CHAPTER 18 All species have a bicornuate uterus with uterine horns and a uterine body. The uterus of the mare has longitudinal folds. Endometrial cups are present in the endometrium between 37 and 150 days of gestation and are the site of production of equine chorionic gonadotropin, formerly pregnant mare serum gonadotropin (Fig. 18-1). These typically form around the pregnant horn at the bifurcation. Their disappearance is an immune-mediated event. The placenta of the horse is diffuse and microcotyledonary. In ruminants, each uterine horn contains four rows of protuberances that become the caruncles. These may be pigmented in sheep. The placenta of ruminants is cotyledonary. The placenta of the pig is diffuse with small ridges. Dogs and cats have a zonary placenta with marginal hematomas. Fig. 18-1 Endometrial cups, uterus, mare. The cell types of the ovary include the epithelium (surface epithelium, subsurface epithelial structures of the bitch, and the rete ovarii), the stroma, germ cells, and follicular cells. Lymphoid cells are usually absent. Control of ovarian function is from the hypothalamus and pituitary through release of gonadotropin-releasing hormone (GnRH), as well as follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (see Fig. 12-3). It is critical that infectious organisms are excluded from the uterus, otherwise fertility or pregnancy can be jeopardized. Portals of entry are listed in Box 18-1. Organisms that cause inflammation of the uterus can enter through the vulva (ascending infection) or can arrive via the blood (hematogenous infection). Reinfection of the external genitalia from nerves is a unique feature of infection with some herpesviruses. Portals of entry are listed in Box 18-2. Most infectious agents and foreign material (intramammary preparations) enter the gland in an ascending fashion via the papillary duct. Small (bacteria) and large (leaches) pathogens can enter the gland via this route. There are some instances in which organisms home to the mammary glands from systemic infection, but their number is small. Viruses, such as the retroviruses of caprine arthritis and encephalitis, ovine maedi-visna, and Mycoplasma spp., are good examples. Penetrating injury is rare. Innate, nonimmune, or physical factors are very important in the defense of the reproductive system. An adaptive immune system occurs after these have failed. In many instances, failure of the physical factors that result in infection of the tract is too late for the fetus, and infertility and failure of pregnancy occurs. Defense mechanisms are listed in Box 18-3. As with the body in general, the mammary glands have a full range of mechanisms to prevent and control infectious disease. It relies heavily on isolation. The structure and function of the papillary duct of the teat and the keratin that accumulates there to form a plug prevent many potential pathogens from entering the gland. Secretions of the gland contain antimicrobial, antiinflammatory, and immune-modulating substances. Within the gland, there also are humoral and cellular defenses. Innate defense mechanisms are listed in Box 18-4. Normal Sexual Development: Sexual development occurs in three sequential processes: (1) chromosomal sex is established at conception, (2) gonadal sex occurs early in fetal development, and (3) phenotypic sex soon follows. Germ cells migrate from the yolk sac to the genital ridge, and without germ cells the ovaries do not develop, and dysgenesis is the result. The undifferentiated and bipotential gonad acquires germ cells, mesenchymal cells, coelomic epithelial cells, and mesonephric epithelial cells. These form the major cell types in the developed gonad: germ cells; supporting, steroid-producing cells and unspecialized mesenchyme; and epithelium. Before differentiation into male and female, the embryo has a double set of ducts: the mesonephric (Wolffian) ducts and tubules and the paramesonephric (Müllerian) duct (Fig. 18-2). In individuals with a chromosomal sex of XX (female), without the sex-determining region of the Y chromosome (SRY negative), there is activation of genes and gene products so that a normal ovary develops. Development of a testis is inhibited. The tubular genitalia of the female develop from the paramesonephric ducts, and the mesonephric ducts disappear. The paramesonephric ducts are paired and join the urogenital sinus. They fuse to form the cranial vagina and the uterine body. The urogenital sinus forms the vulva and posterior part of the vagina. The external genital tubercle forms the clitoris. All stages of the development of the genitalia are under the control of genes and gene products; the female reproductive system is not a “default” system. Fig. 18-2 Schematic diagram of the normal components of the female reproductive system and the embryonic structures, especially the paramesonephric (Müllerian) duct and urogenital sinus and tubercle from which they were derived. Major Anomalies: Major anomalies are those that result in dramatic abnormalities in sexual phenotype and usually result in infertility. Affected animals are phenotypically male, female, or ambiguous or altered. The exact defect cannot be reliably determined from phenotypic characteristics alone. There are a large number of individual steps involved in sexual development and differentiation, and missing or changing one step can have major effects on subsequent differentiation. Common disorders of sexual development (DSD) are listed in Table 18-1. Sex chromosome DSD are those with an abnormal number and/or mixture of sex chromosomes, including XXY (Klinefelter syndrome), X_ (Turner syndrome), and XX/XY (chimerism). XY disorders of sexual development are those with disorders of testicular development, disorders of androgen synthesis or action, and miscellaneous conditions (see Chapter 19). XX DSD includes disorders of ovarian development, androgen excess, or miscellaneous disorders. The greater availability of tests for the SRY gene means a greater ability to define the underlying anomaly. XX disorders are now defined as XX SRY positive and XX SRY negative, and XY disorders are subdivided into XY SRY-positive and XY SRY-negative genotypes. Sex Chromosome Disorders of Sexual Development: True chromosomal DSD is very rare. Cases of X_ (Turner syndrome) and XXY (Klinefelter syndrome) are reported. They usually have gonadal dysgenesis and a feminine phenotype. Chimerism is more common. Chimeras and mosaics have two or more somatic cell types, each with a different chromosomal constitution. Chimeras have two genetically distinct cell types that come from different individuals, whereas mosaicism is a different chromosomal constitution from altered mitosis. The most common chimera in domestic animals is the freemartin calf (Fig. 18-3). Vessels of the placentas from two different fetuses fuse and exchange blood between fetuses. Each fetus becomes a hematopoietic chimera. Anastomosis of placental vessels occurs most often in bovine species and less frequently in other ruminants and pigs. The freemartin is the female of a set of male and female twins. Gene products from the cells of the male fetus induce fetal Sertoli cells and seminiferous cordlike structures in the ovaries of the female twin. The ovaries are small and can have reduced number of or no germ cells or organs partially converted to testes. The paramesonephric (Müllerian) duct derivatives vary from almost normal to cordlike structures, but their lumens do not communication with the vagina. The vagina, vestibule, and vulva are hypoplastic. Vesicular glands are always present; other mesonephric (Wolffian) structures are present to varying degrees. Externally, the animal has a female phenotype, but the vestibule and vagina are short, the vulva is hypoplastic, and the clitoris is enlarged. The male twin is minimally affected. Fig. 18-3 Chromosomal disorder of sexual development, bovine freemartinism, cow. XY Disorders of Sexual Development: XY DSD has a normal male chromosomal component (XY) and a female phenotype. They have abnormal gonadal development that drives phenotypic abnormalities, abnormal androgen synthesis, or lack androgen receptors. They are described according to the presence or absence of the SRY gene and the gonadal type. The most extreme example is XY DSD, SRY-positive with testes and female phenotype. These were called male pseudohermaphrodites, testicular feminization, or XY sex reversal (Fig. 18-4). They usually lack androgen receptors. Serum testosterone is present, but the genitalia are female. A mild form occurs in Miniature schnauzers with persistent Müllerian duct syndrome. They are XY males with a complete paramesonephric system, including uterine tube, uterus, and cranial portion of the vagina. They lack the anti-Müllerian hormone (AMH, previously called Müllerian inhibitory substance) or its receptor. XY DSD, SRY negative with gonadal dysgenesis and female phenotype is another category and is found in horses and other species. They have hypoplastic or undifferentiated gonads and a female phenotype. Fig. 18-4 Disorder of sexual development with testis, male pseudohermaphrodite, reproductive tract. XX Disorders of Sexual Development: The only category of XX DSD reported in animals is XX DSD, SRY negative. Most are XX DSD, SRY negative with ovotestes and a female but ambiguous phenotype. They are usually true hermaphrodites with both male and female gonads (Fig. 18-5). They are phenotypically female, with masculinization, for example, an enlarged clitoris. American cocker spaniels and some other breeds of dog have this autosomal recessive trait. In goats, it is associated with the polled gene. Confirmation of this syndrome requires genotyping because in the case of goats, the presence of mammary development in bucks is not always an indication of an XX DSD. Fig. 18-5 Disorder of sexual development with ovotestis, true hermaphrodite, reproductive tract. Anomalies with Normal Sexual Development: There are many different anomalies found in individuals with normal genotypic, gonadal, and phenotypic sex. They include failure of the normal maturation, hypoplasia, or aplasia of parts of the internal or external genitalia. Because normal development requires such intricate timing of events, including regression of some parts, the joining of tubes and tubules, migration of components from one site to another, the interaction of genes, and hormones and local factors, it is no wonder there are myriad anomalies. Segmental aplasia of the paramesonephric duct can affect any part and little is known of its pathogenesis. A genetic basis is implicated in shorthorn cattle, in which it is linked to the recessive gene for white coat color. The simplest form is failure of the paramesonephric duct to make a proper connection with the urogenital sinus, leaving a persistent hymen, a membrane at the site where the two precursor tissues join (Fig. 18-6). A perforated hymen sometimes persists and is not clinically significant. If the hymen is complete and there is no drainage of fluid from the uterus, the upstream portion of the vagina, cervix, and uterus distend with normal secretions. In the more severe forms of segmental aplasia, one or more segments of the vagina, cervix, uterine body, and uterine horns are absent or rudimentary. Aplasia of a segment of the uterus (Fig. 18-7) occurs in cattle. Prostaglandin can be synthesized and released from the blind ending uterine horn, just as is produced by a normally connected uterine horn. In those with a local utero-ovarian pathway for luteolysis, such as the cow, the absence of a segment of the uterus could result in insufficient PGF2α to cause regression of the corpus luteum. In the pig, where systemic transmission of PGF2α from the endometrium to the corpus luteum is important, prostaglandins from the blind uterine horn can have a lytic effect on the corpora lutea of pregnancy in the contralateral ovary. In the dog and cat, the uterus does not play a role in the regression of the corpus luteum. Fig. 18-6 Persistent hymen, vagina, and vulva, bitch. Fig. 18-7 Segmental aplasia of a uterine horn, uterus, pig. Minor Anomalies: Minor or incidental anomalies are myriad in the reproductive tract. The same factors influencing the development of major anomalies in animals with normal sexual differentiation also operate here. Minor anomalies are incidental and not clinically significant apart from potentially being mistaken for lesions that do cause infertility, subfertility, or are life threatening. Foremost of these are the numerous cysts and tubular remnants. Periovarian cysts are extremely common and can be confused with cystic neoplasms. They can be derived from paramesonephric ducts, mesonephric ducts, and mesonephric tubules. Web Table 18-1 lists the location and names of common incidental cystic anomalies. They are discussed in more detail later. Inclusion cysts of the reproductive tract are isolated cysts not derived from embryonic elements. The serosal inclusion cyst of the uterus in bitches is a common type (Fig. 18-8). It arises from a small group of mesothelial cells trapped beneath the serosal surface during involution of the uterus. These grapelike clusters of semitransparent thin-walled cysts are located on the serosal surface of the uterus. Subsequent distention and enlargement result in numerous cysts developing. Fig. 18-8 Uterine serosal inclusion cysts, reproductive tract, bitch. Many of the commonly seen syndromes of sexual development are listed in Table 18-1. Developmental Anomalies: Agenesis, a total lack of ovarian tissue, can involve one or both ovaries. The entire reproductive tract also can be absent. In cases of bilateral agenesis, the tubular genitalia remain infantile. Cysts In and Around the Ovary: Periovarian (paraovarian) cysts are cysts that are external to the ovary. They are common findings in dogs and cats during ovariohysterectomy (see Web Table 18-1). Intraovarian cysts are the cysts within the ovary. These should be differentiated from cystic neoplasms (see next section). Periovarian cysts: Periovarian cysts are usually cystic remnants of embryonic structures, either paramesonephric ducts or mesonephric tubules or ducts. Location of the cyst helps differentiate them. Cystic remnants of the paramesonephric ducts include the fimbrial cyst and the cystic accessory uterine tube. This is common in the mare and called the hydatid of Morgagni (Fig. 18-9). Hydatid of Morgagni measure up to several centimeters in diameter and are cranial to the ovary in the mesovarium. Cystic accessory uterine tubes are in the mesosalphynx. Histologically, they resemble the normal uterus and have a thin coat of muscle. There are cysts that arise from mesonephric remnants, either ducts or tubules. Cysts of the mesonephric duct are in the cranial or caudal mesovarium and histologically have a thick smooth muscle coat. Intraovarian cysts: Intraovarian cysts are numerous and common. Many are derived from Graafian follicles but others are epithelial cysts from the surface epithelium or from the intraovarian rete ovarii, embryonic structures of mesonephric tubular origin. The most common in the mare is an epithelial inclusion cyst, and the most common in the dog and cat is the cystic rete ovarii (Fig. 18-10). Fig. 18-10 Multiple cystic rete ovarii, ovary, bitch. Epithelial inclusion cysts in mares can cause infertility (see Web Table 18-1). They are located in the ovary around the ovulation fossa. Epithelium from the surface of the ovary becomes pinched off and embedded in the stroma during ovulation. This epithelium produces fluid that causes the structure to enlarge and eventually reach several centimeters in diameter. They are identical in appearance to large follicles, but they do not appear and disappear as follicles should; histologic assessment is necessary for diagnosis. Their size and number may block ovulation. Epithelial inclusion cysts in other species, or cysts of subsurface epithelial structures in bitches form in a similar manner, but they are in the capsule of the ovary and are small and incidental. Cystic ovarian (Graafian) follicles, or follicular cysts, are defined as follicles that are larger than normal. They are especially important in cows and sows. The disease in cows is called cystic ovarian degeneration (COD). Bovine cystic follicles are 2.5 cm or more in diameter (Fig. 18-11) and persist for 10 or more days without the formation of a corpus luteum. The prolongation of the postpartum interval to first estrus (so called days-open) is the main consequence of cystic follicles. Ovulation does not occur. These cysts probably develop because of an abnormality of the hypothalamo-hypophyseal-ovarian axis that causes a deficiency of LH or of the LH receptor in the ovary, although the mechanism is not confirmed. Cystic ovarian degeneration is treated with GnRH, which causes release of LH in the pituitary, and with chorionic gonadotropin (high in LH). Evidence suggests that stress is involved wherein adrenocorticotropic hormone (ACTH) or cortisol inhibits GnRH release from the hypothalamus and prevents upregulation of LH receptors in the ovary. Higher concentrations of progesterone can have a similar effect. The end result is an inadequate LH surge and failure of ovulation. In a similar way, postpartum uterine infection can cause cystic follicles. Escherichia coli infection of the uterus increases concentrations of serum PGF2α metabolites and cortisol. It was proposed that bacterial endotoxins, or the prostaglandins produced because of damage caused by endotoxins, stimulate the adrenal cortical secretion of cortisol; cortisol excess suppresses the preovulatory release of LH, resulting in the development of cysts. Fig. 18-11 Cystic Graafian follicle, ovary, cow (also called follicular cysts). Luteinized cysts are anovulatory luteinized follicular cysts. They develop from follicular cysts by delayed or insufficient release of LH and are therefore part of COD (see Web Table 18-1). It therefore occurs in cows and sows more often than in other species. Luteinized cells line the cystic cavity. Cystic follicles and luteinized cysts can occur in the same ovary. Cystic corpus luteum is a corpus luteum with a cystic center. It is unknown why a follicle fails to luteinize fully. The cystic center is larger than the normal small central cavity that occurs normally in some corpora lutea. Ovulation occurs in a normal follicle, but a large irregular cystic center develops (Fig. 18-12). The length of the estrous cycle is not affected, and most cysts are incidental. Inflammation of the Ovary: Oophoritis, or inflammation of the ovary, is rare in domestic animals. Infectious bovine rhinotracheitis virus (bovine herpesvirus 1 [BoHV-1]) viremia in experimental studies can induce necrotizing oophoritis in the postestrus cow. A cloudy fibrinous fluid fills some follicles. Microscopically, the lesions in the corpus luteum range from focal necrosis and the presence of mononuclear cells to diffuse hemorrhage and necrosis. Most affected ovaries also have necrotic follicles and mononuclear cells in the stroma. Bovine viral diarrhea (BVD) virus, a vertically and horizontally transmitted virus responsible for mild-to-fatal enteric disease and reproductive failure, can localize in bovine oocytes and cumulus cells and cause chronic oophoritis. Infection of ovarian oocytes with BVD virus is one of several possible routes of transmission of the virus from cow to fetus. Bacterial oophoritis occasionally is found in cats and dogs. In cats, it must be differentiated from feline infectious peritonitis (FIP). The inflammation is around the ovary and within the uterine tube, suggesting that the causative bacteria ascended from the uterus. Miscellaneous Diseases of the Ovary: Supernumerary follicles occur in bovine ovaries when FSH is used in doses to cause superovulation in preparation for embryo transfer. There may be more than a dozen well-developed ovarian follicles or corpora lutea. Neoplasms: There are three main groups of primary ovarian neoplasms in domesticated animals: germ cell, sex cord stromal, and epithelial neoplasms. Little is known of ovarian carcinogenesis. Neoplasms rarely metastasize to the ovary of domestic mammals. Germ cell neoplasms: Neoplasms arising from germ cells can differentiate along either embryonic or extraembryonic lines and are benign or malignant. The majority of neoplasms of germ cells are benign and undifferentiated (dysgerminoma) or benign with somatic differentiation (teratoma). Ovarian teratomas are rare and usually well differentiated and benign. They have disorganized elements of at least two of the three embryonic germ layers: ectoderm, including neuroepithelium; mesoderm; and endoderm. Skin with hair is often present (Fig. 18-13). Bone, cartilage, nervous tissue, fat, and respiratory epithelium are frequently seen. Malignant teratomas occur less often, and they are usually poorly differentiated with primitive tissue types. Fig. 18-13 Ovarian teratoma, ovary, bitch. Sex cord stromal neoplasms: Sex cord stromal neoplasms have a phenotype that resembles the tissues derived from the sex cords and/follicles. Most have regions with any combination of granulosa cell, theca cell, luteal cell, Sertoli cell, or interstitial endocrine cell phenotypes. Granulosa cell phenotype usually predominates, thus most are called granulosa cell tumors. Most produce estrogens, androgens, and/or inhibin. The mare can have signs of anestrus (inhibin-producing), nymphomania (estrogen-producing), or stallion-like behavior (androgen-producing); the bitch is likely to have prolonged estrus and may develop pyometra. Granulosa cell tumors: Granulosa cell tumors are the most common ovarian neoplasms reported in large animals. They are unilateral, smooth surfaced, and round and can be 20 to 30 cm in diameter. They can be solid, cystic, or polycystic (Fig. 18-14, A and B). The cysts can vary from microscopic size to several centimeters in diameter. Often, the fluid within the cysts is red-brown. Microscopically, the neoplastic cells resemble normal granulosa cells and often are arranged as they would be in normal Graafian follicles: in single or multiple rows of round to columnar cells lining fluid-filled spaces (Fig. 18-14, C). In less differentiated areas, the neoplastic cells are arranged in sheets. Sometimes, especially in queens, Call-Exner bodies are present. These are rosettes of granulosa cells around a central space. The stroma can be sparse or plentiful. Granulosa cell tumors in the mare and cow are usually benign, are sometimes malignant in the bitch, and are often malignant in the queen; prognostic criteria are not well established. Fig. 18-14 Sex cord stromal neoplasm, granulosa cell tumor, ovary, cow.
Female Reproductive System and Mammary Gland*
Structure
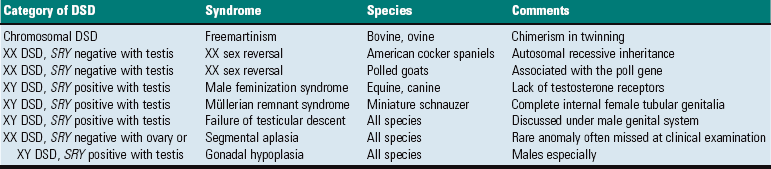
Endometrial cups are plaque-like structures in the endometrium that form when trophoblasts invade the endometrium early in pregnancy. They are present between 37 and 150 days of pregnancy and secrete equine chorionic gonadotropin. The chorionic surface opposite each cup is called the chorioallantoic pouch and is avillous. (Courtesy Dr. K. Read, College of Veterinary Medicine, Texas A&M University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Cell Types
Portals Of Entry
Mammary Glands
Defense Mechanisms
Mammary Glands
Disorders Of Domestic Animals (Horses, Ruminants [Cattle, Sheep, And Goats], Pigs, Dogs, And Cats)
Disorders of Sexual Development
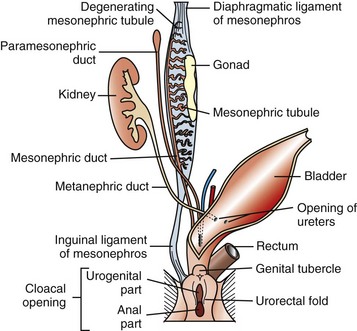
The paired paramesonephric ducts fuse to form the body of the uterus, cervix, and cranial vagina. The mesonephric tubules remain as the microscopic rete ovarii, but the mesonephric ducts usually regress completely.
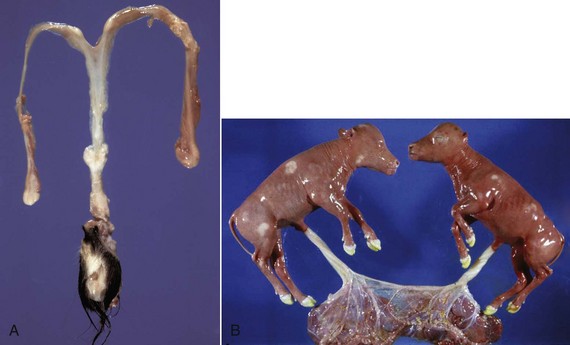
A, Phenotypically female, reproductive tract, cow. The freemartin is the female of a set of male and female twins. Freemartins are chimeras. There is a vulva and vagina with a prominent clitoris. The internal genitalia consist of bulbourethral and vesicular glands, deferent duct, and short incomplete segments of uterus. The gonads are testis with epididymides attached. This major anomaly renders the cow infertile. B, Placenta, twin fetuses. Placental vascular anastomosis, which allows exchange of blood between fetuses, is a requirement for freemartinism. These anastomoses occur most often in the bovine species. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
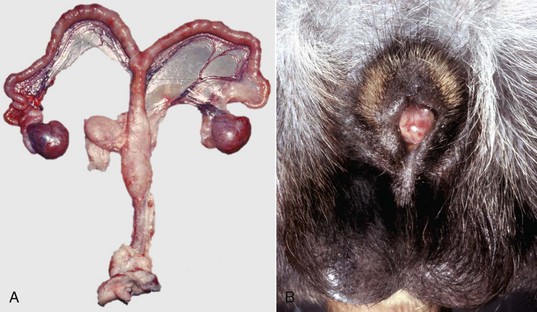
A, Pig. A testis and epididymis are present on each side. Note the well-developed uterus, cervix, and vagina. No ovarian tissue is present. B, Clitoral enlargement, dog. The clitoris protrudes between the labia of the vulva and is visible on the ventral floor of the vulva. Note the formation of a bifid scrotum ventral to the vulva. (Courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois.)
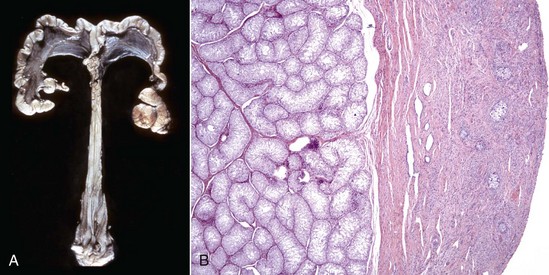
A, Gilt, an ovotestis is on the left and a testis on the right. Note the well-developed uterus, cervix, and vagina. B, Dog, ovotestis, at the periphery (right half of image) is the ovarian component with capsule and stroma. No active follicles are visible. The testicular component contains seminiferous tubules lined by Sertoli cells (left half of image). There is no spermatogenesis in these tubules. H&E stain. (A courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois. B courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
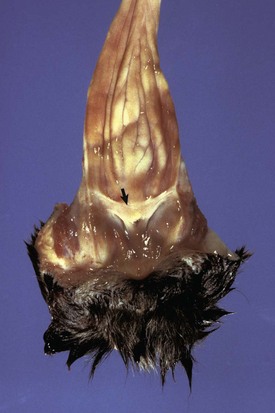
The membrane (arrow) partially separates the vestibule from the vagina and is just cranial to the urethral opening. This minor anomaly is of little consequence and does not interfere with coitus or parturition. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
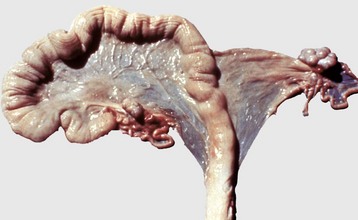
The right uterine horn is completely missing. (Courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois.)
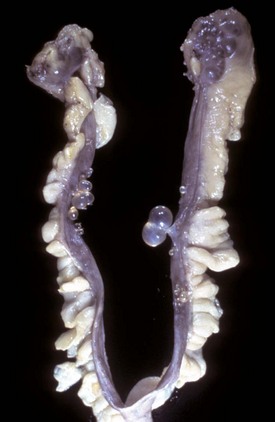
The cysts projecting from the serosal surface of the uterus are believed to arise from mesothelial cells trapped within serosal connective tissue. These cysts are an incidental finding at ovariohysterectomy. Note that there are also multiple thin-walled cysts around the right ovary. These cysts are remnants of embryonal ducts and are called periovarian cysts. (Courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois.)
Common Syndromes
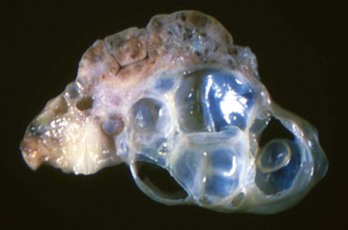
Note the multiple cysts (lower right half of image) within the ovary at the hilus. They are incidental findings in bitches and are of little consequence. They develop from the rete (mesonephric tubules) of the ovary and become cystically distended. In cats, they can be unilocular and very large and cause pressure atrophy of the ovary. They must be differentiated from cystadenomas and cystadenocarcinomas, histologically. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
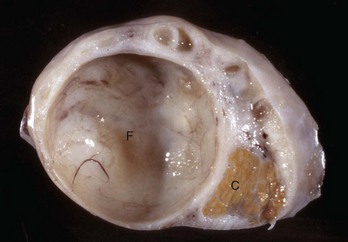
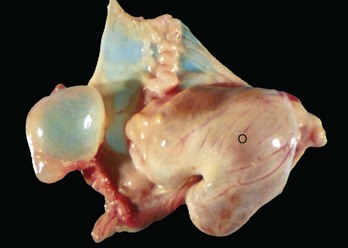
Follicular cysts (F) are larger than normal follicles and usually greater than 2.5 cm in diameter. They are the macroscopic lesions of cystic ovarian disease in the cow. They arise when ovulation of a normal follicle does not occur. C, Corpus luteum. (Courtesy Dr. R.B. Miller, Ontario Veterinary College, University of Guelph.)
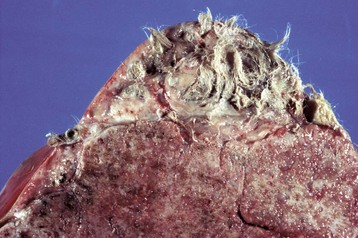
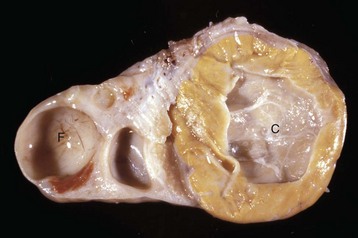
These tumors have cells that represent all three germ cell lines: ectodermal (epithelium, including neuroepithelium), mesodermal (mesenchymal tissue), and endodermal (intestine and respiratory tissues). The most common tissues seen macroscopically include hair, cartilage, and bone. This teratoma was 30 cm in diameter and surrounded by a bursa, but residual ovarian tissue was not found. Hair (top half of image) and bone are the main structures visible. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
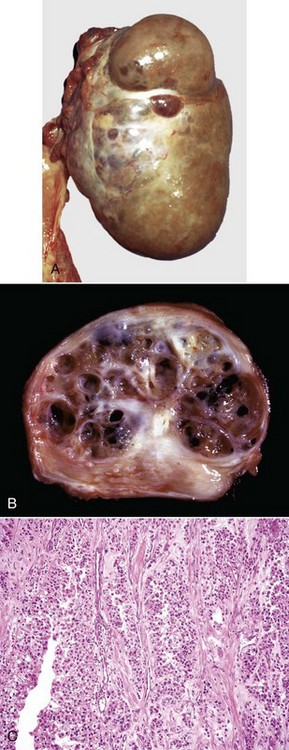
A, This large, lobulated neoplasm has obliterated the normal structure of the ovary. They can be solid (as in this case) or multicystic. B, Multiple fluid-filled cysts and solid areas have caused the dramatic ovarian enlargement. Granulosa cell tumors are part of the group of neoplasms known as sex cord stromal tumors. C, This granulosa cell tumor has solid and cystic regions. The cysts are lined by cells that resemble granulosa cells of the follicle. H&E stain. (A courtesy College of Veterinary Medicine, University of Illinois. B and C courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree