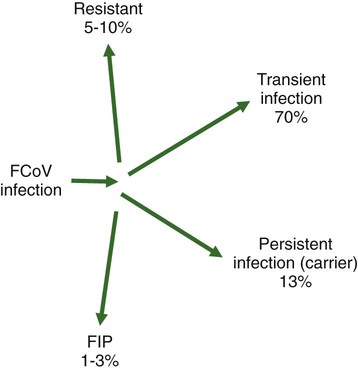
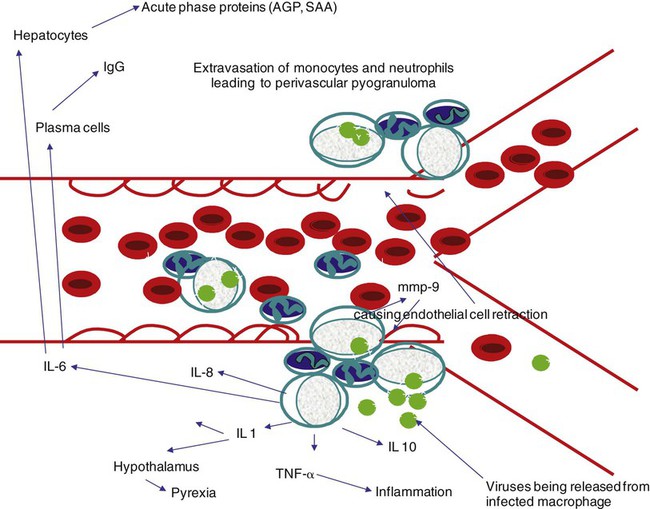
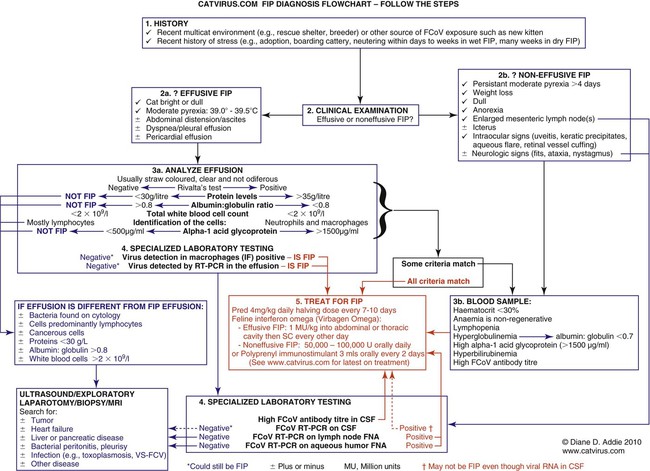
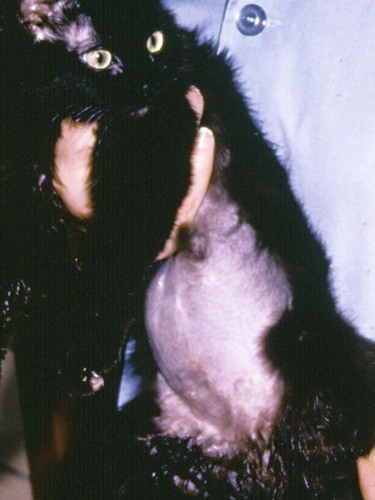
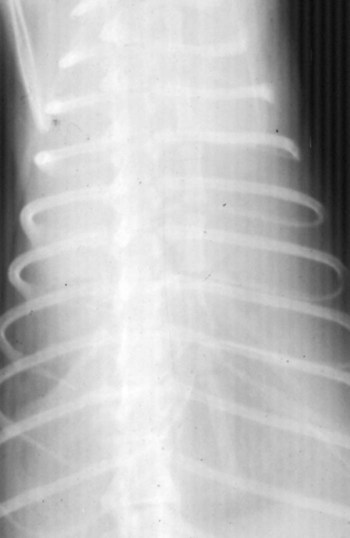
Feline coronavirus (FCoV) causes a ubiquitous enteric infection of cats that occasionally leads to a highly fatal immune-mediated vasculitis named feline infectious peritonitis (FIP). FCoV is a large, spherical, enveloped, positive-sense single-stranded RNA alphacoronavirus (previously termed “group 1”) belonging to the family Coronaviridae. The Coronaviridae, comprising the genera Coronavirus and Torovirus, is part of the Nidovirales order, which also includes toroviruses, arteriviruses, and roniviruses.60 It is proposed that FCoV, along with the coronaviruses of swine and dogs, become part of a new species, called Geselavirus, in reference to the typical genetic arrangement of these viruses “gene seven last” (gsl).60 Coronaviruses possess the largest RNA viral genome known to date: the FCoV genome is 29 kb, encoding a replicase polyprotein, four structural proteins (spike [S], matrix [M], nucleocapsid [N], and envelope [E]), and several nonstructural proteins (3a, 3b, 3c, 7a, and 7b), whose function is unknown. Despite the ubiquitous nature of FCoVs and infected cats, few develop FIP (Fig. 10-1). The explanations proposed for this discrepancy have been controversial and revolved around two basic premises: whether both avirulent and virulent viruses are simultaneously circulating, or virulent viruses arise as a result of de novo mutation within each FIP-affected cat. In this latter theory, a novel mutation, deletion, or insertion must occur in the genome of the infecting FCoV or feline enteric coronavirus (FECV) before FIP can occur.45,244,244 Chang et al. found deletions in the 3c gene from systemic virus, but not from virus in the gut, postulating that an intact 3c gene is essential for viral replication in the gut.45 Pedersen also found intact 3c genes in isolates from the gut.244 In contrast, others having found both deletions/mutations and identical genomes in healthy and FIP cats, or from both systemic and enteric viruses, have questioned the internal mutation theory.38,73,73 Although sharing only 30% genetic homology,254 the 3c gene has been likened to the 3a gene of severe acute respiratory syndrome-related (SARSr)-coronavirus (CoV) on the basis of hydrophilic profile similarity.45,223 The SARSr-CoV 3a protein has been implicated in apoptosis; type 1 interferon (IFN) receptor downregulation, and increased fibrinogen expression.190,206,206 Whether or not deletions in the 3c gene are responsible for the development of FIP is unknown—they could simply be a by-product of rapid viral replication, and 3c deletion mutants made successful vaccine candidates.107 RNA viruses are remarkably prone to genetic change, and it would be expected that in a situation where there is considerable viral replication, many variants would be found in the same host. Such variation is found103,168 not only within organs in the body, but also within different cells in the same pyogranuloma.262 Whether that virus variation is the cause of, or the effect of, the disease process is unknown. Laboratory strains of varying virulence exist; there are strains that are exceptionally virulent, causing FIP in almost every cat infected with them (e.g., the notorious 79-1146 strain). Less virulent FCoV strains vary in their ability to replicate in monocytes, and monocytes vary in their permissiveness for FCoV replication. The interplay of these two factors determines whether or not an individual cat develops FIP.64 Consistently, cats challenged with low viral dose, even with virulent virus such as FIP virus (FIPV) 79-1146, can overcome the infection, whereas increasing doses resulted in almost all cats developing FIP.247,286 Results from a comprehensive genetic analysis of FCoV strains indicated distinct genetic differences between viruses isolated from 48 clinically healthy cats and 8 ill cats with FIP.38 These distinct noncontiguous differences existed in membrane, spike, and nonstructural protein 7b genes. Unfortunately, the 3c genes were not examined. The membrane protein is the most abundant structural protein of the coronaviruses and is likely associated with the pathogenesis of infection, because it is involved in viral budding. Significantly, there were five amino acid differences between the membrane proteins of FCoVs from clinically healthy cats and those with FIP. However, three healthy cats had viruses that contained an FIP amino acid signature (YIVAL), raising the possibility that at least one cat eliminated a FCoV strain capable of causing FIP.38 The genotypes correlated with FIP were more compatible with ancestrally derived and not the result of de novo mutations.38 The majority of cats were not co-infected with multiple strains of FCoVs at the same time, but were generally infected with one predominant strain. However, in two instances cats with FIP were infected with two distinct viral isolates, indicating that superinfection can occur. Another member of the Coronaviridae causes severe acute respiratory syndrome (SARS) in humans. The SARSr-CoV is thought to have originated from the masked palm civet cat (Paguma larvata). Despite its common name, this carnivore is not a feline, but is a member of the mongoose family (Viverridae). Nevertheless, cats may become infected with SARSr-CoV experimentally196 and naturally. A single cat from one household of infected people was found to seroconvert, although it remained clinically healthy. Analysis of data suggest that civet SARSr-CoV is likely a recombinant virus arising from SARSr-CoV strains closely related to the coronaviruses of the horseshoe bat, Rhinolophus sinicus. Frequent recombination coupled with rapid evolution in these animals may have accounted for the cross-species transmission and emergence of SARS.181 There are two types of FCoVs, as classified by their genetic sequence and ability of monoclonal antibodies to recognize them.127,248,316a Type I FCoVs are considered to be unique feline strains. Type II FCoVs have arisen from recombination between type I FCoV and canine coronavirus (CCoV). Although type II FCoVs are mainly type I, they have variable portions of the spike and adjacent genes of CCoV.118,317 Most research has focused on type II because it can be readily propagated in vitro; however, type I is most prevalent worldwide.* Both types can cause FIP. Some investigators126 found a higher prevalence of type II among cats with FIP than among healthy cats in Japan, and others187 found a higher correlation of type II with FIP. However, in other studies the distribution of types I and II in cats with FIP reflected broadly the distribution of the two viral types in asymptomatic FCoV infected cats.31,178 Lin et al. also found a higher genetic diversity among type I FCoVs compared with type II—a feature they attributed to type I FCoV being able to induce persistent infection, whereas type II FCoV probably does not.187 Cats can be simultaneously infected with both types I and II FCoV.187 The type I FCoV receptor is unknown.73,123 The receptor for the type II FCoV is an enzyme, aminopeptidase-N, found in the intestinal brush border.† However, at least in type II FCoV infection of monocytes and macrophages, the receptor is not necessary if there is anti-FCoV antibody present.313 FIPV rivals feline panleukopenia virus as a cause of cat death.42 The apparent increase in the prevalence of FIP can be directly related to the changes in feline husbandry over the past 30 years—more cats are kept indoors and in greater numbers, causing exposure to higher doses of pathogens in feces, which would otherwise have been buried outdoors. The popularity and resulting increased breeding of purebred cats has resulted in loss of immune protection associated with genetic diversity and hybrid vigor.188 An increasing number of cats spend part of their lives in shelters. This life style can result in exposure to a higher coronaviral dose (via the litter tray), increased stress to the naturally solitary cat, and concurrent, sometimes immunosuppressive infections, all of which impair a cat’s ability to prevent infections. Cats are increasingly being prevented from hunting and are instead being fed unnatural foods, often imbalanced in the ratio of omega 6:3 dietary fatty acids, which likely leads to a chronic proinflammatory state. All of these factors favor the spread and increase of FCoV infections and associated FIP. The predominant risk factors associated with the development of FIP are discussed next. Although a cat of any age can develop FIP, kittens and cats up to 2 years of age are at greatest risk,215,244,244 with a second peak in age-related risk in cats over 10 years of age.274 More specifically, kittens developed FIP after weaning,42 and most young cats succumb between 3 and 16 months of age.244 That kittens are at greater risk of developing FIP may be due to the higher viral load generally found in kittens compared with adult cats246; due to their immature immune systems; or due to the many stressful events that generally happen to kittens, such as being vaccinated, rehomed, and neutered. In addition, cats are most likely to develop FIP after their first encounter with FCoV, which is most likely to occur in kittenhood.18 There is little doubt that pedigree cats are more at risk of developing FIP than are nonpurebred cats.* This may be because purebreeding of cats is associated with the loss of genetic diversity188 so that the immune systems of purebred cats may not be as robust as those of outbred cats, indeed a study of the feline leukocyte antigen (FLA, the feline equivalent of the major histocompatibility complex [MHC]) showed that the Burmese breed averaged 2.8 FLA alleles, compared with up to 6 in other breeds.11 Or it may be because cat breeders usually have several cats and they tend to be confined indoors—increasing viral dose to which they are exposed, concurrent stress, and diseases. Cat breeders in the United States344 and 8% of Swedish cat breeders report having had a cat with FIP.304 Results differ between studies—in one study the inheritance of FIP susceptibility was demonstrated in Persian cats.83 In another study Persian, Burmese, exotic shorthair, Manxe, Russian blue, and Siamese cats were not at increased risk of developing FIP, whereas Abyssinian, Bengal, Birman, Himalayan, ragdoll, and rex cats were.259 In a retrospective study of neurologic disorders, Burmese cats were overrepresented as having FIP.36 However, this study was conducted in association with a Burmese cat club, which may have skewed the results for this breed. FCoV infection, disease, and FIP have been reported in a variety of nondomestic felids: European wildcats (Felis silvestris),337 lions (Panthera leo),122,151 tigers (P. tigris), jaguars (P. onca), leopards (P. pardus), sand cats (Felis margarita), mountain lions/panthers (F. concolor),234,273 caracals (Caracal caracal), and servals (Felis serval)143; lynx (Lynx lynx) in Canada,33 but not Eurasian lynx in Sweden282; one bobcat (Lynx rufus)270; and especially cheetahs (Acinonyx jubatus).† As with domestic cats, FCoV is more likely to be a problem in large cats confined indoors or in exhibits than in those allowed to roam outside naturally.‡ Ferrets have two manifestations of coronavirus infection: epizootic catarrhal enteritis342,343 and infectious peritonitis.88,144b,197,258 Although in the same group (1) as FCoV, ferret coronavirus is distinct from FCoV,343 so one would not expect cross-infection between ferrets and cats in the same household; however, RNA viruses are prone to recombination, so it can possibly occur.1 Dogs frequently serve as a source of infection of CCoV. Coronaviruses are frequently transmitted between dogs and cats living in close contact,30,268 giving rise to recombinant variant viruses.118,334 (See Feline Coronavirus Serotypes I and II, discussed earlier.) For more information on CCoV, see Chapter 8. Virus is shed in the feces from 2 days postinfection.246 It is thought that primary viral replication occurs in the epithelial cells of the small intestine,243 but in long-term viral excretion, virus is localized in the ileocecocolic junction.117 A small number of cats are resistant to FCoV infection.10,64,64 It is likely that viral shedding of types I and II is different; laboratory strains, which are typically type II, are shed for only a couple of weeks,303 whereas in natural infection, type I virus is shed by 65% of cats for 2 to 3 months or longer by many cats.10,15 Some cats are co-infected with both types I and II.187 The majority of cats clear the virus after 2 to 3 months of fecal shedding, although in some infected cats (13%) the virus establishes a persistent infection.10,15,15 Experimental infection of specific-pathogen free cats with nonvirulent FCoV resulted in persistent localization of the virus in the colonic epithelium, and to a lesser degree in macrophages of the liver and mesenteric nodes, associated with prolonged fecal shedding.166a A curious feature of lifelong carrier cats is that they shed the same strain of virus continuously in the feces until death15; this is very similar to the situation with chronic carriers of feline calicivirus.52 FCoV carriers rarely develop FIP.15 Chronic carrier cats usually appear to remain in adequate health, though some develop chronic large intestinal diarrhea and fecal incontinence in older age.13 Detection of carrier cats requires positive fecal reverse transcriptase (RT)-polymerase chain reaction (PCR) test results for 9 months.10 Virus is maintained in the cat population by chronic carrier cats and through reinfection of transiently infected cats.10,15,15 The stress of entering a rescue shelter increases viral shedding 101– to 106-fold.256 However, the stress of pregnancy and lactation did not cause infected queens to shed more virus.84 In healthy cats, virus is only shed in the saliva for a very brief period of time (hours).10 Not all (up to 75%) cats with FIP shed virus in the feces,17,45,45 and possibly also in other excretions, such as urine, saliva, and tears. Virus shed in the feces tends to have an intact 3c gene.45,254 Although serologic (antibody to FCoV) testing has limitations (see later discussion), it is clear that cats with seronegative results, as determined by a reliable diagnostic test, do not shed FCoV,7,10,10 whereas approximately one in three cats with seropositive results does shed virus.7 Cats with higher antibody titers are more likely to shed virus,10,84,84 although cats with relatively low indirect fluorescent antibody (FA) titers of 40 to 80 have a 26% to 39% chance of shedding FCoV.11,12,12 Evidence of viral shedding is never a good reason to euthanize a cat because most FCoV shedders stop within a few months, and fewer than 10% develop FIP.8 In addition, if a cat has survived one exposure to FCoV, it may be better to use that animal for breeding rather than introduce new susceptible animals that may not be resistant, because a genetic element may play a role in susceptibility to FCoV infection.244 Cats become infected with FCoV orally, usually indirectly by contact with cat litter contaminated with the virus. FCoV is a highly infectious virus, and in a multicat household, over 90% of cats will seroconvert. FCoV can survive for 7 weeks in a dry environment.139 FCoV is readily inactivated by most household detergents and disinfectants; however, bleach is preferred not only because it is efficacious, but also because it is safe for use around cats.13,139 FCoV has been isolated from a 1-day-old kitten, implying that transplacental transmission could be possible. However, the practice of removing kittens from infected queens, even those who died of FIP, protected the kittens from infection, which would not have worked had transplacental transmission occurred.7,139 Initially, the development of FIP was attributed to properties of the virus, rather than of the host: less virulent laboratory strains have less ability to replicate in monocytes compared with more virulent strains.64 However, monocytes of different cats will support FCoV replication to varying extents64 and some cats’ monocytes will not support viral replication at all, which could be an explanation for the occurrence of FCoV-resistant cats as previously reported.10 Another explanation could be that some cats lack the as yet undetermined receptor for the type I virus. Discoveries regarding the pathogenesis of FIP have been useful in understanding how clinical signs develop and for devising new strategies for therapy. Using immunohistochemistry, Kipar et al.166 demonstrated FCoV within monocytes adhering to blood vessel walls and extravasating (Web Fig. 10-1)—this is the key event in the development of FIP. FCoV-infected macrophages release interleukin (IL)-6,97 IL-1β matrix metalloproteinase (MMP)-9,166 and tumor necrosis factor (TNF)-α.166,311,311 In early infection, IL-6 stimulates hepatocytes to release acute-phase proteins (such as alpha-1 acid glycoprotein [AGP]) and B lymphocytes to proliferate and differentiate into plasma cells.311 It is likely that high IL-6 levels found in cats with FIP are the cause of hypergammaglobulinemia. TNF-α is a major contributor to the inflammatory response and pathogenesis of FIP. TNF-α is very likely the cause of the lymphopenia seen in FIP,311 especially in noneffusive FIP. In vitro, apoptosis of lymphocytes (especially CD8+ lymphocytes) that was induced by ascitic fluid, plasma, and culture supernatant of peritoneal exudate cells from cats with FIP was attributed to TNF-α.311 However, in another study, use of anti-TNF-α or TNF-α neutralizing antibodies was unable to block FIP-induced lymphocyte apoptosis.105 TNF-α upregulates fAPN (the receptor for type II FCoVs)312 and, along with granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor, which are also produced by FCoV-infected monocytes, is a neutrophil survival factor.312 In later infection, TNF production shifts from macrophages to lymphocytes.55 Chronic overproduction of TNF-α also results in cachexia. In addition to the virulence of the infecting strain of FCoV, reduced immunity can predispose a cat to develop systemic infection. Most cats that develop FIP have a history of stress in the previous few months. Stress likely has two effects that increase the cat’s susceptibility to FIP: it decreases the immune system, and increases viral shedding 101– to 106-fold.256 Furthermore, it is hypothesized that the type and strength of immune response determine the outcome of FCoV infection: that a strong cell-mediated immune (CMI) response will prevent FIP, a weak or nonexistent CMI and strong humoral response results in effusive FIP, and an intermediate response results in noneffusive FIP.244 Lesions of noneffusive FIP predominate within the eye and central nervous system (CNS), both sites protected from the immune system. Evidence from experimental infections showed that cats surviving a challenge mount a greater CMI response than those who succumb.62 However, clearance of natural infections also correlated with the presence of a humoral immune response to the FCoV spike protein,103 and it is known that kittens are protected by maternally derived antibody (MDA).7 Therefore, it is possible that some antibody protection also occurs. Humoral immunity associated with secretory IgA is suspected to be important in preventing initial infection of epithelial cells. However, in exposed cats, seroconversion occurs within 18 to 21 days postinoculation,204 which is long compared to most viral infections where antibodies appear 7 to 10 days postinoculation. Although some viruses continually mutate as a means of evading the host immune response, cats persistently infected with FCoV shed the same strain for years.15 Therefore, FCoVs have developed means to suppress the host immune response. It is also evolutionarily beneficial to the virus to delay the humoral response in some way, so that cats become persistently infected and shed virus for longer. Because cats with FIP die and so no longer shed virus, which is not in the evolutionary interest of the virus, FIP might actually be considered as an “evolutionary accident.” Further evidence for viral-associated immunosuppression and impaired clearing of virus is that FCoV-infected cats that succumb to FIP have much higher systemic viral levels than those that survive the infection.159 The means by which FCoVs suppress the host immune response have not been completely elucidated. As stated previously, one way FCoV affects the host’s response is that FCoV-infected cells release a substance that causes apoptosis of lymphocytes,105 and this substance is likely to be TNF-α.311 Once antibodies are present, they cause viral proteins on the surface of the monocyte to be internalized within minutes.50 Perhaps the reason for this is to delay as long as possible the development of anti-S antibodies that are capable of clearing infection.100 Antibody-dependent enhancement (ADE) is a phenomenon that has foiled many attempts to find a successful FIP vaccine and is worrisome to those trying to develop a SARS vaccine.272 In ADE, a greater proportion of cats that had been vaccinated with trial vaccines developed FIP than cats in the unvaccinated control group also exposed to a laboratory strain of FCoV, usually the very virulent 79-1146 type II strain. This strain is not useful because of its extreme virulence. The reason for ADE is not well understood, but one hypothesis is that it is mediated by subneutralizing antibodies that facilitate viral entry into their target cell, the macrophage, via an Fc-receptor-mediated mechanism.* Research shows that addition of antibody to infected macrophages causes rapid internalization of viral proteins from the cell surface.50,65,66,325,326 The significance of this has not yet been fully elucidated, because it is not to evade antibody dependent complement-mediated lysis of infected cells.51 Cats with ADE develop disease in fewer than 12 days, whereas controls take 28 days or more.285 By contrast, field studies have shown that seropositive pet cats that were naturally reinfected by FCoV showed no evidence of ADE.15,18 Indeed, many cats that had become seropositive after natural infection appeared to be resistant to developing FIP (though not to reinfection by the same or another strain of FCoV).15,18 The mortality rate of cats that were in contact at the time of initial FCoV infection was 14%, compared with about 8% at the time of reinfection.18 In practical terms, a seronegative cat introduced into a household in which FCoV is endemic has a 1 in 6 chance of developing FIP, whereas a seropositive cat has a 1 in 12 chance. Cats are at greatest risk of developing FIP in the first 6 to 18 months after infection, and the risk decreases to about 4% by 36 months after infection.18 There is no evidence that the available vaccine against FIP (Primucell, Pfizer) causes ADE (see later discussion). Because ADE has been reported experimentally in cats passively given anti-FCoV antibodies,314 it would be prudent to ensure that blood donors have FCoV seronegative results. Most FCoV infections are subclinical. When FCoV first infects cats, they may have a brief episode of upper respiratory tract signs or diarrhea; although these signs are usually not severe enough to warrant veterinary attention, the diarrhea can occasionally be extremely severe.164 Kittens infected with FCoV generally have a history of diarrhea and occasionally of stunted growth and upper respiratory tract signs.7 Experimentally infected specific-pathogen free cats had diarrhea due to FCoV and can manifest during primary infection, in persistently infected (carrier) cats, and where noneffusive FIP has caused lesions within the colon. Diarrhea, and occasionally vomiting, occurs in kittens and some cats at primary FCoV infection, is a small intestinal diarrhea, and is usually self-limiting within a few weeks. However, occasionally the virus can be responsible for a severe acute or chronic course of vomiting or diarrhea with weight loss, which may be unresponsive to treatment, continue for months, and occasionally result in death.164 However, there are many other causes of diarrhea in cats that should be considered before a diagnosis of FCoV diarrhea can be made (e.g., Tritrichomonas foetus, which tends to affect the same group of cats—young cats living in crowded multicat environments).101 FCoV diarrhea most frequently presents in young kittens from 5 weeks of age. Chronic, large-intestinal diarrhea has been noted in older, otherwise healthy, FCoV carrier cats; it may result in fecal incontinence.3 For details of diarrhea due to FIP, see the later section on Colonic or Intestinal Localization. Web Fig. 10-2 details the FIP-diagnosis algorithm. In step 1 of the algorithm, cats with FIP tend to be young, from multicat environments (breeding and boarding catteries, rescue shelters, veterinary clinics), and have a history of recent stress; FIP incubation is from weeks to months. Approximately one-half of the cats with FIP are younger than 2 years, but cats of any age can be affected.215,244,244 Evaluation of the history of cats with FIP typically reveals that they lived in a multicat environment within the previous year, usually with a cat breeder or in a rescue shelter. Occasionally, they have been to a boarding cattery, cat show, or veterinary clinic. Nevertheless, FIP, especially the noneffusive form, can incubate for months or even years. Cats with FIP usually have a history of stress in the previous few months. Those with effusive FIP are usually taken to their veterinarians within 4 to 6 weeks of arriving in a new home, elective surgery, or a similar stressful situation, whereas cats with noneffusive FIP develop disease after a greater interval. Cats that have spent several years in a single-cat environment are extremely unlikely to have FIP. Cats with effusive FIP have ascites, although very few owners notice the abdominal distention (Fig. 10-2), thoracic effusion (Fig. 10-3), or both. The cat may be bright or dull, anorexic, or eating normally. Abdominal swelling with a fluid wave, mild pyrexia (39° C to 39.5° C [102.2° F to 103.1° F]), weight loss, dyspnea, tachypnea, scrotal enlargement, muffled heart sounds, and mucosal pallor or icterus may be noted. In one survey, FIP accounted for 14% of cats with pericardial effusion, second only to congestive heart failure (28%).281 Abdominal masses can be palpated, reflecting omental and visceral adhesion, and the mesenteric lymph node may be enlarged. Noneffusive FIP is the more chronic manifestation of the disease, occurring weeks to many months after initial infection and the triggering stress. Signs of noneffusive FIP are usually vague and include mild pyrexia, weight loss, dullness, and depressed appetite. Cats may be icteric. Almost all cats with noneffusive FIP have intraocular lesions. Abdominal palpation usually reveals enlarged mesenteric lymph nodes162 and may also reveal irregular kidneys or nodular irregularities in other viscera. If the lungs are involved, the cat may be dyspneic, and thoracic radiographs may reveal patchy densities in the lungs.322 Cats with noneffusive FIP frequently have ocular lesions. The most common ocular sign in FIP is iritis, manifest by color change of the iris. Usually all or part of the iris becomes brown (Fig. 10-4), although occasionally blue eyes appear green. Iritis may also manifest as aqueous flare, with cloudiness of the anterior chamber, which in some cases can be detected only in a darkened room using focal illumination. Large numbers of inflammatory cells in the anterior chamber settle out on the back of the cornea and cause keratic precipitates, which may be hidden by the nictitating membrane (Fig. 10-5). Some cats have hemorrhage into the anterior chamber. If the cat has no sign of iritis, the retina should be checked because FIP can cause cuffing of the retinal vasculature, which appears as fuzzy grayish lines on either side of the blood vessel (Fig. 10-6). Occasionally, pyogranulomata are seen on the retina (see Fig. 10-6); the only other condition likely to produce pyogranulomata on the retina would be mycobacterial infection.68 The vitreous may appear cloudy. Retinal hemorrhage or detachment may also occur306 but is more commonly a sign of hypertension. Similar intraocular signs can also be caused by infections with Toxoplasma organisms, feline immunodeficiency virus (FIV), feline leukemia virus, or systemic fungi (see Chapter 92).306 In cats with noneffusive FIP, 25% to 33% have neurologic abnormalities.81 The onset of neurological signs is a poor prognostic indicator, and decerebrate posture (opisthotonos, forelimb extension, hindlimb flexion) a hopeless one.172,174,257,293 Clinical signs are variable and reflect the area of CNS involvement; the most common clinical sign is altered mental status, then ataxia followed by nystagmus and then seizures.173 An excellent review of ataxia in the cat was written by Penderis.257 Ataxia due to FIP can be cerebellar, sensory (spinal or general proprioceptive), or central vestibular but is not likely to be peripheral vestibular.257 To differentiate central and peripheral vestibular disease: normal postural reactions, ipsilateral cranial nerve VII deficits and Horner’s syndrome, and horizontal nystagmus with fast phase away from the lesion side are present in the peripheral vestibular disease. Central vestibular disease may have these signs; however, any additional deficits make it more likely. Discomfort on opening the mouth is a more common feature of peripheral vestibular disease.257 When FIP causes nonsuppurative granulomatous meningitis, the signs reflect damage to the underlying nervous tissue: unexplained fever, behavioral changes, seizures, paralysis, incoordination, intention tremors, hypermetria, hyperesthesia, and cranial nerve defects. When the FIP lesion is a pyogranuloma on a peripheral nerve or the spinal column, lameness, progressive ataxia, or paresis (tetraparesis, hemiparesis, or paraparesis) may be observed.172,173,214,257 FIP is the most frequent cause of spinal cord lesions in cats up to 2 years of age.194 Cranial nerves may be involved, causing visual deficits and loss of menace response,172,173 depending on which cranial nerve is damaged. An excellent review of diagnosis and treatment of seizures in the cat has been published by Smith Bailey and Dewey.293 Computed tomographic and magnetic resonance imaging (MRI) studies are valuable in the diagnosis of CNS FIP. Occlusion of the aqueduct, causing obstructive hydrocephalus (lateral ventricular width greater than 2 mm) is highly suggestive of a diagnosis of neurologic FIP.81,172,173,257,262 In a study of 24 cats with FIP and neurologic involvement, 75% were found to have hydrocephalus on gross or histologic postmortem examination.173 Other diseases such as cryptococcosis, toxoplasmosis, and lymphoma have not been reported to cause hydrocephalus.173 Isolated fourth ventricle and cervical syringomyelia have also been reported.172,173 After intravenous contrast medium (gadolinium, gadoteridol), enhancement around the third and fourth ventricles, mesencephalic aqueduct, and brainstem on MRI is highly suggestive of FIP (Web Fig. 10-3).81,172,172 Occasionally, the primary or only organ affected by FIP granulomas is the intestine. Lesions are most commonly found in the colon or ileocecocolic junction but may also be in the small intestine.110,327 Cats may have various clinical signs as a result of this lesion—usually constipation, chronic diarrhea, or vomiting.110,327 Palpation of the abdomen often reveals a thickened intestine. A hematologic finding may be increased numbers of Heinz bodies. Lesions have been described in the skin, always in association with other clinical signs of FIP.40 These nonpruritic cutaneous lesions have been characterized as slightly raised, well-circumscribed, intradermal papules of approximately 2 mm in diameter over the neck, forelimbs, and lateral thoracic walls.40,56 Skin fragility similar to that associated with Ehlers-Danlos syndrome has also been reported in a cat with FIP.321 FIP is the second most common infectious cause of mortality in weaned kittens16,42 but causes no deaths from birth to weaning (“fading kittens”). In the 1970s, FCoV was implicated in various reproductive disorders and in fading kitten syndrome,287 but the problem was probably due to taurine deficiency, and FCoV is no longer believed to be involved.16,21 FCoV does not cause infertility.16 However, FCoV infection does result in stunting of kittens (Fig. 10-7) and increased prevalence of diarrhea and upper respiratory signs.7 FCoV can be an important pathogen for domestic and exotic Felidae. Coronavirus infections have produced chronic weight loss, diarrhea, and anorexia. In a survey of captive felids, more than 50% had positive test results for infection based on fecal PCR and serologic testing for type I and type II coronaviruses.150 Mortality from FIP has been observed among captive exotic felids, with cheetahs (A. jubatus) having the highest risk for disease.* Necrotizing colitis caused by FCoV is a major health problem in cheetahs.150 A definitive diagnosis of FIP can often only be made after death, with histopathologic findings consisting of phlebitis or perivascular pyogranuloma.166,227,229,287a In vivo FIP diagnosis is extremely challenging for even the most competent clinician. Even tru-cut biopsy and fine-needle aspirate (FNA) results of the liver and kidney have only 11% to 38% sensitivity in correctly diagnosing FIP.94 At most stages of the diagnostic process, it is easier to rule out non-FIP conditions than to be absolutely sure that FIP is involved. The following discussion will parallel the algorithm given in Web Fig. 10-2. The first steps to a diagnosis of FIP are to obtain a history of the cat; review the clinical signs that have given rise to the suspicion of FIP (see Web Fig. 10-2, boxes 2a and 2b). The next step involves analysis of the effusion or of the blood; however, if abdominal or thoracic effusion is present, its analysis is more useful and will be discussed first (see Web Fig. 10-2, box 3a).109 Nonspecific abdominal ultrasonographic abnormalities can include: peritoneal effusion and abdominal lymphadenomegaly in many cats, and in some cats, hypoechogenicity in the parenchyma of the liver or spleen.185a Approximately 50% of cats with effusions have FIP.200 The FIP fluid may be clear, straw colored, and viscous and because of the high protein content may froth when shaken (Web Fig. 10-4). The effusion may clot when refrigerated. If the sample is bloody, pus-filled, chylous, or foul smelling, then FIP is unlikely,275 although in rare cases it can appear pink and chylous.283 The effusion in FIP is classified as a modified transudate in that the protein content is usually very high (greater than 3.5 g/dL), reflecting the composition of the serum, whereas the cellular content approaches that of a transudate (fewer than 5000 nucleated cells/mL). The high protein content of the effusion parallels the increased levels of gamma globulins; thus a low albumin : globulin (A:G) ratio in an effusion is highly predictive of FIP. An A:G ratio of more than 0.8 almost certainly excludes FIP,288 and with values between 0.45 and 0.8, FIP remains a possibility.295 An A:G ratio of less than 0.45296 in an effusion with greater than 3.5 g/dL of total protein and low cellularity, consisting of predominantly neutrophils and macrophages, is highly predictive of effusive FIP.275 The diseases with similar fluid analyses are lymphocytic cholangitis and occasionally tumors, usually of the liver. Cytology of the effusion, as well as radiographic and ultrasonographic findings, may help to differentiate FIP from neoplasia, cardiomyopathy, and liver disease with portal vascular hypertension.121,288 Additional diagnostic tests can be performed on the fluid to help substantiate a diagnosis of FIP. The Rivalta test is a simple, rapid inexpensive point-of-care test for FIP. One drop of 8% acetic acid is added to 5 mL of distilled water and mixed thoroughly, and a drop of effusion is carefully layered on top. If the drop disappears and the solution remains clear, the test result is negative. If the drop retains its shape, stays attached to the surface, or floats slowly down the tube, then the test result is positive (Fig. 10-8). For the Rivalta test result, the positive predictive value is 0.86, and the negative predictive value is 0.97.284 Positive immunofluorescent staining, indicating FCoV-infected macrophages from an effusion, is definitely diagnostic of FIP, but a negative result does not rule it out (Fig. 10-9).109,233 One difficulty with this test is that often the effusion has few macrophages. The typical hematologic change in both effusive and noneffusive FIP is lymphopenia.230 In noneffusive FIP, a nonregenerative anemia (hematocrit [HCT] less than 30%) associated with chronic inflammation is evident (see Web Fig. 10-2). Cats that are constipated from granulomatous colitis have an increase in Heinz bodies in erythrocytes. FIP was the main cause of thrombocytopenia in cats.175 Serum γ- globulin is a more useful predictive test for FIP than total protein or A:G ratio (Web Fig. 10-5).109,284,284 The specificity of the diagnosis increases in parallel with value used as a cutoff for increased γ-globulin levels; however, the corresponding sensitivity decreases.109,284 The serum A:G ratio decreases in FIP because the albumin level remains within reference limits or decreases slightly and globulin levels increase. The total serum protein level is often high. FIP should be suspected when serum protein electrophoresis reveals a polyclonal increase in γ-globulin. Other possibilities for these increases include B-cell lymphosarcoma, multiple myeloma or other plasma cell dyscrasia, or chronic persistent infections such as FIV.173,193 Other biochemical alterations reflect damage to the organs containing FIP lesions and are not specifically useful for diagnosing FIP. However, they may help the clinician determine whether treatment is worthwhile. Hyperbilirubinemia may be observed and frequently is a reflection of hepatic necrosis. Despite this fact, the alkaline phosphatase and alanine aminotransferase activities are often not increased as dramatically as they are with cholestatic disorders, such as cholangiohepatitis and hepatic lipidosis. Cerebrospinal fluid (CSF) analysis is often the most useful for confirming neurologic FIP, but it may be difficult or impossible to obtain a specimen because of the high viscosity of the fluid as a result of protein and inflammatory cell accumulation.173 The risk of brain herniation is significant in these cases, so care should be taken when performing a CSF tap.215,257 Analysis of CSF from cats with neurologic signs can reveal spectacularly elevated protein levels.81,173,215,257,300 However, in one study, CSF total protein was elevated in only 25% of cats with neurologic FIP.173 Total protein in CSF from healthy cats is less than 0.27 g/L; however, lumbar puncture will give higher total protein levels in CSF than by cisternal puncture.67 Pleocytosis (5 leukocytes/µL or 100 to 10,000 nucleated cells/µL—neutrophils, lymphocytes, and macrophages) is present in 67% of cats with neurologic FIP. * It has been said that more cats have died because of FCoV-antibody test results than of FIP. Serologic testing can be useful if the laboratory is reliable and consistent and the test results have been correlated intelligently with clinical findings. At times, clinicians have mistakenly equated a positive antibody titer result with a diagnosis of FIP, which is partly the fault of commercial laboratories or kit manufacturers who specifically call their tests “FIP tests,” when in fact the tests generally detect only the presence of FCoV itself or FCoV antibodies. False-negative antibody test results can be found if there are numerous virus particles in the sample binding the antibody and rendering it unavailable to the antigen in the test or if the testing is performed too soon after exposure to the virus. Antibodies to FCoV appear 18 to 21 days after infection.204 Antibody presence without infection may be found early in the neonatal period: MDAs disappear by 5 to 6 weeks of age. Approximately one third of seropositive cats are actively infected and shedding coronavirus.10 FCoV antibody titers correlate fairly well with virus shedding.10,246 However, there are many cats with high titers that do not shed virus, and there are cats with low titers that do shed virus. Analysis of antibody titers is especially useful when FCoV in a cat population is being controlled by quarantine or when it has been eliminated. When a test is sensitive enough, a seronegative result in a clinically healthy cat means the cat is uninfected. Methodologies and antibody titer results vary among laboratories, but each laboratory should report two titer levels. One is the least significant level of reactivity (or low positive titer value) and another is the high antibody titer value. High titers have been correlated with a greater chance of FCoV shedding or presence of FIP as demonstrated by confirmation with surgical biopsy or necropsy results.10,246 The absolute antibody titers mentioned in this chapter are those established by the author’s laboratories and should only be used as relative guidelines. When searching for a reliable laboratory, a sample should be divided, stored at −20° C, and sent, without revealing its purpose, to the laboratory in question and an FCoV-referenced laboratory for comparison. See Web Appendix 5 for a listing of some established laboratories for the immunofluorescent antibody test. A final common misconception about antibody titers should be noted. Increasing antibody titers do not indicate that a cat is going to develop FIP—the majority of cats with rising FCoV antibody titers subsequently eliminate the virus and have seronegative results again. FCoV antibody tests based on the 7b protein have been commercially marketed based on data indicating that the less virulent strain, laboratory strain FECV 70-1683, lacked the 7b gene, whereas the highly virulent laboratory strain FIPV 79-1146 had an intact 7b gene.332 The finding was later found to be a laboratory artifact because FCoVs in cell culture frequently develop deletions in the 7b gene.119 This gene is not essential for viral replication and seems to be superfluous in the absence of a host. Both cats with FIP and healthy cats have antibodies to the 7b protein.152 One study showed distinct genetic differences in the membrane and nonstructural protein 7b genes between FCoV strains from cats with and without FIP.38 Other investigators have found consistent deletions in the 3c gene within FIPV biotypes.45,254 The protein encoded by the 3c gene has unknown function but appears to be essential for viral replication in the gut.45 Whether these genetic discoveries can be exploited to develop a diagnostic test reliably predictive of FIP remains to be seen. There are 10 major indications for FCoV antibody testing as outlined in Box 10-1 and Table 10-1. The following discussion considers the various types of antibody tests and their uses. TABLE 10-1 Interpretation of Feline Coronavirus Serology Results FCoV, Feline coronavirus; FIP, feline infectious peritonitis; RT-PCR, reverse transcriptase–polymerase chain reaction. Indirect FA testing is the gold standard for detection of FCoV antibodies; it is useful because it generates indirect FA titers that correlate well with virus excretion.10,246 It is clear that seronegative cats, as determined by a reliable diagnostic test, do not shed FCoV,7,10,10 whereas approximately one in three FCoV-seropositive cats do shed virus.86 Cats with higher antibody titers are more likely to shed virus,10,84,110,246 although cats with relatively low indirect FA titers of 40 to 80 have a 26% to 39% chance of shedding FCoV.7,8,8 Types I and II FCoV and transmissible gastroenteritis virus of pigs can be used in the test.178 Care must be taken to distinguish fluorescence associated with antibodies to FCoV from nonspecific fluorescence caused, for example, by antinuclear antibodies. These can be present because of other factors such as concurrent infections (e.g., FIV, systemic mycoses), autoimmune disease, recent vaccination, or certain treatments for hyperthyroidism (i.e., thiamazole, felimazole, methimazole). Therefore, inclusion of a negative control of uninfected cells for each serum or plasma is essential. Plate enzyme-linked immunosorbent assays (ELISA) or kinetics-based ELISAs are used in commercial and research laboratories. There are no published refereed veterinary assessments of the sensitivity or specificity of these tests apart from the kinetics-based ELISA.25 See Web Appendix 5 for the commercial availability of this assay. There are at least two FCoV antibody test kits: an ELISA, the FCoV or FIP Immunocomb (Biogal Galed Laboratories Kibbutz Galed, M.P. Megiddo, Israel), and the rapid immunomigration (Speed F-Corona, Bioveto, France; Web Fig. 10-6). The FCoV Immunocomb compared favorably with the gold standard indirect FA test.12 Despite frequent criticism, serologic tests are very useful for identifying cats with suspected FIP, but clinicians should be aware of the limitations of these tests. First, many healthy cats (especially if they are purebred) and cats with conditions other than FIP can have seropositive results. Second, some cats with effusive FIP appear to have low titers or to have seronegative titers because large amounts of virus in their bodies are binding to antibody, making them unavailable to bind the antigen in the serologic test. Although exceptions have been reported,173,298 cats with noneffusive FIP usually have a high FCoV-antibody titer and rarely have seronegative results; thus, coronavirus serology can usually be used to rule out a diagnosis of FIP in suspected noneffusive cases. The presence of a high FCoV-antibody titer in a sick cat from a low-risk, one- or two-cat household is also unusual; it is a stronger indicator of a diagnosis of FIP than the same antibody titer in a cat from a multicat household in which FCoV is likely to be endemic. Serologic testing cannot be used alone to diagnose FIP, and the other parameters listed in Web Fig. 10-2 must also be considered. Several popular misconceptions regarding interpretation of antibody titers should be addressed. First, clinically healthy cats with FCoV antibodies do not have noneffusive FIP. Second, cats with neurologic FIP had higher antibody titers but lower FCoV loads than cats with generalized FIP.86 Last, seronegativity in diarrheic cats rules out FCoV as a cause; however, FCoV may or may not be a cause of diarrhea in cats with seropositive results. Serologic tests performed on ascites or thoracic effusions yield the same results as when done on blood samples, provided they have high protein concentrations that approximate blood. High FCoV antibody titers in an effusion are 85% specific and 86% sensitive for predicting FIP.109 As in blood, FIP effusions may appear to have low titers or are seronegative because large amounts of virus in the effusion can bind to antibody, making them unavailable to bind the antigen in the serologic test. One way to resolve this issue is by testing for immune complexes.109 Alternatively, these effusion samples can be examined further for the presence of virus by quantitative RT-PCR. Usually such cats have huge amounts of virus in the effusion.
Feline Coronavirus Infections
Etiology
Feline Coronavirus Serotypes I and II
Epidemiology
Age
Breed
Nondomestic Felidae
Other Pet Species Coronaviruses
Pathogenesis
Virus Shedding
Transmission
Monocyte Infection and Vasculitis
Immune Response to Feline Coronavirus Infection
Antibody-Dependent Enhancement
Clinical Findings
Initial Infection
Coronavirus Enteritis
Multisystemic Inflammatory Vasculitis Disease
Effusive Disease
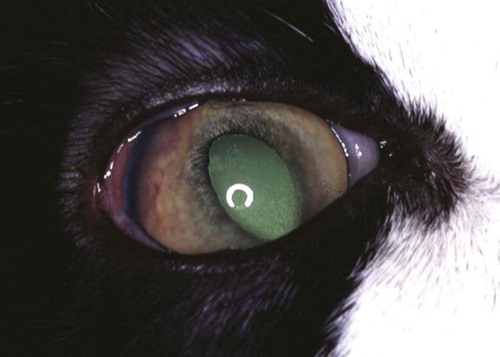
Noneffusive Disease
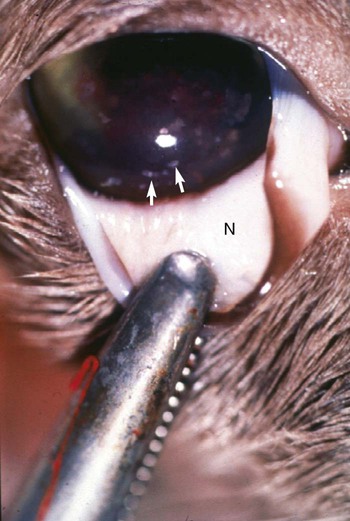
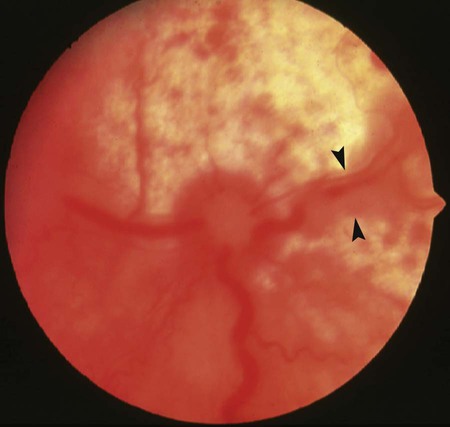
Ocular Signs
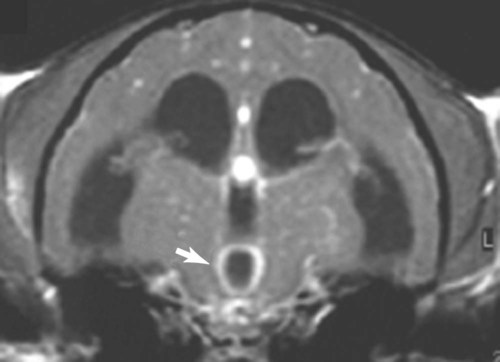
Neurologic Signs
Colonic or Intestinal Localization
Cutaneous Lesions
Neonatal and Prenatal Kittens
Nondomestic Felidae
Diagnosis
Coronavirus Enteritis
Feline Infectious Peritonitis
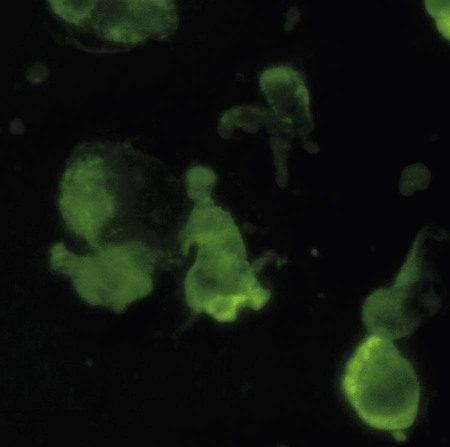
Effusion Analysis
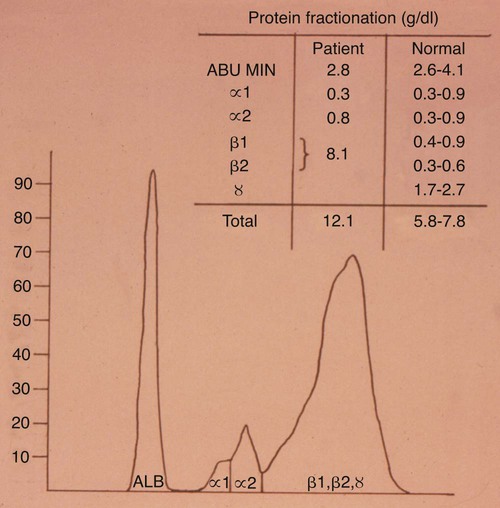
Hematologic and Biochemical Findings
Cerebrospinal Fluid Examination
Feline Coronavirus Antibody Tests
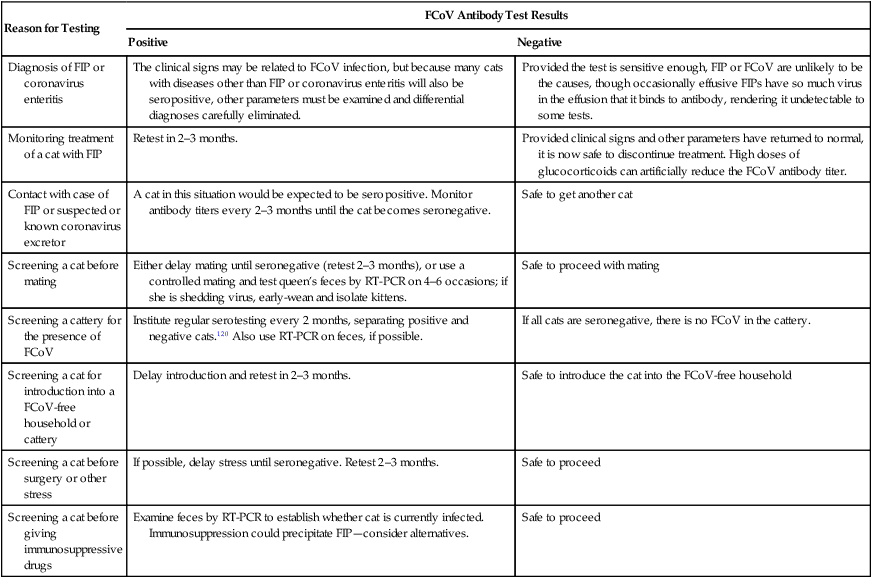
Reason for Testing
FCoV Antibody Test Results
Positive
Negative
Diagnosis of FIP or coronavirus enteritis
The clinical signs may be related to FCoV infection, but because many cats with diseases other than FIP or coronavirus enteritis will also be seropositive, other parameters must be examined and differential diagnoses carefully eliminated.
Provided the test is sensitive enough, FIP or FCoV are unlikely to be the causes, though occasionally effusive FIPs have so much virus in the effusion that it binds to antibody, rendering it undetectable to some tests.
Monitoring treatment of a cat with FIP
Retest in 2–3 months.
Provided clinical signs and other parameters have returned to normal, it is now safe to discontinue treatment. High doses of glucocorticoids can artificially reduce the FCoV antibody titer.
Contact with case of FIP or suspected or known coronavirus excretor
A cat in this situation would be expected to be seropositive. Monitor antibody titers every 2–3 months until the cat becomes seronegative.
Safe to get another cat
Screening a cat before mating
Either delay mating until seronegative (retest 2–3 months), or use a controlled mating and test queen’s feces by RT-PCR on 4–6 occasions; if she is shedding virus, early-wean and isolate kittens.
Safe to proceed with mating
Screening a cattery for the presence of FCoV
Institute regular serotesting every 2 months, separating positive and negative cats.120 Also use RT-PCR on feces, if possible.
If all cats are seronegative, there is no FCoV in the cattery.
Screening a cat for introduction into a FCoV-free household or cattery
Delay introduction and retest in 2–3 months.
Safe to introduce the cat into the FCoV-free household
Screening a cat before surgery or other stress
If possible, delay stress until seronegative. Retest 2–3 months.
Safe to proceed
Screening a cat before giving immunosuppressive drugs
Examine feces by RT-PCR to establish whether cat is currently infected. Immunosuppression could precipitate FIP—consider alternatives.
Safe to proceed
Indirect Immunofluorescence
Enzyme-Linked Immunosorbent Assay
Point-of Care Antibody Tests
Interpretation of Antibody Titer Results
Effusion
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree