Chapter 5 Diseases of the Gastrointestinal System
Diagnostic Procedures
Basic Laboratory Studies
Clinicopathologic data from laboratory studies consisting of a complete blood count (CBC), serum biochemical evaluation (SBE), and urinalysis can be helpful in eliciting the presence of gastrointestinal disease, developing a prognosis and plan for treatment, and monitoring response to treatment. A CBC rarely identifies a specific disease but can be helpful in evaluating the severity of dehydration, anemia, and hypoproteinemia. The clinician must take care to interpret the packed cell volume (PCV) and total protein in light of the hydration status of the animal as noted on physical examination. An anemic or dehydrated hypoproteinemic animal may have normal PCV and total protein values. Both the CBC and SBE can be helpful in determining the presence and severity of an inflammatory disease process. Changes in the total and differential white blood cell counts indicate acute or chronic inflammation; increases in globulins or fibrinogen suggest a chronic inflammatory disease. Low protein levels, especially of albumin, can point to chronic blood loss from intestinal parasitism or infiltrative bowel disease. Liver disease should be suspected if liver enzyme or bilirubin levels are elevated. Of note, however, liver enzyme concentrations can be normal in chronic liver disease. Also, albumin levels rarely drop in ruminants with liver disease as they do in other species.1 Changes in electrolytes can occur with gastrointestinal disease, especially if affected animals are anorexic. Electrolyte measurements also are helpful in formulating a treatment plan. Hypochloremia and metabolic alkalosis occasionally occur in abomasal disease. A mild hypocalcemia may be encountered in some small ruminants with gastrointestinal atony. Many animals with gastrointestinal disease are dehydrated, azotemic, and possibly hypoproteinemic; therefore it may be helpful to rule out urinary tract disorders in these cases.
Normal ranges for clinicopathologic laboratory values are available in Appendix 11 Tables A-D and also have been published in several other textbooks.2–5 However, clinicians would do well to learn the normal values, especially serum biochemistry values, established by the laboratory most commonly used for analysis in their practice.5
Rumen Fluid Analysis
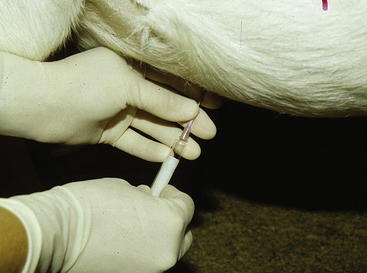
Analysis of rumen fluid can help differentiate among diseases of the forestomachs. An appropriately sized orogastric tube can be passed through the oral or nasal cavity for fluid collection (Figure 5-1). For this procedure, proper restraint of the animal, using a mouth speculum to prevent chewing of the tube if it is passed orally, is essential. If the tube is chewed, its roughened surface may damage the esophagus, and parts of a broken tube can be swallowed. Rumen fluid also can be collected using percutaneous rumenocentesis.1,6–10 For this percutaneous technique, a 16-gauge needle is inserted in the rumen through the abdominal wall caudal to the xyphoid and to the left of midline. The clinician then aspirates fluid with a syringe. Local anesthesia and sedation of the animal may be necessary. This technique avoids the saliva contamination that can occur during collection with an orogastric tube, and it appears to be less stressful. Rumenocentesis carries a slight risk of peritonitis, but this risk can be minimized with immobilization of the animal through proper restraint. Percutaneous rumenocentesis should not be performed on pregnant females.
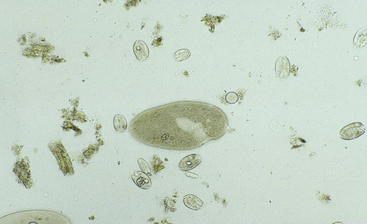
After the fluid is collected, it can be analyzed for color, odor, pH, protozoal species and motility, methylene blue reduction time (MBR), Gram staining characteristics, and chloride levels (Figure 5-2). Normal values are listed in Table 5-1. Anorexia may cause the fluid to appear darker, the pH to increase, and the number and motility of protozoa to decrease. A gray color, low pH, and dead or no protozoa are seen in rumen acidosis from grain overload. The MBR is prolonged with any type of indigestion/digestive disorder. Large numbers of gram-positive rods (Lactobacillus species) also may be seen in rumen acidosis. Elevated rumen chloride concentrations indicate an abomasal or proximal small intestinal obstruction (either functional or mechanical).
TABLE 5-1 Normal Rumen Fluid Characteristics of Sheep and Goats
| Characteristic | Normal Finding |
|---|---|
| Color | Green |
| Odor | Aromatic |
| pH* | 6.5 to 7.5 |
| Protozoa† | Mixed sizes and species rapidly moving |
| Methylene blue reduction time‡ | 3 to 6 minutes |
| Gram stain | Gram-negative rods predominate |
| Rumen chloride | Less than 25 to 30 mEq/L |
* Use pH paper with at least 0.5-unit gradations.
† Place a drop of fluid on a warm slide and cover with a coverslip. Examine under 100× magnification.
‡ Mix one part 0.03% methylene blue to 20 parts rumen fluid. Measure time for blue color to clear to match a control tube of fluid.
Data from Nordlund KV, Garrett EF: Rumenocentesis: a technique for collecting rumen fluid for diagnosis of subacute rumen acidosis in dairy herds, Bovine Pract 28:109, 1994; Keefe GP, Ogilvie TH: Comparison of oro-ruminal probe and rumenocentesis for prediction of rumen pH in dairy cattle, Proceedings of the 30th Annual American Association of Bovine Practice Convention, 1997, p 168; and Smith MC, Sherman DM: Goat medicine, ed 2, Ames, Iowa, 2009, Wiley-Blackwell.
Abdominocentesis
Abdominocentesis is useful in discerning the causes of fluid distention in the abdomen. Two methods can be used. The first technique involves tapping the lowest point of the abdomen slightly to the right of midline; it is useful in ruling out a ruptured bladder as the cause of general ascites1,11 (Figure 5-3). The clinician should take care to avoid the prepuce in males and mammary veins in females. The second technique is useful if peritonitis is suspected. Because localized peritonitis is more common than generalized peritonitis, four sites are tapped.12 The two cranial sites are slightly caudal to the xyphoid and medial to the milk veins on both sides. The two caudal sites are slightly cranial to the mammary gland and to the left and right of midline. For either technique, manual restraint with sedation is recommended; the use of real-time ultrasonography may help locate fluid pockets.
A 20-gauge needle or teat cannula can be used for fluid collection.11 The clinician should prepare the site using sterile technique and provide local anesthesia when a teat cannula is to be used. Fluid should be collected in a small ethylenediamine tetraacetic acid (EDTA) tube for analysis and a sterile tube for culture. Abdominal fluid can be difficult to obtain because of the small amounts normally present in both sheep and goats. It is important to minimize the ratio of EDTA to fluid in the sample, because EDTA can falsely elevate protein levels. Using EDTA tubes made for small animals or shaking excess EDTA out of large tubes resolves this problem. Normal culture results are similar to those for cattle (clear, colorless to slightly yellow, 1 to 5 g/dL protein, less than 10,000 cells)12 (see Appendix Table H). Cytologic examination is needed to characterize the cell population and assess for the presence of phagocytized bacteria.
Radiography
Radiography of the abdomen can be performed in small ruminants using small animal techniques. In adults, the rumen normally fills the entire abdomen. Radiography can detect gas distention of the small intestine, abdominal fluid, and foreign bodies.12,13 Contrast techniques are useful for diagnosing atresia of the rectum or colon. Unlike in other small animals, contrast techniques are not practical for characterizing small intestinal problems in sheep and goats, because the rumen dilutes and slows passage of the contrast media.14
Ultrasonography
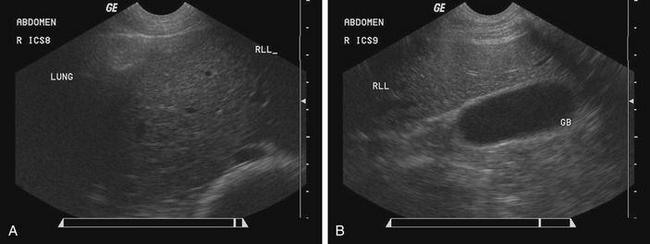
Ultrasonography can be used to provide better characterization of abdominal distention, internal and external abdominal masses, and gross lesions of the liver. With this imaging modality, ascites can be differentiated from fluid in the intestinal tract, and gas distention of the intestines can be differentiated from fluid distention. The normal ultrasonographic examination of the liver in sheep and goats has been described.15,16 The liver can be viewed on the right side from the seventh or eighth rib caudally to the 13th rib (Figure 5-4, A and B).
Laparoscopy
Laparoscopy more commonly is used as a reproductive tool, but it also can be used diagnostically as an alternative to exploratory laparotomy in small ruminants.17–21 General anesthesia is recommended to allow a greater degree of inflation of the abdominal cavity for a more thorough examination, but laparoscopy can be done with use of sedation and local anesthesia at portal incision sites.
The technique for laparoscopic exploration of the abdomen in cattle and llamas can be modified for use in sheep and goats.18–21 Laparoscopic evaluation of the abdominal cavity is usually done through a ventral approach, with the animal secured in dorsal recumbency. The abdominal cavity can be inflated with CO2 delivered by a needle or teat cannula or after placement of a laparoscopic cannula. A time-saving method is to make a “bite” through the skin and into the external rectus sheath with a suture, which can be pulled tight in order to tense the body wall. A stab incision can then be made in the skin and external rectus sheath before introduction of a guarded trocar into the abdominal cavity while tension is applied to the abdominal wall using the previously placed suture. Next, the laparoscope is placed through the trocar and the abdomen inflated under visualization through the scope. The cannula is then placed in the inguinal area as described for laparoscopic insemination (Chapter 8). This technique allows a more efficient use of time and minimizes the likelihood that the omentum will be “ballooned.”
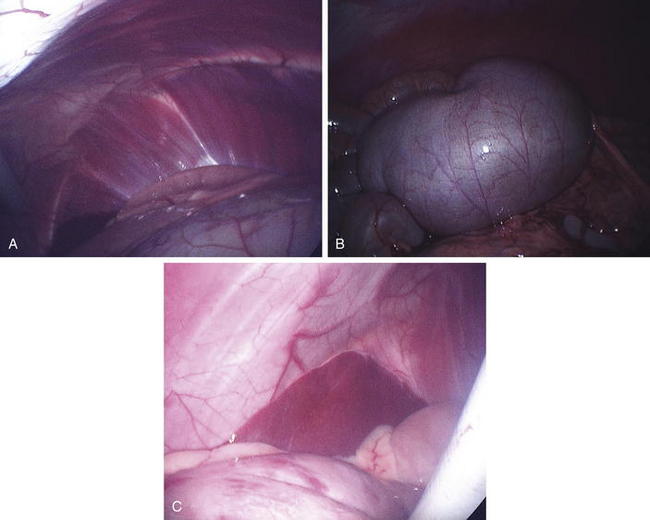
Laparoscopic placement into the right side allows visualization of most of the abdominal organs (Figure 5-5, A to C). Obviously, the clinician should avoid the rumen when introducing the laparoscope into the abdomen. This procedure may be enhanced by lowering the head or rear of the animal, allowing better visualization of the entire abdomen. Visualization of the abdominal cavity and the ability to manipulate organs will be greatly improved by fasting the animal for 24 to 48 hours or at least decreasing the bulk in the diet. Respiration must be monitored closely, and assisted ventilation should be available during this procedure, because inflation of the abdomen and lowering of the head can put pressure on the diaphragm.
Exploratory Laparotomy
Exploratory laparotomy can be a valuable diagnostic tool in evaluating gastrointestinal diseases when other tests indicate abdominal disease. In some cases, therapeutic surgical procedures can be performed at the same time. The technique of exploratory laparotomy used in cattle can be adopted for sheep and goats with the understanding that these animals are more likely to lie down during surgery; therefore standing surgery should be attempted only rarely.22
For this procedure, small ruminants should be heavily sedated or placed under general anesthesia. Animals that show signs of postoperative pain, anorexia, and depression should be treated accordingly with a nonsteroidal antiinflammatory drug (NSAID) (e.g., flunixin meglumine, 1.1 to 2.2 mg/kg intravenously [IV]).12 The decision to use perioperative and postoperative antimicrobial agents should be based on the conditions under which the surgery is performed and the diagnosis made at surgery. Antimicrobial agents are not necessary for elective exploratory surgery performed aseptically, in a hospital setting, and without complications. However, they are indicated in field conditions, if infection is already present, and if the intestinal tract is opened. A combination of ceftiofur (1.1 to 2.2 mg/kg intramuscularly [IM] or subcutaneously [SC] twice a day) and procaine penicillin G (22,000 international units [IU]/kg IM twice daily) can be administered until culture results indicate an absence of microbes (see also Appendix 1).
Liver Biopsy
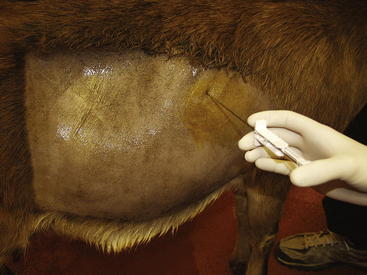
Liver biopsy in sheep and goats is performed using the same technique and instruments as in cattle and llamas.23,24 Sedation and ultrasound guidance are recommended.24 The recommended biopsy site is in the ninth to tenth intercostal space slightly above an imaginary line from the tuber coxae to the point of the elbow (Figure 5-6).
The site should be surgically prepared, and a local anesthetic (2% lidocaine hydrochloride) infused subcutaneously. A small scalpel blade is used to make a stab incision through the skin. A 14-gauge, 11.5-cm liver biopsy instrument is inserted through the incision and the intercostal muscles and into the liver. The biopsy instrument should be directed toward the opposite elbow in most cases, but the use of real-time ultrasonography can help determine the direction and depth needed (2 to 4 cm). The clinician should attempt to avoid large vessels along the caudal border of the ribs. On reaching the liver, the clinician will note a slight increase in resistance. Samples can be submitted for culture (in a sterile plastic or glass vial or tube), histopathologic study (in formalin at a 10:1 ratio of formalin to tissue), or mineral analysis (in a plastic tube). When performing a liver biopsy for mineral analysis, the clinician should rinse the biopsy site with distilled and deionized water after sterile preparation to minimize sample contamination. Samples for mineral analysis should not be placed in formalin.
1. Roussel A.J., Whitney M.S., Cole D.J. Interpreting a bovine serum chemistry profile: part 1. Vet Med. 1997;92:553.
2. Smith M.C., Sherman D.M. Goat medicine, ed 2. Ames, Iowa: Wiley-Blackwell; 2009.
3. Howard J.L., Smith R.A. Current veterinary therapy 4: food animal practice. Philadelphia: WB Saunders; 1999.
4. Howard J.L. Current veterinary therapy 3: food animal practice. Philadelphia: WB Saunders; 1993.
5. Keneko J.J. Clinical biochemistry of domestic animals. San Diego: Academic Press; 1989.
6. Navarre C.B., et al. Analysis of gastric first compartment fluid collected via percutaneous centesis from healthy llamas. J Am Vet Med Assoc. 1999;214:812.
7. Nordlund K.V., Garrett E.F. Rumenocentesis: a technique for collecting rumen fluid for diagnosis of subacute rumen acidosis in dairy herds. Bovine Pract. 1994;28:109.
8. Keefe G.P., Ogilvie T.H. Comparison of oro-ruminal probe and rumenocentesis for prediction of rumen pH in dairy cattle, Proceedings of the 30th Annual American Association of Bovine Practitioners Convention. Guelph, Ontario, Canada. 1997:p 168.
9. VanMetre D.C., Tyler J.W., Stehman S.M. Diagnosis of enteric disease in small ruminants. Vet Clin North Am Food Anim Pract. 2000;16:87.
10. Smith MC: Commonly encountered diseases of goats, Proceedings of the 1996 Symposium on the Health and Disease of Small Ruminants, Kansas City, Mo, 1996.
11. Matthews J. Colic, ed 2. Oxford, UK: Blackwell Science; 1999.
12. House J.K., et al. Ancillary tests for the assessment of the ruminant digestive system. Vet Clin North Am Food Anim Pract. 1992;8:203.
13. Tanwar R.K., Saxena A.K. Radiographic detection of foreign bodies (goat). Vet Med. 1984;79:1195.
14. Cegarra I.J., Lewis R.E. Contrast study of the gastrointestinal tract in the goat (Capra hircus). Am J Vet Res. 1977;38:1121.
15. Braun U., Hausammann K. Ultrasonographic examination of the liver in sheep. Am J Vet Res. 1992;53:198.
16. Soroori S., et al. Ultrasonographic examination of the goat liver. Turk J Vet Anim Sci. 2008;32:385-388.
17. Seeger K.H., Klatt P.R. Laparoscopy in the sheep and goat. In: Harrison R.M., Wildt D.E., editors. Animal laparoscopy. Baltimore: Williams & Wilkins, 1990.
18. Anderson D.E., Gaughan E.M., St-Jean G. Normal laparoscopic anatomy of the bovine abdomen. Am J Vet Res. 1993;54:1170.
19. Lin H.C., et al. Effects of carbon dioxide insufflation combined with changes in body position on blood gas and acid-base status in anesthetized llamas (Lama glama). Vet Anesth. 1997;26:444.
20. Baird A.N., Rodgerson D.H., Pugh D.G. Laparoscopic ovariectomy in llamas. Vet Surg. 1996;25:419.
21. Anderson D.E., et al. Laparoscopic surgical approach and anatomy of the abdomen in llamas. J Am Vet Med Assoc. 1996;208:111.
22. Hooper R.N. Abdominal surgery in small ruminants, Presented at the 1998 Symposium on the Health and Disease of Small Ruminants. Las Vegas: Nev; 1998.
23. Montes A.J., Pugh D.G. A technique for liver biopsy in sheep. In: Dziuk P., Wheeler M., editors. Handbook of methods for study of reproduction physiology in domestic animals. Chicago: University of Illinois, 1992. Section VI, D2, Sheep, p 1
24. Welles E.G., et al. Liver biopsy in llamas. Equine Pract. 1997;19:24.
Diseases of the Forestomachs
Bloat
Bloat is less common in small ruminants than in cattle, with goats being affected less commonly than sheep. Bloat is the accumulation of either free gas or froth in the rumen, which causes rumen distention. The causes of bloat can be divided into three categories1,2:
Frothy bloat—caused by diets that promote the formation of stable froth
Free gas bloat—caused by diets that promote excessive free gas production
Pathogenesis
Frothy bloat usually is associated with the ingestion of legume forages or hay (particularly alfalfa) and with grazing on lush cereal grain pastures, but it also may occur with high-grain diets.3 In the case of frothy bloat from a fine-ground diet (usually corn), mucoprotein released from rumen protozoa stabilizes the foam at a low pH. In legume-associated frothy bloat, plant chloroplasts released into the rumen trap gas bubbles. Regardless of the form of frothy bloat, the small bubbles fill much of the rumen, preventing clearance of the rumen’s cardia and resulting in a cessation of eructation. Free gas bloat also occurs with grain diets, especially if the animals are not adapted to the diet. Failure to eructate has a variety of causes. Physical obstructions of the esophagus such as with choke or swollen mediastinal lymph nodes can cause free gas bloat. Any disease of the rumen wall may result in impairment of contractions and eructation. Hypocalcemia, endotoxemia, pain, peritonitis, and some pharmaceutical agents (especially xylazine) all produce conditions that interfere with rumen function and eructation.1,2,4,5
Clinical Signs
Clinical signs of frothy bloat and free gas bloat from either food intake or physical obstruction of the esophagus usually are more severe and immediately life-threatening than those associated with bloat due to rumen wall diseases and systemic influences. Abdominal enlargement occurs, particularly in the dorsal left paralumbar fossa. This ruminal enlargement may be subtle in sheep or Angora goats with full fleece. Signs of colic and anxiety are common. The rumen may be either hypomotile or hypermotile. Respiratory distress is obvious, with mouth breathing evident in some animals; death can ensue if the bloat is not treated.3
Diagnosis and Treatment
Presence of bloat constitutes a medical emergency, so diagnosis and treatment should occur almost simultaneously. If the animal is not in immediate danger of dying, an orogastric tube can be passed. Most cases of free gas bloat are relieved with passage of the tube. A thorough history and complete physical examination are then indicated to find the cause of the bloat. If the bloat is not relieved with passage of an orogastric tube, the tube should be removed and examined for evidence of froth. Frothy bloat can be treated with poloxalene (44 mg/kg) or dioctyl sodium sulfosuccinate (DSS) (28 mL [1 oz]) delivered by orogastric tube. The froth encountered in frothy bloat, caused by the ingestion of finely ground grain, has a pH of less than 5.5. If frothy bloat develops while animals are being fed concentrates, mineral oil (100 mL) may work better. Peanut oil (20 to 50 mg/kg), vegetable oil (100 to 200 mL), and hand soap (10 mL) also have been recommended in emergency situations.3
If the animal is in severe respiratory distress, the clinician should insert a trocar or large needle into the rumen at the paralumbar fossa. If gas does not escape, or froth is seen coming out of the trocar, an emergency rumenotomy is indicated (see later under “Rumenotomy”).3 With occurrence of bloat in multiple animals of a pastured group, the entire group should be removed from the pasture and reintroduced slowly after gradual acclimation. If only one or two cases of bloat are encountered, the healthy animals can remain on the offending pasture, but grazing should be limited, to ensure gradual acclimation.
Prevention
Free gas bloat from concentrate feeds can be controlled by slow introduction to these feeds to allow for rumen adaptation and by the inclusion of ionophores in the diet.1 Monensin (15 mg/head/day in ewes and 1 mg/kg/day in goats) and lasalocid (0.5 to 1 mg/kg/day in sheep and goats) both decrease the formation of free ruminal gas. By enhancing propionic acid formation, these drugs not only reduce the amount of methane produced in the rumen but also improve the efficiency of nutrient assimilation from feedstuffs.
Bloat in lambs and kids can have the same causes as in adults but also can be caused by improper milk feeding. Overfeeding, feeding of large infrequent meals, and feeding spoiled or cold milk all have been associated with bloat in lambs and kids.6 Rapid overdistention of the abomasum and improper chemical or physical composition of milk replacers both will inhibit rumen motility, leading to bloat. Even though the feeding of cold milk has been associated with bloat, the practice can be used effectively in orphan feeding programs. Lambs and kids tend to limit their intake of cold milk after they have become accustomed to a free-choice feeding system that delivers refrigerated milk. Milk usually is placed in the rumen when animals are tube-fed; this may result in milk spoilage.1,6
Simple Indigestion
Simple indigestion is a mild form of upset of reticulorumen function caused by a change in feeding routine. Such changes typically involve alterations in the type of feed or in the amount of feed offered. The most common causes of simple indigestion are the addition of grain to the diet, an increase in the amount of grain fed, and an increase in the energy density of the diet. Examples of such dietary changes are replacing oats with corn and changing from whole to ground corn. If the changes are drastic, rumen acidosis (discussed next) can occur. Other common causes are changes in hay or pasture, consumption of moldy hay, and ingestion of weeds and toxic plants after overgrazing or drought. Clinical manifestations include mild anorexia that lasts for 1 to 2 days; mild diarrhea and bloat also may be present. Rumen fluid pH may be unchanged, increased, or decreased, depending on the inciting cause. Most animals improve with no treatment.1
Rumen Acidosis
Pathogenesis
Rumen acidosis usually occurs in animals that have been fed predominantly forage-based rations and suddenly are given access to large amounts of highly fermentable concentrates or concentrated forms of energy. It also can occur in animals that have been receiving concentrates previously if the amount is suddenly and drastically increased; if access is denied for a time and then suddenly returned (e.g., during weather changes or with alterations in water availability); or if ration mixing errors occur (e.g., leaving out monensin and rumen buffers).
As highly digestible carbohydrates are fermented, rumen pH drops. Lactobacillus species, which are lactic acid producers, proliferate in the acidic rumen environment and further lower rumen pH. As the rumen pH drops, rumen protozoa and many of the lactate users begin to die. Lactic acid production causes the osmotic pressure in the rumen to increase. Fluid is drawn from the systemic circulation into the rumen, resulting in dehydration and possibly hypovolemic shock. Lactate concentrations increase in the blood, potentially leading to systemic lactic acidosis. The lactic acid in the rumen also is toxic to the rumen epithelium. Damage to the epithelium can result in leakage of bacteria and toxins into the portal and systemic circulation. Chronic sequelae to rumen acidosis include fungal rumenitis and occasionally formation of liver abscesses.1,7 Liver abscesses are less commonly encountered in sheep and goats than in cattle. Laminitis also can occur but may be more of a problem in sheep than in goats.8 The severity of the disease depends on the composition of the feed, particle size, amount of feed consumed, and the period of adaptation to the diet.
Clinical Signs
Clinical manifestations vary with the amount and type of feed ingested and the time since ingestion. Signs first appear 12 to 36 hours after ingestion of the offending feed; they range from anorexia, depression, and weakness to recumbency in an animal suffering from severe circulatory shock. Dehydration usually is severe, and evidence of toxemia is present (e.g., injected mucous membranes, increased scleral injection). Colic, bilateral ventral abdominal distention, rumen stasis, and a “splashy” feel to the rumen also may be noted. Diarrhea can develop, adding to dehydration.1,8,9 The diarrheal output can range from paste-like feces to very watery droppings with foam, occasionally with pieces of easily recognizable grain. Dehydration, lactic acidosis, and toxemia may result in ataxia, head pressing, opisthotonos, seizures, and other neurologic abnormalities.10 The body temperature initially is elevated but may drop as the condition worsens or the animal becomes toxic. Secondary thiamine deficiency also can contribute to neurologic changes.11
Diagnosis
The rumen fluid pH may fall below 5.5. The fluid itself is milky gray, and particles of the inciting feed may be noted. Protozoa usually are reduced in number or absent, and large gram-positive rods (Lactobacillus species) may be seen on Gram staining.9 Clinicopathologic laboratory data are consistent with dehydration (increased PCV and total protein, prerenal azotemia) and metabolic acidosis.9 Liver enzymes (gamma-glutamyl transpeptidase [GGT], aspartate aminotransferase [AST], lactate dehydrogenase [LDH]) may be elevated on serum biochemical analysis.1,11 The leukogram can vary in appearance, ranging from normal to a degenerative left shift, depending on the severity of the case. Urinalysis reveals an increased specific gravity.
Treatment
Treatment is aimed at correcting cardiovascular shock, dehydration, acidosis, and toxemia and removing or neutralizing the offending feedstuffs. Intravenous fluids containing 5% sodium bicarbonate should be administered.1,12 Oral fluids are contraindicated because absorption is diminished, potentially increasing the rumen distention and worsening the animal’s discomfort. NSAIDs are indicated to control the pain and inflammation of toxemia (flunixin meglumine, 1.1 to 2.2 mg/kg IV).1,12 Oral administration of magnesium hydroxide and magnesium oxide (1 g/kg) may neutralize the acidic pH and is sufficient in mild cases. However, if much of the feed is still in the rumen, these two alkalizing agents will only work temporarily. Oral antibiotics have been recommended to kill rumen microflora and stop fermentation. We believe that these agents are contraindicated, however, because the gram-negative anaerobes that need to flourish to reestablish normal rumen microflora are susceptible to most antimicrobials effective against Lactobacillus species. Removing the substrate for growth of Lactobacillus organisms is more effective. Because orogastric tubes with large-enough bores for reflux of feedstuffs are too large for sheep and goats, rumenotomy is indicated in severe cases to remove the feed (see earlier under “Rumenotomy”). After the rumen pH is corrected, transfaunation of the rumen microflora with approximately 1 qt of rumen fluid from another small ruminant is beneficial (Box 5-1). Thiamine supplementation (vitamin B1, 5 mg/lb SC, three to four times a day) is indicated until rumen function returns.11 In certain instances, calcium may be indicated and can be added to the intravenous fluids (as calcium gluconate). The clinician should avoid mixing calcium salts and sodium bicarbonate.
BOX 5-1 Collection, Handling, and Storage of Rumen Fluid for Transfaunation
Storage
Rumen fluid ideally should be administered immediately. However, it can be stored for 24 to 48 hours, as follows: The surface of the fluid is covered with a layer of mineral oil to maintain an anaerobic environment and the open container is refrigerated. Caution: Do not store rumen fluid in a closed container, because it may explode.
Bacterial leakage into the rumen wall, liver, and systemic circulation makes antimicrobial therapy necessary. The systemic antimicrobial agent of choice is penicillin (procaine penicillin G, 22,000 IU/kg IM twice daily), because anaerobes are the most likely offending organisms. With aggressive treatment, the prognosis for short-term survival is good. Feed (grass hay only) and water should be limited until rumen contractions return, to prevent overdistention of the rumen. The chronic sequelae discussed previously influence long-term survival.
Reticulitis, Rumenitis, and Parakeratosis
Pathogenesis
After the mucosa has been damaged, secondary infection by bacteria or fungi can occur.13 Previous treatment with oral antibiotics may predispose animals to development of fungal infections of the rumen wall, especially if the mucosa is already damaged. Actinobacillosis, actinomycosis, and tuberculosis rarely affect the rumen wall. Tumors of the rumen wall also have been reported.1,14 Not all of these causes of reticulitis and rumenitis have been reported in sheep and goats, but all are potential problems.
Diagnosis
Confirming a diagnosis of these diseases also may prove difficult. Samples of rumen fluid may show only changes associated with anorexia (alkaline pH, decreased numbers and motility of protozoa, prolonged MBR time; see Table 5-1 for normal values). Occasionally, fungal organisms may be seen on Romanowski (Diff-Quick)-stained slides of rumen fluid. In such cases, a diagnosis of fungal rumenitis should be made. An exploratory laparotomy and rumenotomy may be required to identify foreign bodies or masses. Rumen parakeratosis is characterized by dark, thickened, and clumped rumen papillae. It is seen mainly in feedlot lambs that consume finely ground or pelleted rations.15 The parakeratotic rumen papillae are fragile and vulnerable to damage, which can increase the risk for development of rumenitis.1
Treatment and Prevention
Treatment depends on the inciting cause. Dietary changes should be made to decrease energy density and increase fiber intake. Mild rumenitis may subside with time and supportive care (i.e., transfaunation, fluid support, high-quality feed). Fungal rumenitis can be treated with oral thiabendazole, 25-44 mg/kg, when available.16 Severe changes may lead to scarring and permanent impairment of rumen function.
Diseases of the Reticulorumen
Traumatic Reticuloperitonitis
Traumatic reticuloperitonitis is not as common in small ruminants as in cattle, but it has been reported. Goats are affected more commonly than sheep. The overall lower incidence probably is related to the dietary habits of small ruminants, which tend to be selective grazers and do not “vacuum” the ground as cattle do. Offending foreign bodies that cause traumatic reticuloperitonitis include pieces of wire and needles.17–19 The clinical signs are identical to those in cattle and may include anorexia, depression, colic, signs of heart failure, and evidence of draining tracts from the chest cavity. Treatment usually is difficult.
Rumen Impaction
Rumen impaction can occur after dehydration, blockage of the omasal orifice by a foreign body, sand ingestion, or consumption of diets high in fiber and low in digestibility.20 Rumen impaction with plastic trash bags present in the environment has become a growing problem worldwide.18 Clinical manifestations are nonspecific, but the firm rumen usually can be palpated in the left flank. The feces may be scant and dry. Oral fluids containing magnesium sulfate (60 g) may loosen impactions, but a rumenotomy is required in severe cases.20
Rumenotomy
To reduce rumen fill in sheep or goats requiring rumenotomy, ideally feed should be withheld for 24 hours before surgery. Such preparation usually is impossible, however, because in most cases rumenotomy is an emergency procedure. The perioperative administration of antimicrobial agents is essential, because even with meticulous technique, some contamination of the incision site and possibly the peritoneal cavity is inevitable. Because the rumen microflora is composed predominantly of anaerobic bacteria, penicillin (22,000 IU/kg) is the antimicrobial agent of choice and should be administered 2 to 4 hours before surgery. If the rumenotomy is being performed on an emergency basis, penicillin salts (potassium or sodium), which can be given by the intravenous route, provide therapeutic concentrations more rapidly than can procaine penicillin. NSAIDs (e.g., flunixin meglumine, 1.1 to 2.2 mg/kg IV) also are recommended before surgery. If necessary, treatment of cardiovascular shock and dehydration with intravenous fluids also should begin before surgery and continue until the animal is rehydrated and in stable condition (see Chapter 3).
Rumenotomy more commonly is done with the small ruminant in right lateral recumbency, because the standing patient is likely to become recumbent anyway during the procedure; either the animal will need to be restrained on some elevated surface, or the practitioner will have to operate while kneeling. The procedure can be safely done with use of local anesthesia, usually assisted by sedation in most animals. The use of general anesthesia may be considered in fractious or very valuable animals, to decrease the potential for abdominal contamination (see Chapter 18). The clinician should clip and surgically prepare a square area from 5 cm in front of the last rib to the tuber coxae, and from the dorsal midline to the lower abdomen, encompassing the entire left paralumbar fossa.
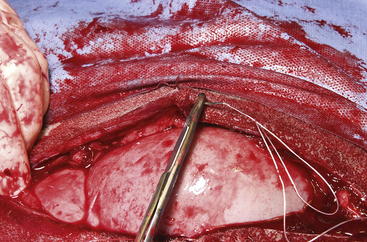
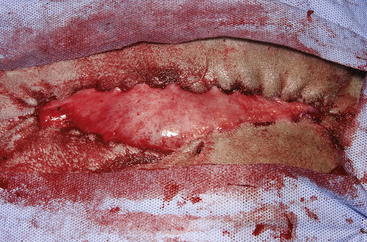
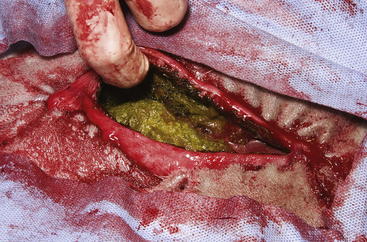
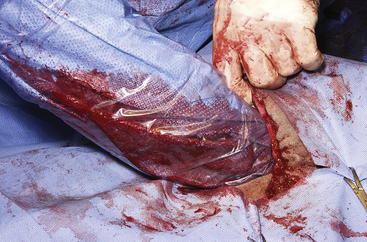
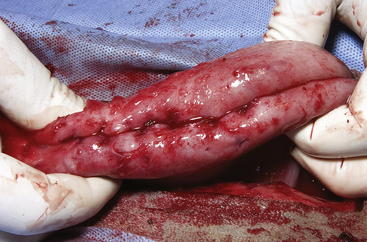
The routine skin and body wall incision is made in the middle of the left paralumbar fossa. The surgeon makes a skin incision approximately 5 cm longer than a handwidth, 5 cm caudal and parallel to the last rib. The incision is continued through the muscle layers into the abdomen. Because the abdominal wall is relatively thin, the surgeon should take care not to enter the rumen or bowel. The body wall incision must be more than adequate in size to facilitate entry of the practitioner’s hand and possibly forearm, to allow exploration of the rumen and evacuation of its contents. The rumen incision will be smaller than the body wall incision after the rumen is secured to the skin. After the body wall incision has been made, a thorough exploration of the abdominal cavity should be performed before the rumen is secured to the skin. (Note: Exploration of the abdominal cavity is absolutely contraindicated after the rumen has been opened.) After abdominal exploration, the rumen is secured to the skin by creating a watertight seal with continuous suture. The watertight seal is critical in preventing abdominal contamination. The rumen is secured to the skin with use of monofilament (or coated) suture with minimal drag in the tissue on a cutting needle in a Cushing pattern. The first “bite” is taken in the skin at either 3 or 9 o’clock (Figure 5-7); then the second bite is taken in the rumen at that location in the opposite direction. The suture is then tied and the tail left long. To prevent or minimize leakage, the rumen suture bites should be through the seromuscular layer but not penetrate the mucosa, which could lead to leakage at closure. The Cushing pattern is continued from the midpoint of the incision to the dorsal- and ventralmost aspects of the body wall incision, where a modification resembling a W stroke is done to ensure a seal at those areas. If the practitioner starts at the 9 o’clock point and sutures dorsally, the following technique is used at the dorsalmost part of the body wall incision: The standard bite (approximately ¼ inch) is made in the rumen near the end of the body wall incision; then the suture is passed superficial and dorsal to the end of the incision by approximately 2 inches before a bite is taken in the skin toward the incision. The suture then is passed superficially to the dorsalmost part of the rumen, where a transverse bite (perpendicular to the suture line) is taken in the rumen. The suture is again passed superficial and dorsal to the body wall incision, where a skin bite is made parallel to and at the same level as the previous skin suture. Then the Cushing pattern is continued from the dorsalmost (12 o’clock) position down to the 3 o’clock location, where the suture is tied. The same procedure then is followed from 3 o’clock ventrally to 6 o’clock, where the dorsalmost suture pattern is repeated and continued to the originating 9 o’clock position, where the suture is tied. Two separate suture lines are used to limit the circumferential decrease in lumen size created by one suture line pulled tightly. If an assistant is available, each operator can work simultaneously on the two separate suture lines. The rumen can now be rolled over the skin to create a watertight seal (Figure 5-8). The rumenotomy incision is then made in the center of the exposed, secured rumen (Figure 5-9). Once the rumen has been secured and opened, no other modifications should be made to the rumenotomy. The rumen contents can be evacuated by hand, which will lead to significant contamination of the field—thereby showing that a watertight seal is imperative (Figure 5-10). If the contents are very liquid, a large tube can be used to siphon the rumen. In such instances, care must be taken to guard the end of the tube, to prevent occlusion of flow by suction of the rumen wall over the end of the tube.
Closure of the rumen is performed in two layers. Absorbable suture in a simple continuous pattern is used to close the rumen lumen for the first layer, and any drapes should be removed. The surgical field should be reprepared; all soiled materials (e.g., gloves, gown, drapes) are removed and replaced, and sterile instruments are readied for the second part of the closure. Absorbable suture in an inverting pattern (e.g., Lembert, Cushing) is used for the second layer of the rumen closure. Suturing of this second layer should start at one end of the rumen incision, and retention sutures securing the rumen to the skin are removed as needed to free enough rumen for closure. When the second layer closure is complete (Figure 5-11), the rumen is cleaned with moist sponges before being returned to the abdominal cavity. (Note: Exploration of the abdominal cavity at this time is associated with an increased incidence of septic peritonitis.) The muscular body wall and skin are closed in routine fashion using the practitioner’s technique of choice.
1. Garry F.B. Indigestion in ruminants. In Smith B.P., editor: Large animal internal medicine, ed 2, St Louis: Mosby, 1996.
2. Guard C. Bloat or ruminal tympany. In Smith BP, editor. Large animal internal medicine, ed 2. St Louis: Mosby, 1996.
3. Matthews J. Diseases of the goat, ed 2. Oxford, UK: Blackwell Science; 1999.
4. Brikas P., Tsiamitas C., Wyburn R.S. On the effect of xylazine on forestomach motility in sheep. J Vet Med. 1986;33:174.
5. van Miert A.S., van Duin C.T., Anika S.M. Anorexia during febrile conditions in dwarf goats: the effect of diazepam, flurbiprofen and naloxone. Vet Q. 1986;8:266.
6. Chennells D. Bloat in kids. Goat Vet Soc J. 1981;2:16.
7. Nour M.S.M., Abusamra M.T., Hago B.E.D. Experimentally induced lactic acidosis in Nubian goats: clinical, biochemical, and pathological investigations. Small Rumin Res. 1999;31:7.
8. VanMetre D.C., Tyler J.W., Stehman S.M. Diagnosis of enteric disease in small ruminants. Vet Clin North Am Food Anim Pract. 2000;16:87.
9. Braun U., Rihs T., Schefer U. Ruminal lactic acidosis in sheep and goats. Vet Rec. 1992;130:343.
10. Lal S.B., et al. Biochemical alterations in serum and cerebrospinal fluid in experimental acidosis in goats. Res Vet Sci. 1991;50:208.
11. Karapinar T., et al. Severe thiamine deficiency in sheep with acute ruminal lactic acidosis. J Vet Intern Med. 2008;22:662.
12. Smith MC: Commonly encountered diseases of goats, Proceedings of the 1996 Symposium on the Health and Disease of Small Ruminants, Kansas City, Mo, 1996.
13. Perez V., et al. Generalized aspergillosis in dairy sheep. J Vet Med. 1999;46:613.
14. Norval M., et al. Rumen papillomas in sheep. Vet Microbiol. 1985;10:219.
15. Kutas F., Galfi P., Neogrady S. Effect of monensin on development of ruminal parakeratosis in fattening lambs. Zentralbl Veterinarmed. 1983;30:506.
16. Kersting K.W., Thompson J.R. Lactic acidosis. In: Howard J.L., Smith R.A., editors. Current veterinary therapy 4: food animal practice. Philadelphia: WB Saunders, 1999.
17. Sharma K.B., Ranka A.K. Foreign body syndrome in goats—a report of five cases. Indian Vet J. 1978;55:413.
18. Maddy K.T. Traumatic gastritis in sheep and goats. J Am Vet Med Assoc. 1954;124:124.
19. Akkoc A. Traumatic reticulopericarditis in a Saanen goat. Turk J Vet Anim Sci. 2007;31:283.
20. Smith M.C., Sherman D.M. Goat medicine, ed 2. Ames, Iowa: Wiley-Blackwell; 2009.
Diseases of the Abomasum
Abomasitis and Abomasal Ulcers
Abomasitis and abomasal ulcers in adult sheep and goats are associated with rumen acidosis or chronic rumenitis but also can be caused by infections.1–5 Finely ground feeds, pelleted rations, systemic stress, and feeding lush forages all have been implicated. Anecdotal associations with mineral deficiency (i.e., copper) have gone unproved.
Treatment
Effective therapy can be difficult to achieve. Oral medications such as coating agents must first pass through the rumen and therefore arrive at the abomasum diluted. Intravenous (not oral) ranitidine (15 mg/kg once a day) may be beneficial.6 Herd problems of rumen acidosis may be addressed with addition of buffers to the feed.
Abomasal Hemorrhage
A syndrome of abomasal hemorrhage, bloat, and ulceration is encountered in lambs and kids 2 to 10 weeks of age. Sarcina-like bacteria, Clostridium falax, Clostridium sordelli, and Clostridium septicum have been isolated in many of these cases.7–11 C. septicum infections of the abomasum commonly are called braxy.1 Free-choice milk replacer feeding regimens, iron deficiency, and bezoars have been implicated as predisposing factors.11,13
Clinical Signs
The clinical manifestations of this syndrome are severe, acute abdominal distention and colic, possible ulceration, with progression to death in all cases.7–10
Prevention
Adding formalin to milk replacers and vaccinating for clostridial diseases may decrease the occurrence of abomasal hemorrhage.12,14 Lambs or kids on farms where such disease has been a problem can be vaccinated with multivalent bacterins against Clostridium infections during the first week of life.
Abomasal Impaction
Similar to rumen impaction, abomasal impaction usually occurs when poor-quality roughage is fed, but it also can be seen with foreign body obstruction of the pylorus.4,15–17 Goats appear to be more commonly affected than sheep, and Boer goats are more commonly affected than Angora goats.17 Pregnant animals may be more prone to this condition.
Clinical Signs and Diagnosis
Affected animals usually are anorexic. Mild distention of the ventral abdomen is characteristic, and in some cases the firm abomasum can be palpated through the abdominal wall on the right side.18 Weight loss may be apparent. Clinicopathologic evaluation may be normal, or mild hypochloremic metabolic alkalosis may be present, with elevated rumen chloride concentrations (greater than 50 mEq/L).18
Treatment
Dietary changes and oral administration of mineral oil are the most commonly used treatments. Abomasotomy can be attempted, although it has rarely been reported in small ruminants and does not usually improve the long-term prognosis. For this procedure, the animal is positioned in dorsal recumbency and placed under general anesthesia. The abomasum can best be visualized through an incision parallel and to the right of midline, caudal to the xyphoid process. The prognosis is poor with or without surgery.15
Abomasal Emptying Defect
Abomasal emptying defect is a disease that manifests in similar fashion to that for abomasal impaction but is recognized only in Suffolk sheep. The underlying cause is unknown, but the proposed pathomechanism is an acquired dysautonomia from neurotoxicosis.19 Unlike abomasal impaction, this disease is associated with concentrate feeding and often occurs around lambing time. The clinical signs are chronic weight loss, abdominal distention, and anorexia. Clinicopathologic laboratory findings and rumen chloride levels are the same as those described for abomasal impaction. At necropsy, the abomasum is greatly distended, and the contents may be liquid or dry. Treatment with laxatives, cathartics, motility modifiers, and abomasotomy has been mostly unsuccessful.20–22
Azalea, Laurel, and Rhododendron Toxicity
Clinical Signs
Animals browsing a new area, those fed clippings from trimmed azalea hedges, and underfed, hungry animals with access to these plants are likely candidates for intoxication. Animals that ingest as few as two or three leaves may show salivation, tooth grinding, nasal discharge, colic, epiphora, and acute digestive upset within 6 hours of ingestion. As the intoxication progresses, animals become depressed, with a slowed pulse, and exhibit projectile vomiting and frequent defecation. Fatally intoxicated animals become paralyzed and comatose. Aspiration pneumonia secondary to the intoxication-induced impairments may develop in both sheep and goats.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree