The choice of antiinflammatory agent has not been evaluated in farm animals, but nonsteroidal antiinflammatory drugs are thought to be beneficial. Seizures should be controlled using diazepam (0.01 to 0.2 mg/kg every 30 minutes).1 In neonates with failure of passive transfer, plasma should be administered (15 to 25 mL/kg IV).
1. Fecteau G., George L.W. Bacterial meningitis and encephalitis in ruminants. Vet Clin North Am Food Anim Pract. 2004;20:363.
2. Bajmocy E., et al. Disease caused by Mycoplasma mycoides subspecies mycoides LC in Hungarian goat herds. Acta Vet Hung. 2000;48:277.
3. Berthelin-Baker C., George L.W. Meningitis (suppurative meningitis, bacterial meningitis). In: Smith B.P., editor. Large animal internal medicine. 4th ed. St Louis: Mosby Elsevier; 2009:998-1002.
Clostridium perfringens Enterotoxemia
Etiology and Pathophysiology
Clostridium perfringens types C and D (and possibly type E) are important pathogens of sheep and goats that cause infection manifesting primarily as enteric disease and peracute death, with associated pathologic changes in the nervous system. The organisms are ubiquitous in the environment and feces of farm animals. C. perfringens type C produces α and β toxins, which are not degraded in young animals (less than 10 days of age), owing to low intestinal concentrations of proteolytic enzymes. The β toxin induces ion-conductive channels in membranes of excitable cells, leading to irreversible depolarization and neurologic disease.1 Disease associated with C. perfringens type D usually is seen in young animals consuming overly plentiful diets, allowing clostridial overgrowth and production of α and ε toxins in the small intestine; however, neonatal lambs in unvaccinated flocks also may be affected.2 Activation of ε toxin from its precursor is induced by enteric proteases. The active toxin causes disruption of tight junctions of vascular endothelial cells and vasogenic edema in different organs, including brain, lungs, and kidneys.3 The systemic effects of ε toxin may be facilitated by intestinal activity of β2 toxins4 (see Chapters 12 and 16).
Clinical Signs
The duration of disease is limited to a few hours, and clinical signs preceding death may not be observed. Abdominal discomfort and signs of colic, such as teeth-grinding and vocalization, may be noted. Hemorrhagic diarrhea may be present in some cases and is regularly observed in goats with type D enterotoxemia.5 Neurologic signs include depression, tetany, opisthotonos, convulsions, and coma.5,6 In type D enterotoxemia, focal encephalomalacia may develop, which is characterized by aimless wandering, blindness, and walking into inanimate objects.5
Treatment
Affected animals usually die before systemic antibiotics (affording coverage of the gram-positive spectrum) and fluid therapy could have any effect. Administration of C and D antitoxin probably is more beneficial to prevent disease in at-risk herdmates than as a treatment of moribund animals.6
Prevention
Routine vaccination of sheep and goats against C. perfringens C and D is paramount. Regular boosters should be administered to all breeding stock. Administration of C and D antitoxin shortly after birth should provide protection to lambs and kids of unvaccinated dams for approximately 2 weeks.6
1. Shatursky O., et al. Clostridium perfringens beta-toxin forms potential-dependent, cation-selective channels in lipid bilayers. Infect Immun. 2000;68:5546.
2. Scholes S.F., et al. Clostridium perfringens type D enterotoxaemia in neonatal lambs. Vet Rec. 2007;160:811.
3. Watson P.J., Scholes S.F. Clostridium perfringens type D epsilon intoxication in one-day-old calves. Vet Rec. 2009;164:816.
4. Uzal F.A., et al. Ulcerative enterocolitis in two goats associated with enterotoxin- and beta2 toxin-positive Clostridium perfringens type D. J Vet Diagn Invest. 2008;20:668.
5. Songer J.G. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216.
6. Rings D.M. Clostridial disease associated with neurologic signs: tetanus, botulism, and enterotoxemia. Vet Clin North Am Food Anim Pract. 2004;20:379.
Lentiviral Encephalitis: Caprine Arthritis-Encephalitis and Maedi-Visna
Etiology and Pathophysiology
In small ruminants, lentiviral leukoencephalomyelitis is caused by caprine arthritis-encephalitis virus (CAEV) in goats and either maedi visna virus (MVV) or, less commonly, ovine progressive pneumonia virus (OPPV) in sheep. In North America, OPPV usually is associated with respiratory disease; however, neurologic disease in sheep also has been described.1 Small ruminant lentiviruses (SLRVs) belong to the genus Lentivirus, family Retroviridae, and have a worldwide distribution, although prevalence varies widely among herds.2 While differing in certain clinical aspects, SLRVs share many virologic, epidemiologic, and pathophysiologic features. The nomenclature may indicate SLRVs to be species-specific, but natural infection of sheep with CAEV and goats with MVV is possible, and crossing of species barriers is not uncommon.3,4
The SRLVs are enveloped RNA viruses that, on infection of host cells, transcribe their genome into a double-stranded DNA that is inserted into the host’s genome, resulting in life-long infection.2 Monocytes and macrophages are the primary cells infected by SRLVs, and invasion of target organs is believed to be in macrophages.5,6 Transmission of SRLVs is primarily horizontal. Infection of lambs or kids by ingestion of colostrum and milk from infected dams is a main route of transmission. Horizontal transmission by the respiratory route may cause infections in older animals. In utero infections are possible, but their relative importance is unclear.2,6 Inflammatory changes associated with SRLV infection may occur in the CNS, lungs, udder, joints, lymph nodes, and blood vessels, potentially resulting in progressive dysfunction of the affected organ(s).
Clinical Signs
Clinical signs associated with SLRV infection are slowly progressive and initially nonspecific. Neurologic disease (leukoencephalomyelitis) is a relatively rare form of infection in comparison with respiratory disease in adult sheep and arthritis in adult goats.1,7 In sheep, clinical signs usually occur in animals older than 1 to 3 years of age; however, clinical disease has been reported in younger sheep.2,7,8 Initial signs include weight loss, hindlimb weakness, and abnormal stance with progression to ascending paresis and paralysis. Death occurs several months after initial signs are noticed and may be preceded by neurologic signs localized to the head, such as lip twitching, nystagmus, and blindness.
The clinical course of neurologic disease is more rapid in goats than in sheep. Affected goats usually are 1 to 5 months of age; however, adult goats may be affected.6 Clinical signs include a short, choppy gait and unilateral or bilateral posterior paresis and ataxia. Initially, the mentation appears to be unaffected, and animals eat, drink, or nurse normally. Progression of the disease leads to tetraparesis, and head tilt, circling, torticollis, opisthotonos, and blindness may develop.
Diagnosis
Neurologic disorders associated with SRLV infection are relatively rare, and other conditions should be considered in the workup. Presence of interstitial pneumonia may suggest SRLV infection, but clinical signs of respiratory disease often are absent. CSF analysis may reveal increased protein concentrations and mononuclear pleocytosis. Identification of SRLV antibodies is performed by AGID or ELISA techniques. The presence of antibodies in serum implies infection but not disease causation. PCR techniques may be used to detect SLRV infections before antibodies are produced.9 Postmortem diagnosis is based on identification of a nonsuppurative demyelinating encephalomyelitis and lymphocytic infiltration of the CNS.
Treatment
Effective therapies to slow or halt the disease do not exist, and affected animals should be humanely euthanized (see Chapter 16).
Prevention
Successful vaccination strategies suitable for field conditions are not available.10 Eradication of SRLVs from a herd is possible with use of test-and-slaughter programs, which may not be feasible in herds with high prevalence rates. Control measures should be chosen according to herd prevalence of infection.10 In herds with mixed populations of sheep and goats, cross-specific transmission is possible, and measures for control of both CAEV and MVV-OPPV must be instituted.11
Prevention is based on identification of seropositive animals and segregation of infected from uninfected animals, removal of infected animals, and continued serologic testing. Prevention of perinatal transmission is achieved by separation of lambs and kids from their dam immediately after birth, and by cleaning uterine and placental fluids off newborns.2 Because consumption of colostrum and milk from infected dams is an important method of transmission, these products should be harvested only from uninfected dams. Alternatively, heating of colostrum to 56° C (130 F) for 60 minutes decreases the load of infectious virus and appears to decrease immunoglobin concentrations only minimally.12,13 Use of pasteurized milk or milk replacers is recommended in kid- and lamb-raising protocols. Iatrogenic transmission by needles, tattooing equipment, and other surgical instruments must be prevented through use of disposable instruments or sterilization2 (see Chapters 11 and 16).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree