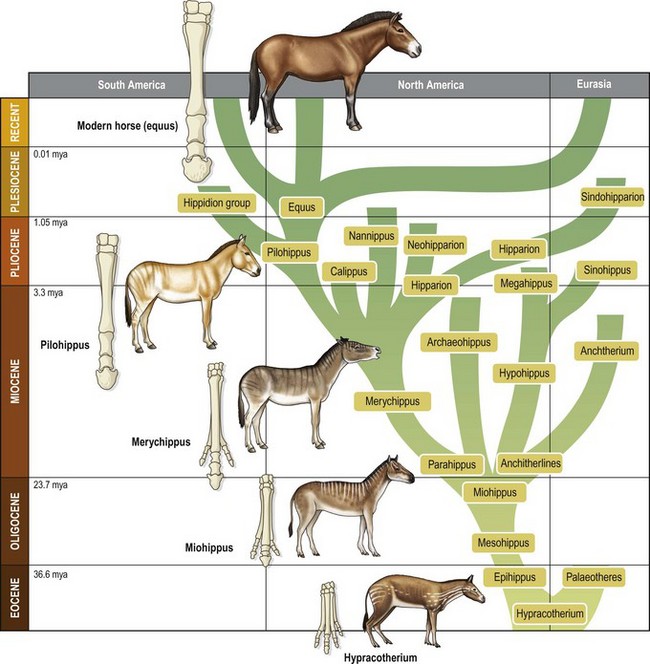
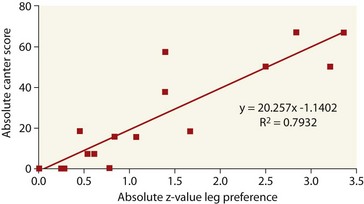
Chapter 16 Through the process of domestication the horse has gained survival advantages such as food, shelter, veterinary care and protection, but this has been at the cost of restriction of movement, limited breeding opportunities and a requirement to expend energy for the benefit of another species. In fact it is likely that many of the demands made on the domestic horse conflict with the evolutionary process that shaped the behavior of its predecessors (Goodwin, 1999). The evolution of the horse began some 60 million years ago (Fig. 16.1). The ancestor of the domestic horse was Eohippus or the ‘Dawn Horse’ (Hyracotherium). Eohippus was a small creature about the size of a fox, with adaptations for life in the swampy regions of North America, with four toes on its front feet and three on the hind feet. The ‘Dawn horse’ died out, and by the Oligocene period its successors had grown to the size of a large dog with three toes on each foot. Pliohippus was the first equid to walk on one digit, the two ‘other’ toes being reduced to long thin vestiges, more commonly referred to as the splint bones in the domestic horse. The various breeds of Equus caballus are derived from a 13–14 hand (130–140 cm) at the withers, social, Steppe-dwelling animal that thrived on low-quality forage through the development of hypsodontic molars as well as by evolutionary adaptations to the digestive tract. The domestic horse is a non-territorial member of the family Equidae (Goodwin, 2002), and is often described as a follower species, because the precocial young stand and move to feed within a few hours of birth, after which they will follow the mother as she moves off with the herd. Thus the horse performs locomotory behavior from only a few hours of age. This makes perfect evolutionary sense, since as a prey animal the horse relies upon early predator detection and flight as its primary defense mechanisms. In terms of locomotion, in its natural state, the horse has been observed to range over considerable distances. In ethological terms, the horse’s home range contains resources important for survival such as watering holes and suitable grazing areas. Home ranges vary in size between and within areas where feral horses have been studied depending on the availability resources, and the available area ranges have been reported from 0.9 to 48 km2 and more in different study sites (Berger, 1986; Boyd & Keiper, 2005; Tyler, 1972). In addition, the members of a horse band (the structured social unit within a herd) follow similar movement patterns within a common home range. Changes in band composition may occur through natal dispersal, which is the movement of young horses from one maternal group to another or to a bachelor band, as occurs in many feral herds. For example, Berger (1986) reported that fillies moved on average 4 km and males moved between 10 and 15 km away from their natal herd in the Navada region of the USA, and in Japan, the Misaki horses will emigrate over much greater distances. Having evolved from animals adapted to live in the unpredictable and open environment of the Steppe region of Asia, it is not surprising that they exhibit anatomical specializations for running. These adaptations include anatomical changes in the 3rd phalanx (the caput ridge on the distal metacarpi and metatarsi) as well as the development of the ‘spring mechanism’. Due to the spring mechanism, the energy the horse needs for locomotion is halved through storing and returning elastic strain energy in spring-like muscle–tendon units, and is optimized by the anatomical construction of the tendons and the number of distal joints affected by the tendons (Wilson et al., 2001). These features allowed the horse to respond quickly through early detection of danger due to their well-developed senses (as social behavior developed – it is likely that their sensory development may have become enhanced), followed by flight as their first defense mechanism against predators. Therefore sociality and flight became neurologically ‘blue printed’, and are considered a biological need even for domesticated and intensively managed horses. Equus caballus (the modern ancestor of the little dawn horse) exhibits similar motivation in relation to much of their locomotion and behavior. Simple movements initiated by the developing fetus can be detected from only a few months old and include limb stretching, becoming more complex and coordinated as it matures. Once born, the foal becomes mobile through a series of stages described by Fraser (1992) that result in teat seeking and sucking. Foals differ in their ability to achieve each stage of mobility, but usually they are stable on their limbs within hours of birth (Fig. 16.2). Its interesting that although most foals are able to walk, canter and suckle very soon after birth, they need a few days to develop the skills needed for lying down and standing up. Many foals will literally fall asleep on their feet, during their first days of life, illustrating that even in precoccial species, complex locomotory movements need to be learned and practiced. It is worth considering how much the intensive housing of foals at this early stage may hamper the development of many behaviors, including locomotion. Fig 16.2 A foal just after birth quickly learns to balance and to move around so it can follow its dam, even in canter. However, complex behaviors like lying down take much longer to learn. © Arnd Bronkhorst/www.arnd.nl. One of the primary activities foals engage in is stretching, which seems to be of greatest importance during the first few weeks after birth. Foals have been observed to perform this activity 40–60 times each day (more than sucking) (Fraser, 1992). It is suggested that both recumbent and upright stretching are important for athletic development, growth and joint formation. Especially in the first 10 days of their life, foals are engaged in many short bouts of solitary locomotory play around their dams, allowing their muscles to develop. During the first week of life the mare and foal spend approximately 90% of their time within 5 m of each other (Waring, 2003). During these early weeks, the mare repeatedly leaves the foal and moves a short distance away, and the foal follows. This following behavior is an instinctive tendency in a foal, refined through learning during the first week of life. Over time, the mare and foal will spend more time apart, until by the age of 1 year the youngster may be grazing for approximately 44 min/h alongside the rest of the herd. Grazing involves moving whilst foraging, an activity that horses in their natural state will perform for around 16 h each day depending on their physiological and reproductive state, the time of year, available forage and weather conditions. Thus horses have evolved to spend much of their time moving slowly with their head and necks lowered, whilst selecting suitable forage. In the natural environment, horses may travel up to 80 km during grazing and for water, and even when kept on restricted pastures, horses are estimated to move around 20 km each day (McGreevy, 2004). Increased locomotory activity has been found in horses kept socially isolated from others in which case they will walk and trot three times more frequently than horses kept in groups or in visual contact with another horse (Houpt & Houpt, 1989), apparently to try to get into more close contact with conspecifics. Apart from movement associated with following and grazing with the herd, the foal engages in various locomotory activities described as play (Fig. 16.3). There appears to be no universally agreed function or homogeneous character to define play, but it is apparent that play has survived the pressures of natural selection and is an almost universal trait in mammals. There are a number of proposed functions which include development of social skills (Fraser, 1992); motor strength and co-ordination; behavioral flexibility (Spinka et al., 2001) and cognitive training. In horses, play occurs most frequently in younger animals, with colts performing more than fillies (Kurvers et al., 2006). When the spontaneous locomotion activity of foals kept under various management conditions was compared (Kurvers et al., 2006), it was found when foals were kept for 24 h/day at pasture, they exercised at a level comparable with feral foals. Interestingly the total observed daytime workload in the first month of life was approximately twice that of the following months, while locomotion activity decreased with increasing age. Foals that were stabled for some portion of the day appeared to engage in compensatory locomotion activity. However this was insufficient to reach the level of locomotion of foals kept continually outside (Kurvers et al., 2006). There have been relatively few studies of play in non-domestic equids and where recorded this has generally been anecdotal, but social and solitary-locomotor play have been recorded in Hartmann’s zebra foals (Joubert, 1972) and also in captive juvenile Przewalski horses (Zharkikh, 1999) as well as in free-ranging and feral horse populations in the UK and USA (Tyler, 1972; Berger, 1986). In these groups, the majority was social play behavior with activity patterns including: play fighting, neck wrestling and chasing and with solitary-locomotor play including gamboling, high speed turns and sudden stops (see Goodwin & Hughes, 2005). During play, absolute force values as exerted on the developing tissues are probably not substantially different from those created by cantering, but play activities result in other loading directions, including eccentric loading and loading in the sagittal plane, which might be important for the development of musculoskeletal structures (Kurvers et al., 2006) (see Chapter 13). Equine laterality (or sidedness in horses) has been investigated in relation to equine performance and training as well as trying to understand the underlying factors that lead one horse to favor a left lead over a right leading leg. In a series of studies, (van Heel et al., 2006; Kroekenstoel et al., 2006; van Heel et al., 2010) healthy jumping bred KWPN foals were born and raised outside at the same location, and were then followed weekly for a year to observe the development of laterality (Fig. 16.4). Fig 16.4 Fifty percent of foals develop a side preference while equally distributed left and right. © Arnd Bronkhorst/www.arnd.nl. They were assessed at 3, 15, 27 and 55 weeks of age for pressure distribution under their feet (to determine the Centre of Pressure), external and internal development of the foot including phalangeal development and ‘handedness’ (or side preference). In addition several conformation measures were made such as relative leg length and head size, heel height and toe angle. It was shown that about 50% of the foals developed a significant preference for protracting the same limb systematically while grazing, which resulted in changes to the balance of their feet and subsequently uneven loading patterns. There was no overall preference for either left or right sidedness, with an even distribution between the two sides. However it appears that foals with relatively long limbs and small heads were predisposed to develop laterality and, consequently unevenness (van Heel et al. 2006, Kroekenstoel et al. 2006). Seventeen of these horses were tested again when 3 years old, after they had only been handled for normal management procedures like trimming and de-worming (van Heel et al., 2010). The assessments revealed that the laterality as well as the unevenness still existed, but the relationship between the body conformation and the unevenness had weakened. In addition when these horses were also assessed in free trot to canter transitions and free jumping, laterality and unevenness were strongly related to incorrect transitions (either disunited canter or counter-canter) (Fig. 16.5). Fig 16.5 Relationship between canter (% correct lead when horse is asked to canter; 15 repeats per horse) and side preference (15 repeats) of 3-year old horses (n = 17). Reprinted with permission from van Heel et al. (2010). Since in ridden horses there is a preference for left sidedness (McGreevy & Rogers, 2005; McGreevy & Thomson, 2006), the results of studying 3-year-olds suggests that left sidedness can be induced by the human interaction with the horse, which, in most cases, is predominantly from the left side of the horse. Apart from the concern that horses with strong lateral preferences might be more prone to overloading injuries of the distal limb it is also interesting to note that this could relate to performance problems later in life. For example: horses may be less balanced when running around racetracks that run to the left or right, or have problems with picking up the correct lead in canter when under saddle. There is a need for more applied work in this area to determine how motor laterality can be influenced through breeding, environment and training. Circadian rhythms reflect extensive programming of biological activity that meets and exploits the challenges and opportunities offered by the periodic nature of the environment. Bertolucci et al. (2008) found that athletic horses monitored for activity during four different times of the year (vernal equinox, summer solstice, autumn equinox and winter solstice) experienced seasonal variations in their daily activity rhythms. Athletic Thoroughbred horses kept in individual boxes (stables) with access to a paddock showed the highest daily amount of activity during the vernal equinox (spring) and the lowest during the winter solstice but in all cases horses were most active in the middle of the photoperiod. The authors suggest that information regarding the natural activity patterns of the horse should be used to plan the training schedules and competition management of athletic horses. However a recent study of the impact of biorhythm changes associated with long distance travel for competition purposes suggests that horses are far more flexible than humans in relation to coping with change (Murphy, 2008). In humans a change in the light/dark cycle has a number of effects including disturbed sleep patterns and lethargy. Given that racehorses and other high level sport horses are frequently transported long distances between hemispheres there are questions about how quickly they recover from any jetlag in order to perform well. The findings of the study by Murphy (2008) showed that melatonin levels in the two test horses that experienced an abrupt change in light/dark cycles, were reset within 24 h, whereas regulation of body temperature took approximately 3 days. These results suggest that horse performance is less compromised by the time change than human athletes and that horses possess the ability to rapidly adjust to any changes in photoperiod. For example, various studies of feral horses have shown that horses will spend approximately 60–80% a day on feeding, during which they walk around 5–10 km, and even up to 20 km a day (VanDierendonck et al., 1996; Feist & McCullough, 1976; McGreevy, 2004). By contrast in stabled horses both feeding and moving time are significantly reduced due to the restricted space and the fact that there is no need to move in order to obtain food (Mills & Clarke, 2002; Sweeting et al., 1985; Vervuert & Coenen, 2002; Waring, 2003). When there is a high motivation for a stabled horse to perform certain behaviors but expression is restricted due to environmental factors this can lead to frustration and hence to the development of abnormal behavior (Mills & Clarke, 2002; Rushen et al., 1993) including behavioral pathologies that are extremely difficult to manage. Foraging in itself is an appetitive behavior and is a biological need which a horse must be able to perform otherwise its welfare could be hampered (VanDierendonck, 2006). One of the problems with the intensive management of horses kept in stables or stalls is their restricted movement associated with the acquisition of food. These horses are provided with all their daily requirements in ‘meals’ and no foraging is required. Providing stabled/stalled horses with multiple forage types in different locations within the indoor housing environment can increase movement and has also been shown to reduce abnormal activities such as weaving (see Thorne et al., 2005) The effects of diet on equine locomotory responses has received surprisingly little scientific attention, despite the fact that many sports horses are fed high-energy diets in order to boost performance. A study by Nicol et al. (2005) examined the effect of different feeds on the behavior of foals aged from 2 to 40 weeks. Each foal received either a starch and sugar (SS) diet, or a fat and fibre (FF) diet, and their behavior was monitored immediately after weaning. The results showed that horses receiving the FF diet cantered less frequently and for a shorter duration and the authors described the foals receiving the fat/fiber diet as more settled. This suggests that at least during a stressful time such as occurs at weaning, high-energy diets appear to be associated with increased locomotion. However, inappropriate or over-feeding high-energy foods to horses is also known to be associated with unwanted locomotory responses, such as explosive reactions to signals (aids), and this is particularly the case for horses housed in stables (Riveria et al., 2002). Hyperactivity responses, including shying, pulling, bolting, and rushing could all be considered to be the result of a restricted environment, lack of exercise and too much sugar/starch in the diet. It is likely that the behavioral problems often experienced by horse owners and that lead to poor owner–horse relationships, and thus lower equine welfare, could be prevented through improved understanding of the effect of food and feeding on horse behavior and by providing the horse with a more natural time-budget including access to an area to enable it to perform a normal locomotion pattern.
Ethology and welfare aspects
Introduction
Development of locomotion
Early kinetic activity

Grazing
Play
Development of laterality (sidedness)

Natural rhythms of activity
The impact of intensive management on locomotion
Feeding
Veterian Key
Fastest Veterinary Medicine Insight Engine