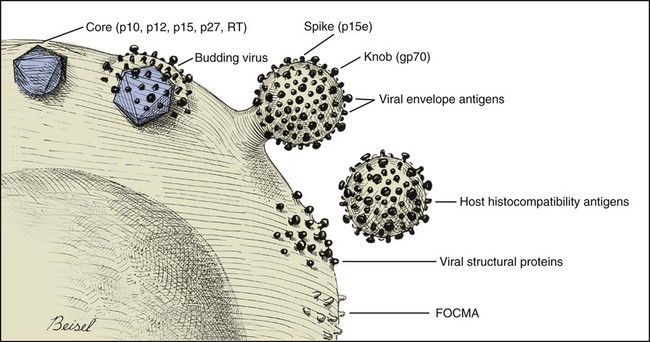
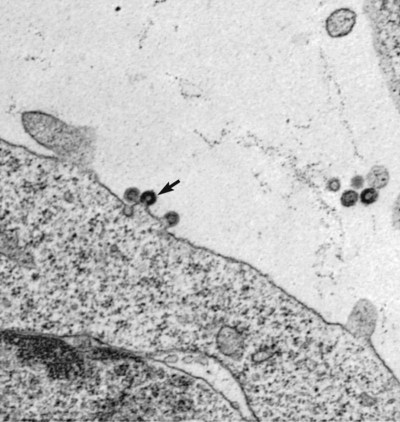
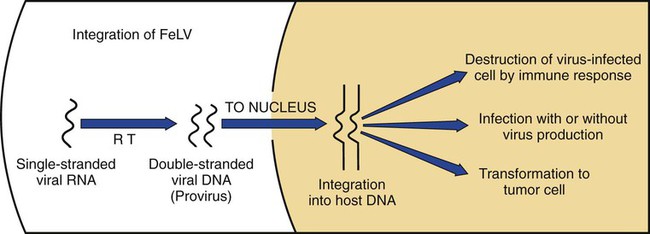
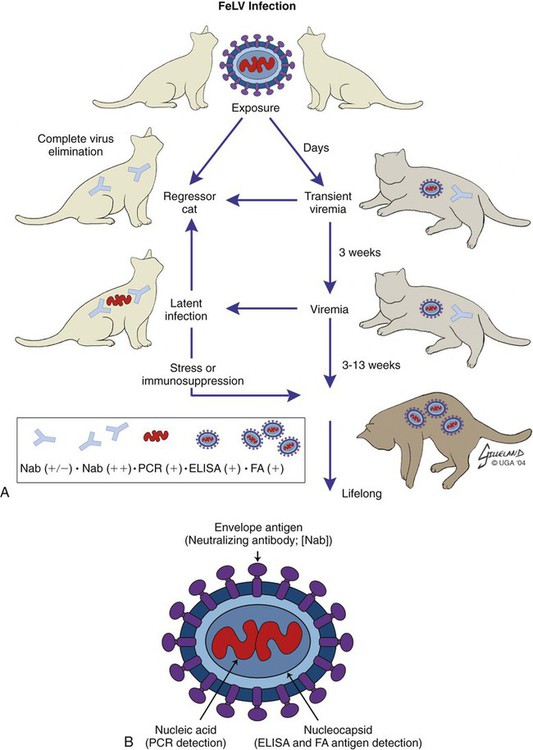
Feline leukemia virus (FeLV) infection occurs worldwide.62,93 For many years after its discovery, FeLV was considered to (1) be the principal scourge in cats, (2) account for most disease-related deaths in pet cats, and (3) be responsible for more clinical syndromes than any other single agent.375 FeLV was first described in 1964 by William Jarrett and co-workers, when virus particles were seen budding from the membrane of malignant lymphoblasts from a cat with naturally occurring lymphoma (Figs. 11-1 and 11-2).218,219 The virus was shown to produce a similar tumor when experimentally injected into healthy cats and thus was proven to be capable of transmitting neoplasia. Although clusters of lymphoma cases occurring in households had always been observed, it was not until the discovery of FeLV that an infectious etiology was finally proven. After this discovery, it was assumed for many years that all hematopoietic tumors in cats were caused by FeLV, independent of whether the cats were found to be FeLV-positive.146 Later, it had been estimated that at least approximately one third of all cancer deaths in cats were caused by FeLV, and an even greater number of infected cats died of anemia and infectious diseases caused by suppressive effects of FeLV on bone marrow and immune system.62 However, today these assumptions are being reconsidered because the prevalence and importance of FeLV as a pathogen in cats are decreasing, primarily because of testing and eradication programs and routine use of FeLV vaccines. It is currently accepted that tumor-causing factors other than FeLV play more important roles, specifically in older cats.279 FeLV, a γ-retrovirus of domestic cats, is a member of the Oncornavirus subfamily of retroviruses. It contains a protein core with single-stranded RNA protected by an envelope. FeLV is an exogenous agent that replicates within many tissues, including bone marrow, salivary glands, and respiratory epithelium. If the immune response does not intervene after initial infection, FeLV spreads to the bone marrow and infects hematopoietic precursor cells. All retroviruses, including FeLV, rely on a DNA intermediate for replication. The single-stranded RNA genome is reversely transcribed into DNA, which is randomly integrated into the host’s cell genome (the integrated DNA is called “provirus”) with the help of an integrase (Fig. 11-3). After reverse transcription, synthesis of viral proteins occurs with assembly of the virions near the cell membrane and budding from the cell (see Fig. 11-1). Infection of a cell by a retrovirus does not usually lead to cell death. Once the provirus is integrated, cell division results in daughter cells that also contain viral DNA. The ability of the virus to become part of the host’s own DNA is crucial for the lifelong persistence of the virus after bone marrow infection. Consequently, every infected cell has to be recognized and destroyed to “cure” an infection. Once the pool of hematologic and immune stem cells becomes infected, true elimination of the virus becomes impossible.48,187,187 Both exogenous (foreign, “pathogenic”) and endogenous (inherited, “nonpathogenic”) retroviruses occur in cats.347a Pathogenic exogenous viruses that can be transmitted horizontally from cat to cat include FeLV, feline immunodeficiency virus (FIV, see Chapter 12), and feline foamy virus (also known as syncytium-forming virus, see Chapter 15), which is widespread but has a low pathogenicity. On the basis of similarities in nucleotide sequences, it is likely that FeLV evolved from a virus in an ancestor of the rat. It is likely that this event took place in the late Pleistocene up to 10 million years ago in the North African desert. Ancestral rats and cats roamed freely, and the virus was transmitted to cats through ingestion or a rat bite. The initial spread of FeLV among cats might have been inhibited by the aridity of the North African desert.29 Certain endogenous, nonpathogenic retroviruses (e.g., enFeLV, RD-114 virus, MAC-1 virus) are normally present in the genome of the cat population and inherited by transmission from mother to kitten through germline. These endogenous fractions of proviral DNA (also called “proviral sin”) cannot produce infectious virus particles themselves. They are present in every feline cell but not replicating. Their main relevance relies on the fact that these DNA fractions can potentially recombine with FeLV-A DNA in cats with FeLV-A infection and thus increase the pathogenicity of FeLV-A. EnFeLV is thought to have originated hundreds of thousands of years ago from cats that had eaten mice viremic with a murine leukemia virus (MuLV) that was able to incorporate its genome into the germline cells of the predator. This MuLV was then inherited by all the feline offspring. The enFeLV genome is not complete and, therefore, is not competent to replicate by its own.409 The amount of enFeLV varies between different breeds of cats, including the wild cat (Felis silvestris), suggesting that this exposure to MuLVs is a continuing phenomenon,347a,426 and an association between enFeLV loads and FeLV-A replication but not with outcome of FeLV-A infection was demonstrated.425 RD-114 is of primate origin and is most closely related to an endogenous baboon retrovirus and only distantly related to FeLV. It is thought to have originated hundreds of thousands of years ago from an ancestor cat that had preyed on an early primate infected with this RD-114 virus.23 RD-114 is replication competent. Although no evidence shows pathogenicity of or any immune response to RD-114 virus in cats, it may play some role in normal fetal differentiation.58,62,62 It also appears important to monitor RD-114 virus production in feline cell lines used for biological products as substrates, and assays to screen for RD-114 infection in cell culture have been developed.383 FeLV exists in several subgroups that are mainly defined by host cell spectrum, on the basis of their ability to replicate in nonfeline tissues, interference testing, and virus neutralization (Table 11-1). The three most important FeLV subgroups are FeLV-A, FeLV-B, and FeLV-C, all immunologically closely related. Other less important subgroups have been described, including subgroup T, which is highly cytolytic for T lymphocytes and causes severe immunosuppression.24,250,250 A particular “FeLV feline acquired immunodeficiency syndrome” (FAIDS) is composed of FeLV-A virus and highly immunopathogenic variants that infect CD4+ and CD8+ lymphocytes and B lymphocytes in blood, lymph nodes, and myeloid cells.354 This widespread proliferation greatly impairs the immune response. TABLE 11-1 Feline Leukemia Virus Subgroupa Modified from Jarrett O. 1990. Feline leukemia virus subgroups, pp 473-479. In Hardy WD, Essex M, McCleland AJ (eds), Feline leukemia virus. Elsevier, New York; Nakata R, Miyazawa T, Shin YS, et al. 2003. Reevaluation of host ranges of felline leukemia virus subgroups. Microbes Infect 5:947-950. Only FeLV-A is contagious and passed horizontally from cat to cat in nature. The other subgroups evolve de novo in a FeLV-A-infected cat by mutation and recombination between FeLV-A and cellular or endogenous retroviral sequences contained in normal feline DNA. Subgroup B originates from recombination of FeLV-A with enFeLV. Subgroup C is less common and is the result of mutations in the env gene. It has been suggested that FeLV-C arises in FeLV-A-infected cats through intermediates that are multitropic in their receptor use.392 Replication of FeLV-B and FeLV-C is only possible with the help of FeLV-A, because important genomic sequences are replaced in these recombinant viruses. Proposed FeLV-A helper functions include enhanced replication efficiency, immune evasion, and replication rescue for defective FeLV-B and FeLV-C virions. However, in certain experiments, it was possible to induce replication without FeLV-A. In newborn specific-pathogen free kittens, experimental FeLV-B or FeLV-C infection has been established without presence of FeLV-A.27,387 Nevertheless, all naturally infected cats carry FeLV-A either alone or in combination with FeLV-B, FeLV-C, or both. Thus, if antibodies against subgroup A are produced, the cat is protected against any FeLV infection. Pathogenicity of FeLV-B and FeLV-C, in combination with FeLV-A, is higher than that of FeLV-A alone.374 However, in one experiment, infection of FeLV-A in combination with FeLV-B under experimental conditions was associated with an attenuated infection compared to infection with FeLV-A alone when inoculation of different subgroups was performed simultaneously.344 Different properties of the envelope proteins in the various subgroups have been shown to be the major pathogenic determinant, but the mechanisms by which envelope differences influence pathogenesis are not well understood.317 FeLV-B is commonly associated with malignancies; FeLV-C is mainly associated with nonregenerative anemia. In experimental infections, a FeLV-B strain (Rickard strain) caused lymphoma in nearly 100% of kittens by 1 year of infection, whereas FeLV-C isolates repeatedly produced fatal nonregenerative anemia.338 FeLV-B has been associated with a majority of cats with thymic lymphomas.4 FeLV is a typical retrovirus, containing single-stranded RNA that is transcribed by the enzyme reverse transcriptase (RT) into DNA, the so-called provirus that is subsequently integrated into the cellular genome. The gene sequence contains long terminal repeats (LTRs), which are repeated sequences that have regulatory function and control expression of the other viral genes but generally do not code for a protein product. From the 5′ to the 3′ end, the gene order is LTR-gag-pol-env-LTR. LTR regions play a critical role in tissue tropism and pathogenic potential of the viruses. Within the LTRs, recurrent enhancer sequences or upstream region enhancers (UREs) are frequently found in cats with myeloid leukemias and thought to play some role in oncogenesis.298,325 Of the UREs, the U3-LTR of FeLV upregulates specific cellular genes in an integration-independent way. The U3-LTR region does not encode a protein but instead makes a specific RNA transcript. It was demonstrated that FeLV U3-LTR upregulates the NFκB signaling pathway via activation of Ras-Raf-IκB kinase and degradation of IκB, providing new explanations of LTR-mediated cellular gene transactivation that might play a role in oncogenesis.2 The gag (group-associated antigen) gene encodes the internal structural proteins, including p15c, p12, p27, and p10 (Table 11-2). The gag protein p27, which is routinely used for diagnosis of FeLV infection, is produced in virus-infected cells in amounts exceeding what is necessary for assembly of new virus particles. Thus, p27 is abundant in the cytoplasm of individual infected cells and also in the blood of infected cats, which is why most available immunochromatographic tests, such as the enzyme-linked immunosorbent assay (ELISA) and immunofluorescence assays, are designed to detect this protein, in blood or intracellularly, respectively. Free p27 not only circulates in blood but is shed in tears and saliva, where it also can be detected. The pol (polymerase) gene specifies the viral enzyme RT, which is responsible for synthesis of DNA on the RNA template. The env (envelope) gene encodes the envelope components gp70 and p15e. The env protein gp70 defines the virus subgroup and appears to be important for inducing immunity. Antibodies to gp70 are subgroup-specific and result in neutralization of the virus and immunity to reinfection. Thus, gp70 is important in natural resistance and, therefore, as a target for vaccine production. The transmembrane protein p15e is thought to interfere with host cell immune responses, thus facilitating viral persistence. TABLE 11-2 Summary of Genetic Map and Function of FeLV Proteinsa ELISA, Enzyme-linked immunosorbent assay; env, envelope; FeLV, feline leukemia virus; gag, group-associated antigen; gp, glycoprotein; ICGA, immunochromatography assay; IFA, immunofluorescent antibody; P, protein (number is molecular weight in kilodaltons); pol, polymerase; RT, reverse transcriptase. aAs listed in chart, genes are located from the 5′ to the 3′ end with long terminal repeat (LTR) sequences at each end. In vitro, FeLV can replicate also in nonfelid cell lines (see Table 11-1). For example, FeLV-B replicates in cells derived from cats, dogs, cows, pigs, hamsters, monkeys, and humans; FeLV-C replicates in cells of cats, dogs, guinea pigs, and human beings.214,217,217 It was thought that FeLV-A only replicates in cat cells in vitro, and that infection in vivo that always requires FeLV-A, therefore, cannot occur in nonfelids. However, it has been found that two independent FeLV-A isolates from United Kingdom and United States also have infected various nonfeline cell lines including cells from human beings, rabbits, pigs, and minks.322 Although malignant transformations do not occur in nonfelid cell cultures,272 experimental FeLV infection with development of lymphomas could be induced in young dogs and marmosets.367 In experimental infections with FeSV, fibrosarcomas also could be produced in nonfelids in vivo.8 However, no reports have been made on natural transmission of FeLV to nonfelids. Documentation of FeLV in nondomestic felids, however, becomes more and more common, and FeLV appears to be enzootic in some wild felids. Introduction of FeLV into free-living and captive nondomestic felid populations has serious consequences for their health and survival. FeLV infects small wildcats including F. silvestris71,460 and European and Iberian lynxes.278,306 FeLV also has been detected in the Florida panthers (Puma concolor coryi) and causes severe problems in this species, in which vaccination programs now have been instituted.37,70 A multicentric T-cell lymphoma associated with FeLV infection was found in a captive Namibian cheetah (Acinonyx jubatus).297 FeLV was also detected in an 11-month-old captive-bred male neutered bobcat (Felis rufus) showing signs of lethargy, anorexia, neutropenia, lymphopenia, and nonregenerative anemia.405 Although in one study, FeLV was not found in 12 ocelots (Leopardus pardalis) in Barro Colorado Island,116 FeLV proviral DNA was detected in one male captive ocelot and one female little spotted cat (Leopardus tigrinus) in a wildlife center in southern Brazil.139 There is no evidence of FeLV infection in African lions (Panthera leo) or Asian lions (P. leo persica).90,156a,358,359 FeLV infection exists in domestic cats worldwide. Prevalence studies have focused on the detection of FeLV mainly in third-world countries or on remote islands, where the prevalence of virus infections in cats was unknown. In these studies, FeLV has been detected almost everywhere.31,54,72,284,307 Only cats on Grenada Island, West Indies, and Isabela Island, Galapagos, were free of FeLV infection.91,261 In contrast to FIV infection, in which the prevalence varies significantly, the FeLV infection rate of free-roaming cats is similar throughout the world, ranging from 1% to 8% in healthy cats.20,126,264,269,410 Prevalence is as high as 38%, if only sick cats are included in the surveys.15,157,157 Originally, certain diseases, such as lymphoma, were associated with very high rates (up to 75%) of FeLV infection. Cats that have positive test results for FeLV have become less common because the overall prevalence of FeLV infection has decreased, presumably as a result of control measures. A number of reports document that the overall rate of FeLV infection is decreasing. For example, the Tufts Veterinary Diagnostic Laboratory, where approximately 2000 serum samples are tested yearly for FeLV antigen, reported a decrease from 8% in 1989 to 4% in 1995.61 In Germany, a steady decrease in FeLV prevalence from 6% to 1% was observed when investigating the FeLV infection rate from 1993 to 2002.126 Studies report a prevalence of 2.3% to 3.3% in North America, 0% to 2.9% in Asia, and 1.0% to 15.6% in Europe.* There are a number of possible explanations for the decrease in prevalence. It is most likely the result of test and removal programs at breeding facilities, the practice of testing cats at animal shelters before adoption, and the widespread use of vaccination. None of the available vaccines have been shown to provide 100% protection against progressive infection, but the common practice of vaccination likely has had an impact on the prevalence of FeLV. Although vaccination contributes to the decrease, epidemiologic studies suggest that testing and removal practice is more effective than vaccination.380 The first vaccine was introduced in 1985, but the observed decline in the overall infection rate began before this time.257 Many deterministic models have been constructed to predict the dynamics of FeLV in cat populations. These models predicted that FeLV dynamics depend on the size of the host population and the relationship between host density and the pattern of contacts of individual cats. They found no threshold population size for virus persistence in large populations, but the possibility of FeLV extinction in small populations.118 Models take into account that cat populations can be connected to each other by dispersal of individuals, which favors roaming of cats and spread of disease.117 These models explain the geographic discrepancies of FeLV prevalence. Although the absolute number of pet cats is remarkably higher in Northern European countries (e.g., 10 million in the United Kingdom, 8 million in Germany, 10 million in France) than in southern European countries (e.g., 4 million in Spain), living conditions differ considerably. Hence, the higher number of free-roaming cats in southern Europe increases the contact rate in these countries, which, as a consequence, increases the overall prevalence of FeLV infection.117 Additionally, discrepant results in FeLV prevalence are based on the health status of the cats under consideration.269 Whenever only clinically healthy cats are included, the prevalence is noticeably lower than in surveys of sick cats.15,201,201 Certain risk factors contribute to a higher prevalence. Prevalence of FeLV is higher in cats that are allowed to roam outside,126,264 because direct contact is required for transmission. In a study in the United States, antibody prevalence (which predicts exposure) was clearly related to the time spent outdoors and the degree of exposure to other cats. Of cats in a study in Boston and Detroit, of which many were allowed to roam outside, 63% and 47% had positive serum FeLV antibody test results, respectively, whereas only 5% of New York cats that were primarily confined to high-rise apartments had FeLV-specific antibodies.338 One study looked into risk of disasters on FeLV infection rates among cats exported from the 2005 Gulf Coast hurricane disaster area, but could not demonstrate an increase in infection rates in this situation.262 Risk groups for FIV and FeLV infections are only slightly different. Although fighting, free-roaming, intact male cats are still considered mainly at risk for acquiring FIV infection, the same risk factors also facilitate FeLV infection. FeLV can no longer be considered primarily an infection of “social cats,” although FeLV is easily spread through social contacts. In earlier studies, FeLV infection rate was found to be almost equal in male and female cats. In one older study, 733 feral free-roaming cats in Raleigh, North Carolina, and 1143 feral free-roaming cats in Gainesville, Florida, were tested for FIV and FeLV infection, and prevalence of FeLV infection was not significantly different between males (4.9%) and females (3.8%).252 However, two more contemporary studies, in the United States and Germany, found a significantly higher risk of FeLV infection among male cats.126,264 Although FeLV transmission commonly occurs between infected queens and kittens and among cats living in prolonged close contact, it seems that aggressive behavior, a common male attitude, plays a greater role than previously reported.129 Thus, the common opinion that FeLV was a disease of “friendly” cats should be reconsidered. This is also supported by the findings that cats exhibiting aggressive behavior have a higher risk of FeLV infection,127 and more than 8% of cats examined by veterinarians for fighting injuries were FeLV antigen-positive, a prevalence considerably higher than in the clinically healthy cat population.129 Although no breeds are predisposed to being infected with FeLV, infection is less commonly found in purebred cats, mainly because they are commonly kept indoors. In addition, awareness in the cat-breeder community leads to frequent testing. In older studies, young age also was considered to be a risk factor for FeLV infection, but this statement has to be reconsidered, too. In a study in the United States, in which 18,038 cats at 345 veterinary clinics and 145 animal shelters were tested, adults cats were more likely to be FeLV-infected than juveniles,264 and in another study, the median age of FeLV-infected cats was not significantly lower than that of non-FeLV-infected cats,127 at least in countries with good veterinary care. This is unexpected because the susceptibility of cats to FeLV is age-dependent,194,201 but because of the increasing awareness, more cats are tested for FeLV, FeLV infection is recognized earlier, and medical care is provided during the initial stage of disease. In addition, awareness among cat breeders and animal shelters has led to routine testing of new pets entering the household or shelter. Moreover, euthanasia of infected asymptomatic cats is less common. As demonstrated earlier, there is a significant decrease in prevalence of infection in many countries. However, with few exceptions, FeLV prevalence studies are uniquely based on detection of FeLV p27 antigen in blood using ELISA or similar immunochromatographic assays. But the pathogenesis of FeLV infection is complex, and free antigen can only be detected in the blood of cats with productive viremia, because those with regressive infections only harbor provirus in their bone marrow cells after overcoming antigenemia.377 Thus, antigen testing may underestimate the true prevalence of infection. In a study in Switzerland it was shown that in addition to 7% of cats with both viral p27 antigen and provirus in blood, 10% of cats had negative results for p27 antigen and positive results for proviral DNA in blood.189 This result is surprisingly high and raises the question whether the same situation occurs in other countries. FeLV is contagious and spreads through close contact between virus-shedding cats and susceptible cats. Transmission of FeLV occurs primarily via saliva, where the concentration of virus is higher than in blood. Viremic cats constantly shed millions of virus particles in saliva, and shedding through saliva occurs relatively consistently in FeLV-viremic cats.131,132 The concentration of virus in saliva and blood of healthy viremic cats is as high as it is in those with signs of illness. FeLV is passed effectively horizontally among communal cats that have prolonged close contact. Fighting and biting behavior,127,129 as well as social behavior, such as sharing food and water dishes, mutual grooming, and using common litter areas, are the most effective means of transmission. Although the virus may enter many tissues, body fluids, and secretions, it is less likely to spread via urine and feces, and urine and feces were not considered an important source of infection. However, it was shown that antigenemic cats shed FeLV RNA and DNA in feces and urine, and infectious virus was isolated from feces and urine.50,130 It was even shown that naïve cats exposed to virus-containing feces developed anti-FeLV antibodies, showing that infection through feces without direct cat-to-cat contact took place, but these cats remained negative for FeLV antigen and provirus in blood. These results suggest that fecal shedding of FeLV may play a role in transmission, but is probably of minor importance under natural circumstances. Nevertheless, sharing of litter pans by susceptible and viremic cats could increase the environmental infectious pressure.130 Fleas have been considered a potential source of transmission because FeLV RNA has been detected in fleas and their feces,448,449 but flea transmission does not seem to play a major role in nature. Iatrogenic transmission can occur via contaminated needles, instruments, fomites, or blood transfusions.279 Vertical transmission from mother to kittens occurs commonly in FeLV-viremic cats. Neonatal kittens can be infected transplacentally or when the queen licks and nurses them. Transmission also can occur in queens that are regressively infected (and therefore, have a negative result on routine tests) because latent infection may be reactivated during pregnancy. In addition, isolated FeLV transmission via milk to offspring, from queens with antigen-negative test results, has been described. If in utero infection occurs, reproductive failure in the form of fetal resorption, abortion, and neonatal death is common, although up to 20% of vertically infected kittens may survive the neonatal period to become persistently infected adults.257 It is possible to observe that newborn kittens from infected queens have negative FeLV antigen test results at the time of birth but may have positive test results over the following weeks to months once the virus starts replicating. Thus, if the queen or any kitten in her litter is infected, the entire family should be treated as if infected and should be isolated from uninfected cats. Susceptibility to becoming persistently FeLV viremic is highest in young kittens. Studies in a household with many FeLV-infected cats showed that 7 of 10 kittens placed there at 3 months of age became viremic within 5 months, whereas only 3 of 17 adults in the same household became viremic over 7 years.58,59 Experimental infection is difficult if not impossible in healthy adult cats. Depending on the FeLV strains used, experimental infection can even be difficult to achieve in kittens older than 16 weeks of age.194 Age resistance to FeLV also exists in nature. Prevalence of anti-FeLV antibodies increases steadily over time, indicating an increased exposure to the virus throughout life, and although exposure to FeLV accumulates with age, susceptibility to develop persistent viremia after infection simultaneously decreases. The described age resistance is independent of immunity from previous contact or vaccination. An explanation for the age resistance is that the number of cellular receptors necessary for FeLV-A to enter target cells seems to decrease in older cats, and thus, establishment of infection becomes more difficult. Age resistance also may be related to maturation of macrophage function.191 However, age-related resistance is not absolute and depends on the infection pressure. Risk of developing persistent viremia increases in kittens but also to a certain extent in adult cats when they are housed together with FeLV-shedding cats. This is shown by the increased rate of viremic cats in households with endemic FeLV infection and by natural exposure studies in which a certain percentage of cats becomes FeLV-positive over years when they are housed together with infected cats. However, the risk of an adult cat becoming persistently viremic after one short contact with a FeLV-shedding cat is certainly very low and probably lower than the risk of developing vaccine-associated sarcomas after FeLV vaccination. Therefore, use of FeLV vaccination should be considered carefully in adult cats. The cellular receptors of FeLV are not fully identified despite intensive ongoing research. FeLV subgroups use different receptors,40,308,364,402 and strain-dependent differences seem to exist. A binding receptor for FeLV-A has been detected that seems to be identical with the feline thiamine transport protein 1 (THTR1) receptor.308 FeLV-C uses the host receptor known as FLVCR1, but binding of FeLV-C to FLVCR1 seems to involve interaction of two receptor-binding domains (including the carboxy terminal C domain) with the host receptor FLVCR1.364 FeLV-B uses a cellular protein (phosphate transporter 1, Pit-1) as receptor.40,402 FeLV-T also can use Pit-1 as a receptor but the host ranges of FeLV-B and FeLV-T are not exactly the same, suggesting a different Pit-1 use at the postbinding level.402 FeLV-T cannot infect cells unless a classic multiple membrane-spanning receptor molecule and a second co-receptor or entry factor are present. This cellular protein can function as either a transmembrane protein or a soluble component to facilitate infection.10 The outcome of FeLV infection is very different in each cat. Although outcome mainly depends on immune status and age of the cat, it is also affected by pathogenicity of the virus, infection pressure, and virus concentration.161 Outcome of FeLV infection also reflects genetic variation both in the virus and the naturally outbreeding host population. Mutational changes identified in FeLV strains were shown not to alter receptor usage, but to significantly increase the efficiency of receptor binding. Longitudinal studies of infected animals showed that certain mutations resulted in a significantly more rapid disease onset, whereas other substitutions in certain genes changed the disease outcome entirely, suggesting that the distinctive LTR and surface unit (SU) genes mediate a rapid pathogenesis with distinctive clinical features and oncogenic mechanisms.257 Discussions of FeLV infection, which has different courses, outcomes, and classifications (Figs. 11-4 and 11-5 and Table 11-3) are controversial. Diagnostic tools, including very sensitive polymerase chain reaction (PCR) methods, have provided new data that question the traditional understanding of FeLV pathogenesis. Previously, most FeLV pathogenesis studies were conducted assaying parameters such as virus isolation and antigen detection. Accordingly, infection was characterized by undetectable, transient, or persistent viremia. Using real-time PCR, the spectrum of host response categories to FeLV infection was refined by investigating proviral DNA and viral RNA loads. Cats believed to be immune to FeLV infection were found to have positive provirus test results. FeLV provirus was found to persist for years; recurrence of viremia and disease development was observed in some cats. Thus, cats with negative antigen and positive provirus test results are FeLV carriers and, after reactivation, may act as an infection source. However, integrated viral DNA may also be essential for solid protection and long-lasting maintenance of protective immunity.187 Therefore, the potential courses of FeLV infection have been reclassified, and the stages of FeLV infection are described as (1) abortive infection (comparable to the former “regressor cats”), (2) regressive infection (comparable to the former “transient viremia” followed by “latent infection”), (3) progressive infection (comparable to the former “persistent viremia”), and (4) focal or atypical infection (see Table 11-3).186,187,187 TABLE 11-3 Characteristics of Stages of Feline Leukemia Virus Infection Progressive = persistent viremia; Regressive = transient viremia followed by latent infection; Abortive = complete elimination. From Ref. 260. In the past, approximately one-third of cats were believed to become persistently viremic and up to two thirds of cats eventually clear the infection.191 Newer research suggests that most cats remain infected for life after exposure but may revert to an aviremic state (regressive infection) in which no antigen or culturable virus is present in the blood but in which FeLV proviral DNA can be detected in the blood by sensitive PCR methods.189,343,343 The clinical relevance of cats with antigen-negative and provirus-positive results is not yet clear. The provirus is integrated into the cat’s genome, so it is unlikely to be cleared over time.49 Although these cats are unlikely to shed infectious virus in saliva, proviral DNA might be infectious via blood transfusion.52 The continuous presence of provirus might explain the long persistence of virus-neutralizing antibodies in “recovered” (recovered from viremia, but not from latent infection) cats. Before the development of PCR, a status of “latent infection” was described in which the absence of antigenemia was accompanied by persistence of culturable virus in bone marrow or other tissues but not in blood.* The “latent infection” is now considered a phase through which cats pass during regressive infection.33 FeLV provirus and plasma viral RNA are usually detectable by PCR within 1 week of FeLV exposure, even if FeLV antigen is not. All cats with progressive and regressive infection seem to undergo this phase and to develop similar proviral and plasma viral RNA loads in the peripheral blood during early infection.187 After FeLV exposure, FeLV infection has four possible outcomes, described next (see Table 11-3). After initial infection, which most commonly occurs via oronasal routes, virus replicates in the local lymphoid tissue in the oropharyngeal area. In some immunocompetent cats, viral replication may be stopped by an effective humoral and cell-mediated immune (CMI) response; these cats never become viremic. This abortive exposure has been observed infrequently after experimental FeLV inoculation and is characterized by negative test results for culturable virus, antigen, viral RNA, and proviral DNA.436 These cats were formerly called “regressor cats.” They have high levels of neutralizing antibody, but neither FeLV antigen nor viral RNA or proviral RNA can be detected in the blood at any stage. In these cats, virus never spreads systemically, and infection usually remains undetected. Abortive infection likely is caused by low-dose exposure to FeLV, as shown in an experimental study in which, after exposure to low doses of FeLV, cats only developed antibodies as the sole parameter of infection.292 It is currently unknown how often this situation truly occurs in nature, because newer studies using very sensitive PCR methods have found that in many of the formerly considered “regressor cats,” virus actually can be retrieved later on, and it appears likely that no cat or only very few can completely clear FeLV infection from all cells. This might explain why virus-neutralizing antibodies persist in recovered cats for many years (or even lifelong) in the absence of overt infection or exposure to viremic cats. If this is the case, the risk of such persistence leading to potential reexcretion of virus or the development of FeLV-associated disease must be extremely low, because recovered cats appear to have the same life expectancy as cats that have never been exposed to FeLV.279 This explains why the majority of cats in a population show evidence of exposure by the presence of anti-FeLV antibodies after contact with FeLV, but only a small proportion actually become viremic. These cats build a very effective immunity and are protected against new viral challenges, probably for several years if not lifelong. Protective immunity is partly humoral and partly cellular, and antibody production is not necessarily required for protection; about 2% are effectively protected without detectable antibodies. Regressive infection is accompanied by an effective immune response, and virus replication and viremia are contained before or shortly after the time of bone marrow infection. After initial infection, replicating FeLV spreads systemically within mononuclear cells (lymphocytes and monocytes). During this first viremic episode, free FeLV-p27 antigen is detectable, and cats have positive results on tests that detect free antigen in plasma (e.g., ELISA) and can shed the virus during that period. The initial viremia may be characterized by malaise, fever, or lymphadenomegaly resulting from lymphocytic hyperplasia. The virus spreads to target tissue including thymus, spleen, lymph nodes, and salivary glands. In cats with regressive infection, this viremia is terminated within weeks or months (formerly called “transient viremia”). In most cats, the viremia lasts only 3 to 6 weeks (with a maximum of 16 weeks). During this time, cats shed virus and are infectious. Many cats are able to clear viremia very early before bone marrow becomes infected. It was thought that these cats not only terminate the viremia, but also completely eliminate the virus from the body. However, studies question the fact that virus can be completely cleared and that virus may be found in these cats at a later time. These cats also develop a very effective immunity and are protected against new exposures to virus. They have a low risk of developing FeLV-associated diseases, although FeLV is integrated into their genome (and thus, FeLV can be detected by PCR). After virus replication is contained, viral shedding does not occur.109,110,110 In some cats, viremia may persist longer than 3 weeks. After this time period, bone marrow cells may become infected, and affected hematopoietic precursor cells produce infected granulocytes and platelets that circulate in the body. In this circumstance, a high level of viremia develops, and lymphoid organs and salivary glands become infected with up to 1 × 106 viruses/mL of saliva. From this time point on, viral antigen is also detectable intracellularly in platelets and granulocytes by tests such as direct fluorescent antibody (FA) assays that can only detect large quantities of intracellular antigen. In contrast to antigen tests (e.g., ELISAs) that can detect lower quantities of free p27 antigen and become positive during the first viremia, direct FA test results become positive later and only after infection is established in bone marrow. This explains discordant ELISA-positive and direct FA-negative results. Even if bone marrow becomes infected, a certain percentage of cats can clear viremia (and therefore develop regressive infection); however, the longer the viremia lasts, the less likely it is that these cats will clear their infection. Once bone marrow cells develop an established infection (after 3 weeks of viremia), cats cannot eliminate the virus from the body and from the bone marrow even if they terminate viremia because the information for virus replication (its proviral DNA) is present in bone marrow stem cells. This stage has been called “latent infection” (now considered a stage of regressive infection). Although proviral DNA remains, no virus is actively produced, and cats with regressive infection have negative results from routine tests (ELISA and FA) that detect FeLV antigen. Regressive infection can only be diagnosed by in vitro culture of bone marrow samples or using PCR to detect provirus. Growth can be facilitated by adding glucocorticoids to the cell culture. Productive viral infection can be reactivated in vivo, spontaneously or in response to immune suppression, and latently infected cats can become viremic and show positive results again in antigen tests. This usually occurs after stress and can be experimentally induced in cats by administration of high doses of glucocorticoids.377 Regressive infections may reactivate in pregnancy as a result of immunosuppression from endogenous progesterone, which also may explain the reemergence of FeLV infection in kittens. Mammary glands of regressively infected queens may begin to produce infectious viral particles during the induction of lactation.334 Regressive infection and the latent state are unique features in FeLV infection. The molecular basis of latency is the integration of a copy of the viral genome (provirus) into cellular chromosomal DNA. During the replication cycle, the enzyme RT produces a DNA copy using the viral RNA as a template. The copy is integrated into the cellular chromosomal DNA and maintained as a provirus for the life span of the cell. During cell division, proviral DNA is replicated and the information given to the daughter cells. Thus, complete cell lineages may contain FeLV proviral DNA. However, the proviral DNA is not translated into proteins, and no infectious virus particles are produced. Therefore, regressively latently infected cats do not shed FeLV and are not infectious to other cats. Although latency is a sequel to FeLV infection, the majority of cats completely eliminate the viral genes from their cells by 9 to 16 months after infection, and all but 10% have done so after 30 months.334 Virus can remain integrated in a small number of cells for a long time, while being kept in check by a partial immune response. As antibody concentration increases, virus production decreases. No harmful virus is produced during regressive infections, and clinical signs (with few exceptions such as neoplasia or myelosuppressive syndromes) do not occur. In a study in Switzerland, where 7% of cats had both positive p27 antigen and positive proviral test results, 10% of the cat population had negative p27 antigen results and positive proviral test results in blood, which indicates latent infection.189 Regressive infection can be reactivated because the genetic information for producing complete viral particles is present and can potentially be reinduced when antibody production decreases (e.g., after immune suppression). Reactivation is more likely the earlier the stress factor occurs after the viremic phase. In the first weeks after viremia, viral replication can be experimentally reactivated in most cats. As the time passes, regressive infections become more difficult to reactivate, even with high doses of glucocorticoids. Although possible by 1 year after infection, reactivation is considered unlikely and is very difficult after 2 years. This may be explained by genetic code-reading mistakes that may occur if the information is frequently reproduced in these fast-dividing cells. Thus, information to produce infectious viral particles gets lost, and reactivation becomes more and more unlikely over time. The proportion of experimentally infected cats that had regressive FeLV infections in their bone marrow decreased with time after disappearance of viremia.340 In the first 3 months after recovery from viremia, integrated virus could be isolated from the bone marrow of approximately 50% of experimentally infected cats. A pronounced decrease in the incidence of regressive infections occurred by 190 days after the viremia.334,340 More than 1 year later, only 5 of 19 previously challenge-exposed cats that had negative FeLV ELISA test results still had FeLV detectable in several tissues (e.g., bone marrow, spleen, lymph node, small intestine).174 At 3 years postviremia, only about 8% of cats still harbored latent infections in bone marrow, myelomonocytic cells, and stromal fibroblast cells.189,290,334,340,377 Regressive infection is probably a stage in the elimination process of the virus. Most regressive infections are not clinically significant because viral reactivation is unusual under natural circumstances. As long as the infection remains confined, the cats are not contagious. However, viral latency explains relapsing viremias, protracted incubation periods, and persistent high titers of antibodies. A question always arises regarding whether regressive FeLV infection can be responsible for clinical signs. However, for the majority of pathogenic mechanisms by which FeLV causes clinical signs, active virus replication is necessary; but this is not the case in regressive FeLV infections, in which the virus is harbored in a “dormant” and nonproductive form. Regressive, as compared to productive, FeLV infection has been found to occur most commonly in older cats that originated from animal shelters and, rather than lymphoma, was more commonly associated with anemia, panleukopenia and purulent inflammatory processes.419a Regressive infections help to explain how myelosuppression or hematopoietic malignancy could be FeLV-related in cats with negative FeLV antigen test results. In one study, 2 of 37 cats (5%) with nonregenerative cytopenias and negative FeLV antigen test had positive results with bone marrow PCR, suggesting that regressive FeLV infection can cause myelosuppression.419 Some studies also detected FeLV provirus in lymphomas of cats that had negative results on blood testing for FeLV antigen.122,204 FeLV provirus can be inserted at many different sites in the host’s genome, carrying potent regulatory signals. In the development of myelosuppressive disorders or tumors, integrated FeLV provirus may interrupt or inactivate cellular genes in the infected cells, or regulatory features of viral DNA may alter expression of neighboring genes. In addition, because bone marrow microenvironment cells (e.g., myelomonocytic progenitor cells and stromal fibroblasts) provide a reservoir for regressive FeLV infections, it seems possible that the integrated provirus may alter cellular functions and contribute to the pathogenesis of myelosuppressive disorders. Finally, FeLV not only contributes its genes to the host, it also has been shown to appropriate cellular genes. Several such transducted genes that are also present in regressively infected cells have been implicated in viral oncogenesis.365,373,373 In one SPF cat, experimentally infected with FIV and FeLV, regressive infection occurred and the cat became aviremic for 8.5 years. A genetically altered FeLV variant of this virus reappeared in the blood, in conjunction with the development of multicentric lymphosarcoma.175a
Feline Leukemia Virus Infection
Etiology
Virus Origin
Feline Leukemia Virus Subgroups
Viral Subgroups
Frequency of Isolation in FeLV-Positive Cats
Associated Disease
Comparison by Species of in Vitro Replication
A
100% in infected cats, mildly pathogenic but highly contagious, mildly cytopathogenic
Immunosuppression and other FeLV-associated diseases, replicating and contagious
Cat, rabbit, pig, mink, human
B
Occurs with subgroup A in 50% or more of cats with neoplastic disease (lymphoma)
Hematopoietic neoplasia, nonreplicating and noncontagious, virulent in recombination with subgroup A
Cat, dog, cow, hamster, pig, human
C
Rarely isolated, mainly in cats with nonregenerative anemia
Nonregenerative anemia and erythremic myelosis, nonreplicating and noncontagious, virulent in recombination with subgroup A
Cat, dog, guinea pig, human

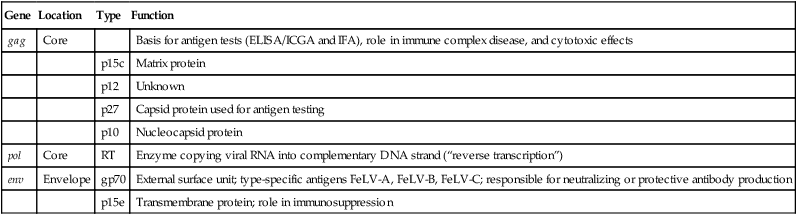
Feline Leukemia Virus Genome and Proteins
Gene
Location
Type
Function
gag
Core
Basis for antigen tests (ELISA/ICGA and IFA), role in immune complex disease, and cytotoxic effects
p15c
Matrix protein
p12
Unknown
p27
Capsid protein used for antigen testing
p10
Nucleocapsid protein
pol
Core
RT
Enzyme copying viral RNA into complementary DNA strand (“reverse transcription”)
env
Envelope
gp70
External surface unit; type-specific antigens FeLV-A, FeLV-B, FeLV-C; responsible for neutralizing or protective antibody production
p15e
Transmembrane protein; role in immunosuppression
Epidemiology
Host Range
Prevalence
Transmission
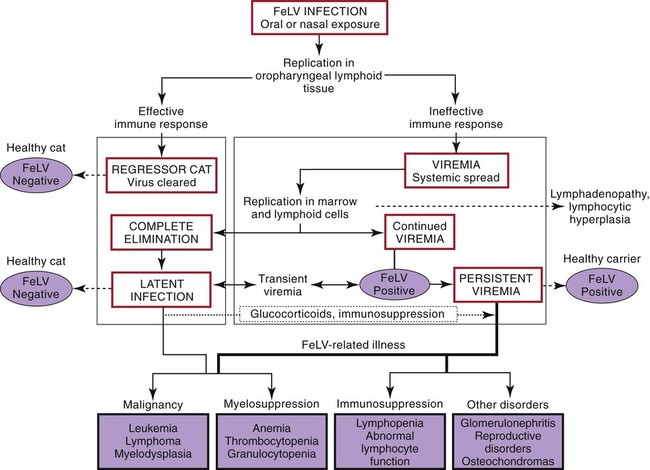
Pathogenesis
Stages of Feline Leukemia Virus Infection
Outcome of FeLV Infection
FeLV p27 Antigen in Blood
Virus Blood Culture
Viral RNA in Blood
Viral DNA in Blood
Viral Tissue Culture
Viral Shedding
FeLV-Associated Disease
Progressive
Positive
Positive
Positive
Positive
Positive
Positive
Likely
Regressive
Negative
Negative
Negative
Positive
Negative
Negative
Unlikely
Abortive
Negative
Negative
Negative
Negative
Negative
Negative
Unlikely
Focal
Negative
Negative
Not tested
Not tested
Positive
Variable
Unlikely

Abortive Infection
Regressive Infection
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine