39 Feeding management pre- and post-surgery
Introduction
General anesthesia in the horse has a much higher mortality and morbidity than most other species. The confidential enquiry into perioperative equine fatalities (CEPEF) (Johnston et al 1998, 2002) recorded a mortality rate of 1.6%, which decreased to 0.9% when anesthesia for colic surgery and obstetrical procedures was excluded. Another study of mortality during elective procedures from a single center recorded a death rate of 0.063% (Mee et al 1998), and a more recent study from one large veterinary hospital in Kentucky recorded a rate of fatalities directly related to anesthesia of 0.12%, rising to 0.24% with the inclusion of horses killed or dying within 7 days post general anesthesia (Bidwell et al 2007). Sick and shocked horses that require emergency surgery (primarily horses affected by colic) carry a higher mortality rate (1.9%) (Johnston et al 2002). Mee et al (1998) found that mortality associated with general anesthesia was 4.25 times more likely for emergency procedures not associated with colic than for similar procedures carried out electively; emergency general anesthesia for colic carried an increased risk of mortality of 9.86 times that of elective cases. In another study, pre-operative and anesthesia-related variables associated with intra- and postoperative mortality were assessed in 774 surgical colic cases; cardiovascular compromise, level of pain, age, and breed were all shown to be associated with the risk of mortality (Proudman et al 2006). Various metabolic and endocrine changes occur during anesthesia and surgery in horses, and these may have profound, albeit temporary, effects on the nutritional status of the horse. Feeding practices around the time of surgery, and the underlying disease status of the horse, will also have important implications to the metabolic condition.
Hormonal and metabolic changes associated with general anesthesia and surgery
Horses develop a stress response to anesthesia and surgery, which results in numerous endocrine, humoral and metabolic changes designed to restore or maintain homeostasis (Wagner 2009). Among other effects, the stress response results in the activation of the adrenocortical system, redistribution of blood flow, mobilization of substrates such as glucose and free fatty acids, and activation of the immune system (Muir 1990). The interaction between nutritional status and feeding practices with the stress response in horses undergoing surgery and anesthesia has received little attention, but is likely to be significant.
The degree of stress imposed by anesthesia and surgery is difficult to quantify. Traditional approaches include measuring physiological parameters such as heart rate and plasma cortisol concentrations. In humans, anesthesia without surgery does not produce a stress response, but surgery does, and the magnitude of the cortisol response varies with the severity of the surgical procedure (Traynor & Hall 1981). In contrast, inhalation anesthesia alone in normal horses is associated with a marked increase in plasma cortisol (Taylor 1985, 1998, Luna & Taylor 1995, 2001, Robertson et al 1996, Luna et al 1997, 1999). Relatively benign surgical procedures in horses, such as arthroscopic surgery, produce only minor and short-lived further increases in cortisol (Robertson et al 1990). Horses affected by serious intestinal diseases causing colic have pre-existing hypercortisolemia (Hoffsis & Murdick 1970, Hodson et al 1986, Santschi et al 1991, Hinchcliff et al 2005, Edner et al 2007, Sherlock & Mair 2008). Although abdominal surgery is also known to cause increases in serum cortisol levels (Taylor 1985, Stegmann & Jones 1998), the surgery itself causes only slight increases in the stress response above that already induced by the disease and anesthesia.
The cortisol reaction contributes to a variety of protective responses (Zaloga & Marik 2001, Wagner 2009) including: (i) maintenance of blood pressure by increasing the synthesis of catecholamines, sensitizing smooth muscle cells to the effects of catecholamines, and by inhibiting vasodilation by decreasing nitric oxide synthesis; (ii) increasing the provision of nutrients through stimulating hepatic gluconeogenesis and decreasing peripheral utilization of glucose (which result in hyperglycemia), increasing protein catabolism and decreasing protein synthesis, and promoting lipolysis; and (iii) prevention of an excessive inflammatory reaction in response to injury by stabilizing lysosomal membranes, decreasing capillary permeability and by altering the expression of pro- and anti-inflammatory cytokines. Glucocorticoids also stimulate tissue cells to produce lipocortins (peptide hormones that interact with the immune system to decrease production of prostaglandins, thromboxanes and leukotrienes) and decrease the migration of inflammatory cells into tissues (Muir 1990). The long-term effects of increased circulating cortisol levels for prolonged periods of time can include delayed wound healing, muscle wasting, immune deficiencies, and increased susceptibility to infection. Lipocortin also inhibits prostaglandin production in the gastrointestinal tract, which might promote gastrointestinal ulceration (Breazile 1987).
In addition to a cortisol response, anesthesia and surgery result in other endocrine and metabolic responses, including increases in circulating catecholamines (Robertson 1987, Wagner et al 1990) and changes in insulin and glucose levels. The pre-anesthetic and anesthetic induction drugs can also affect insulin responses and glucose metabolism (Robertson 1987). Alpha-2-adrenergic agents (such as xylazine) can inhibit insulin release by stimulating alpha-2-adrenoceptors in pancreatic beta cells, thereby resulting in hyperglycemia (Thurmon et al 1982). Feed intake pre- and post-surgery will also affect the insulin response, with fasting resulting in a suppression of the insulin response, and refeeding enhancing insulin release (Robertson 1987, Taylor 1989, Robertson et al 1990). Surgery in humans generally causes hyperglycemia (Clarke 1973), but in horses this response appears to be more variable, and some anesthetic drugs may actually result in hypoglycemia (Robertson 1987, Taylor 1989, Robertson et al 1990).
Plasma concentrations of non-esterified fatty acids are also affected by stress and surgery. Increased sympathetic activity associated with pain or fear will cause lipolysis and an increase in non-esterified fatty acid concentration (Snow 1979), but decreased sympathetic activity associated with sedation and anesthesia may have the opposite effect. The insulin response will also affect nonesterified fatty acid concentration because insulin is antilipolytic; suppression of insulin therefore tends to cause an increase in nonesterified fatty acid concentration. Preoperative fasting is also likely to increase nonesterified fatty acid concentration, but this may fall once the horse is sedated, and in the immediate postoperative period the concentration may be normal or low (Robertson 1987, Robertson et al 1990).
As in humans, the stress response in horses to anesthesia and surgery is temporary and is unlikely to be clinically relevant in healthy individuals undergoing surgical treatment. However, severely malnourished humans suffer more serious complications following abdominal and thoracic surgery, and preoperative total parenteral nutrition may be beneficial in these patients (Veterans Affairs Total Parenteral Nutrition Cooperative Study Group 1991). The significance of the stress response to anesthesia and surgery in malnourished horses has received little attention.
Perioperative glucose regulation and control
Development of hyperglycemia in humans after major surgery is common and is modulated by many factors. These include perioperative metabolic state, intraoperative management of the patient, and neuroendocrine stress response to surgery. Acute insulin resistance also develops perioperatively and contributes significantly to hyperglycemia, which is associated with poor outcomes in critically ill post-surgical patients (Bochicchio et al 2005a, b). Many studies have shown that intensive insulin therapy can combat insulin resistance, decrease blood glucose levels, and induce anabolic processes, thus, decreasing morbidity and mortality. Initial studies in humans demonstrated improved outcomes in critically ill, postsurgical patients who received intensive glycemic control (IGC) including insulin therapy. These results were quickly extrapolated to other clinical areas, and IGC was enthusiastically recommended in the perioperative period. However, recent prospective trials have not been able to show the benefit of IGC (Finfer et al 2009); neither an appropriate therapeutic glycemic target nor the true efficacy of perioperative glycemic control has been fully determined. IGC also increases the risk of hypoglycemia significantly, which is not inconsequential in critically ill patients (Akhtar et al 2010).
The use of insulin therapy in critically ill horses and horses after surgery has received relatively little attention. Endotoxemia and the systemic inflammatory response syndrome are frequent consequences of many gastrointestinal diseases (including strangulating obstructions and colitis) (Fig. 39.1) in horses (Bryant & Moore 2008). Endotoxin has been shown to decrease insulin sensitivity in horses (Toth et al 2008). The pathophysiology and systemic manifestations of endotoxemia in horses and sepsis in humans are very similar and the maintenance of normoglycemia with the use of insulin in endotoxemic horses may contribute to a significant reduction in morbidity and mortality. With the use of controlled delivery pumps and regular blood glucose monitoring, maintenance of normoglycemia with a constant rate infusion of insulin is an achievable goal in equine critical care, especially in neonates (Sykes & Furr 2005). In adult horses with acute colic, hyperglycemia has been found to be common, and severe hyperglycemia has been shown to be associated with a worse prognosis for survival (Hollis et al 2007, Hassel et al 2009); these observations suggest that glycemic control in adult horses during and after colic surgery may also warrant investigation.
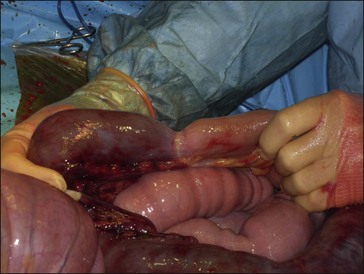
Figure 39.1 Intraoperative view of small intestinal strangulation (the intestine to the left is strangulated).
Key Points
• Anesthesia and surgery can induce several metabolic and endocrine alterations, including increases in circulating cortisol and catecholamines, hyperglycemia and insulin resistance
• This stress response is temporary and unlikely to be clinically relevant in healthy animals undergoing surgical treatment but may exacerbate disturbances in horses with critical illness such as surgical colic. Additionally, poor nutritional status at the time of surgery may impact the extent of endocrine and metabolic derangements due to surgery and anesthesia.
Feeding management pre-surgery
Most authors recommend withholding food but not water for 6 to 12 hours prior to inducing general anesthesia (Taylor & Clarke 2007, Robertson & Scicluna 2009). The justification for preoperative starvation is that it reduces gut content and increases the functional residual capacity of the lung. This may help oxygenation, but the beneficial effect is probably very limited, as it is impossible to empty the gastrointestinal tract completely. Starvation beyond 12 to 18 hours is likely to induce significant stress and metabolic derangements.
Relatively short periods of perioperative fasting can have significant effects on the metabolic status of systemically healthy horses. However, the effects on physiological functions are minor. Reinprecht et al (2007) studied the metabolic and clinical effects of feed deprivation in 20 horses subjected to orthopedic surgery. The patients were fasted from 12 hours before until 4 hours after surgery. Plasma glucose and free fatty acids increased after surgery and returned to pre-operative levels 72 hours after surgery. A significant rise in segmented granulocytes occurred 24 hours after surgery. Serum aspartate amino transferase (AST) reached its highest activity 24 hours after surgery, whereas creatine kinase (CK) activities were highest at 2 hours after surgery. Abdominal sounds were significantly reduced until 24 hours after surgery, although appetite was not depressed.
Although the metabolic and clinical effects of preoperative starvation in systemically healthy horses are short-lived and unlikely to be clinically significant, horses affected by acute abdominal disease requiring surgery (i.e., surgical colic) are compromised due to the disease, often starved for long periods of time and sometimes exposed to the stress of long trailer rides. Edner et al (2007) studied some metabolic parameters in 20 healthy horses given anesthesia alone and 20 horses undergoing abdominal surgery for colic, and found that the post-anesthetic period was associated with increased lipolysis and weight loss in the horses with colic. Plasma cortisol, free fatty acids, glycerol, glucose, lactate and creatine kinase (CK) were elevated and serum phosphate and potassium were lower in colic horses before anesthesia. Anesthesia and surgery resulted in a decrease in plasma free fatty acids and glycerol in colic horses whereas levels increased in healthy horses. During anesthesia plasma lactate and phosphate both increased in both groups. In the colic horses, plasma lactate continued to rise after surgery. Plasma free fatty acids and glycerol increased for 8 hours after standing in the colic horses. In both groups serum CK increased and phosphate decreased after anesthesia. By day 7 after anesthesia most parameters were similar in both groups. However, colic horse lost on average 8% of their initial body weight. These results suggest that the colic horses were in a negative energy balance for the first week after surgery.
Postoperative colic and ileus
Postoperative ileus (the failure of effective aboral propulsion of gastrointestinal tract contents) (Fig. 39.2) is a well-recognized clinical entity in horses (Becht & Richardson 1981, Adams 1988, Little et al 2001, Senior et al 2004). Postoperative ileus affecting the stomach and small intestine is manifested as pain, toxemia and gastric reflux, and is a major postoperative complication following colic surgery, especially surgery involving ischemic damage to the small intestine (Edwards & Hunt 1985, Hunt et al 1986, MacDonald et al 1989, Van der Velden & Klein 1993, Proudman et al 2002, Blikslager et al 1994, Freeman et al 2000, Cohen et al 2004, Mair & Smith 2005, Garcia-Seco et al 2005). Mortality rates between 13 and 86% have been reported for this condition (Hunt et al 1986, Blikslager et al 1994), and the condition is also associated with increased duration of hospital stay and increased costs to owners (French et al 2002). The etiology and pathogenesis of postoperative ileus following intestinal surgery are unclear, although current theories suggest that intestinal inflammation associated with the primary disease, intestinal distension and surgical trauma to the small intestine is likely an important factor (Koenig & Cote 2005, Doherty 2009).
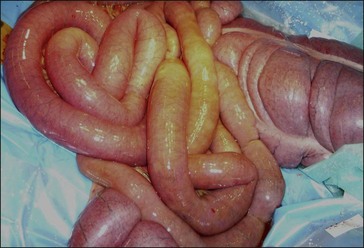
Figure 39.2 Intraoperative view showing diffusely distended small intestine due to postoperative ileus.
Postoperative colic unassociated with colic surgery or other types of abdominal surgery is recognized as a clinical problem in horses. For example, Mircica et al (2003) reported that five of 84 horses showed signs of “gastrointestinal discomfort” following nonabdominal surgery, an incidence of nearly 6%. The majority of postanesthetic colic cases occur within 72 hours of anesthesia (Senior et al 2004). There are several possible causes; recent changes in management (including recent transport, changes to exercise routine and dietary changes) are recognized as important risk factors for many types of naturally-occurring colic (Tinkler et al 1997, Cohen et al 1999, Hillyer et al 2002), and similar factors are probably associated with post-operative colic. Sympathetic nervous activity involving the release of endogenous catecholamines increases as part of the perioperative nocifensive and stress responses to surgery (Weismann 1990). These and exogenous catecholamines decrease gut motility in man (Steinbrook 1998). One study demonstrated an increased prevalence of postanesthetic colic in horses undergoing surgery compared to those anesthetized for noninvasive procedures (i.e., magnetic resonance imaging) (Andersen et al 2006).
In humans, postoperative ileus is known to affect all parts of the gastrointestinal tract after surgery, but different segments are not equally susceptible (Woods et al 1978, Livingston & Passaro 1990). Motility of the large intestine of horses may also be adversely affected by general anesthesia (Gerring 1992). General anesthesia depresses gut motility and the development of post-operative colic is not considered unusual (Taylor & Clarke 2007). This may vary from transient mild discomfort to more severe colic and impaction of segments of the large intestine. Development of impaction of the large colon has been associated with several factors, including stall confinement after musculoskeletal injury and hospitalization for reasons other than gastrointestinal tract disease. In one study, 19 of 147 horses with impaction of the large colon had undergone general anesthesia and arthroscopy prior to developing impaction (Dabareiner & White 1995). Cecal impaction has also been associated with hospitalization, surgery and the administration of anesthetic agents (Ross et al 1985, Collatos & Ronano 1992). Anesthetic and analgesic drugs may have marked depressant effects on gut motility, and may be important factors in the development of these conditions. Starvation depresses gut motility and may also predispose to impaction and other forms of colic. For example, starvation for 18 hours and the use of a muzzle preoperatively was blamed as the likely cause of colic in six horses after non-abdominal surgery (Jones et al 1991).
There appear to be very few published studies that have evaluated factors affecting postoperative fecal production in horses, and the effects of pre- and postoperative feeding practices on return of bowel function after anesthesia remain largely unknown. Little et al (2001) found that reduced fecal output following surgery unrelated to the gastrointestinal tract (defined as ≤ 3 defecations per 24-hour period after surgery) was associated with age ≥5 years old, orthopedic procedures of >60 minutes’ duration and no phenylbutazone therapy after surgery. 12% of the total number of horses in the study developed postoperative colic, all of which demonstrated reduced fecal output. These results support the view that colic can occur as a complication of surgery for problems that do not involve the gastrointestinal tract and suggest that there is an intermediate clinical phase manifested as reduced fecal output before overt signs of colic are seen.
An increased risk of post-operative colic may also occur with the use of opioids in the anesthetic protocol, although the results of several retrospective studies have conflicted: Mircica et al (2003) did not find an association whereas Senior et al (2004) did show that morphine was a risk factor for postanesthetic colic. Senior et al (2004) reviewed the records of 496 orthopedic operations on 428 horses to estimate the prevalence of, and identify the risk factors for, the development of colic in horses after surgery. Fourteen of the horses developed colic; eight of them were undiagnosed, three were classified as impactions, one as tympanic colic of the colon, one as incarceration of the small intestine in the epiploic foramen, and one as left dorsal displacement of the colon in the nephrosplenic space. Morphine was associated with a fourfold increased risk of colic compared with the use of no opioid or butorphanol, and out-of-hours surgery was also associated with an increased risk. This association between the use of morphine and postoperative colic probably results from opioid-suppression of normal gut motility. Morphine is mainly a µ-agonist (Branson & Gross 2001), and it has been shown that µ-receptor agonists can decrease the propulsive motility and cause sphincter closure in the gastrointestinal tract (Sojka et al 1988, Roger et al 1994). Postoperative colic is most likely to occur with higher doses, and probably prolonged systemic administration.
In addition to anesthetic and analgesic drugs, other factors such as preoperative starvation and the administration of other drugs around the time of surgery are likely to be important in the etiopathogenesis of postoperative colic (Taylor & Clarke 2007). A multicenter prospective case-control study of post anesthetic colic was reported by Senior et al (2006). The estimated mean prevalence of colic in the study population was 5.2%; however the prevalence varied between each of the four participating centers. The most commonly diagnosed cause of colic was large intestinal impaction. Multivariable analyses showed that the center involved and the type of surgery performed were associated with an increased risk of postanesthetic colic. Preoperative food deprivation and the use of opioid drugs were confounding factors.
Key Points
• Postoperative ileus is a major complication following colic surgery, especially when there has been ischemic damage to the small intestine
• Postoperative colic not associated with colic surgery or other types of abdominal surgery also occurs in horses. The severity of colic may vary from transient mild discomfort to impaction of segments of the large intestine
• Anaesthetic and analgesic drugs depress gut motility, and may be important factors in the development of these postoperative complications
• There is very little information on the impact of feeding practices pre- and/or post-surgery on risk for development of postoperative complications
Postoperative feeding management
There appears to be a wide variation in opinion between different surgeons and hospitals with respect to the timing of reintroduction of feed postoperatively. Most horses undergoing nonabdominal surgery or simple intestinal surgery (such as management of intestinal displacements without enterotomy) will recommence partial enteral feeding within 6 to 12 hours of recovery from anesthesia (Spier & Meagher 1989, Durham 2005, White 2009). However, following small intestinal resection and anastomosis (Fig. 39.3), horses are frequently starved for longer lengths of time. In one study of surgical colic cases, postoperative feed withdrawal for more that 10 days was reported in some cases (Cohen et al 2004
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


