Chapter 3 Esophagoscopy
Indications
Esophagoscopy is indicated for the diagnostic evaluation of animals with signs of esophageal disease, including regurgitation, dysphagia, odynophagia, and excessive salivation. Esophagoscopy is also indicated for evaluation of animals known or suspected to have ingested a potential foreign body (see Chapter 7). This minimally invasive procedure allows visual examination of the esophageal mucosa and lumen and facilitates the procurement of specimens for biopsy, cytology, and culture. Thus esophagoscopy is most effective for obtaining a definitive diagnosis of conditions involving the mucosa or abnormalities within the lumen, including esophageal foreign body, esophagitis, esophageal stricture, esophageal neoplasia, and gastroesophageal intussusception. Compared with contrast radiography, esophagoscopy is less definitive for diagnosing megaesophagus and other motility disorders, diverticulum, hiatal hernia, and compression by periesophageal masses, although it often provides valuable diagnostic information in these situations. Esophagoscopy can also be used as therapeutic intervention to guide balloon catheters for dilating esophageal strictures, to assist deployment of esophageal stents, to remove esophageal foreign bodies (see Chapter 7), to place indwelling gastrostomy (see Chapter 9) or esophagostomy feeding tubes, and to ablate neoplastic tissue with lasers.
The age of onset is important because regurgitation beginning at the time of weaning suggests a vascular ring anomaly, congenital idiopathic megaesophagus, or congenital stenosis. An acute onset of regurgitation suggests the presence of an esophageal foreign body or acute esophagitis, whereas a chronic history of regurgitation is more consistent with idiopathic megaesophagus, vascular ring anomaly, hiatal hernia, chronic reflux esophagitis, or esophageal neoplasia. Intermittent signs are often seen in animals with hiatal hernia and reflux esophagitis. The history may indicate potential exposure to foreign bodies or caustic medications (e.g., doxycycline or clindamycin), a recent anesthetic procedure that could cause reflux esophagitis and esophageal stricture, or signs of neurologic or neuromuscular dysfunction that could be associated with secondary megaesophagus.
Instrumentation
Esophagoscopy can be performed with flexible or rigid endoscopes; however, flexible video endoscopes are most versatile because of their superior optics, illumination, insufflation, recording functions, and maneuverability. Flexible video endoscopes for examination of the gastrointestinal (GI) tract should have a working length of at least 110 cm, a diameter less than 9.8 mm, suction and air/water insufflation capability, four-way tip deflection of at least 180 degrees by 90 degrees, and a 2.0- or 2.8-mm instrument channel (see Chapter 1). Flexible endoscopes readily enable thorough evaluation of the stomach and duodenum during the same procedure, which is not possible with rigid scopes.
Rigid endoscopes for esophagoscopy are available in various lengths and diameters and are most useful for removal of certain types of esophageal foreign bodies. Even when a flexible endoscope is the primary instrument, one or two multipurpose rigid scopes should be available for selected situations, such as esophageal foreign body removal with rigid grasping instruments or flexible endoscope extraction of sharp foreign bodies with the rigid scope used as a protective “overtube” (see Chapter 7). Most rigid endoscopes marketed for procedures in people have a light source, an air insufflation mechanism, and a blunt-tipped obturator that fits inside the scope to facilitate insertion into the lumen of the esophagus. Pediatric rigid scopes have a diameter of approximately 12 mm, whereas adult rigid scopes are approximately 25 mm in diameter.
The accessory instruments needed for esophagoscopy include biopsy forceps, cytology brushes, foreign body retrieval instruments, injection needles, and a set of balloon catheters or bougies of various sizes (6- to 30-mm diameter) for dilation of esophageal strictures. Accessory instruments for foreign body removal are discussed in Chapter 7. Oval-cupped alligator jaw biopsy forceps (especially without a central spike) are adequate for obtaining mucosal biopsy specimens of the stomach, small intestine, and large intestine, but they are generally less effective for obtaining diagnostic biopsy specimens from the tough mucosa of the esophagus. Forceps with a central spike can improve the results of esophageal biopsies.
Patient Preparation and Restraint
Esophagoscopy requires general anesthesia, and food should be withheld for a minimum of 12 hours before the procedure. Throughout the procedure the anesthetized patient should have an endotracheal tube and mouth gag in place. The patient is positioned in left lateral recumbency.
Esophagoscopy Procedure
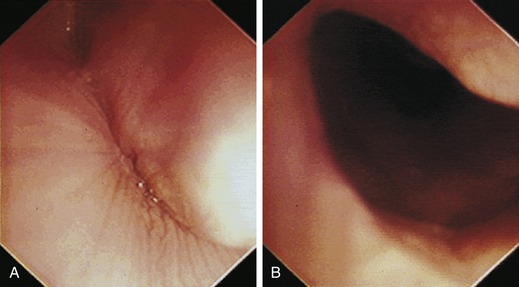
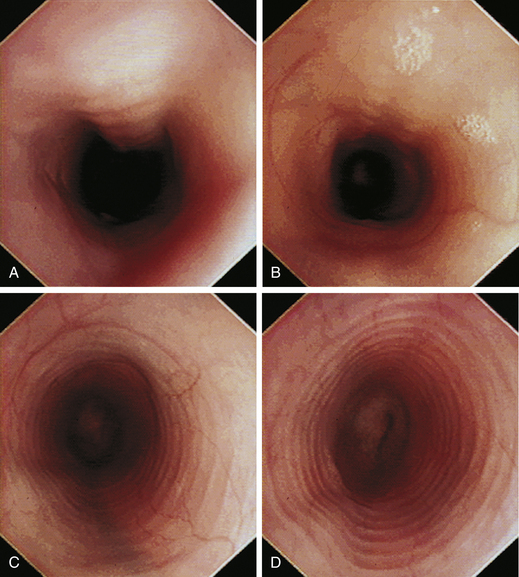
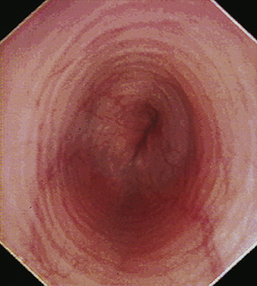
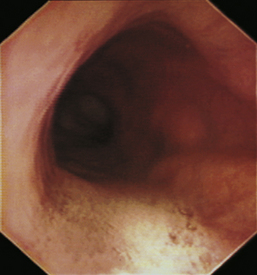
With the animal’s head and neck extended, the endoscope is directed centrally through the oropharynx and guided dorsal to the endotracheal tube and larynx so that the cranial esophageal sphincter (CES) comes into view. The CES is the entrance to the esophagus and is normally closed, appearing as a star-shaped area of folded mucosa dorsal to the larynx (see Figure 3-1, A-B). With insufflation and minimal pressure of the endoscope tip against the CES, the scope is easily advanced through the low-resistance sphincter into the cervical esophagus. Resistance is occasionally felt when the endoscope tip is misdirected into one of the piriform recesses located on either side of the larynx. Withdrawing and redirecting the endoscope more toward the dorsal midline easily corrects this problem. If the endoscope tip is passed blindly in large-breed dogs, it may inadvertently be advanced through the laryngeal opening into the proximal trachea. Resistance is felt when the endoscope comes into contact with the endotracheal tube. If this occurs, the endoscope tip should be pulled back to the oropharynx and redirected into the CES.
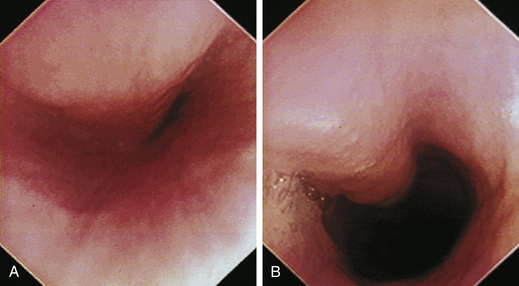
The cervical esophagus is normally collapsed, so as the endoscope passes through the sphincter, a brief “red out” usually obscures visibility. Insufflation of air should begin immediately and continue until the esophageal lumen is clearly visualized ahead of the scope as it advances (see Figure 3-2, A-B). The esophageal lumen forms a straight tube except for a slight flexure at the thoracic inlet, where the cervical and thoracic regions of the esophagus meet. The endoscope should meet little or no resistance as it is advanced. The endoscopist should advance the scope down the esophagus in a slow continuous motion, using only minor adjustments in tip deflection and torque to maintain a full panoramic view of the lumen and mucosal surfaces. Air should be insufflated intermittently, as needed, to keep the lumen distended ahead of the scope. To maintain distension in some patients, it may be necessary to have an assistant help occlude the esophagus just caudal to the larynx to prevent the escape of insufflated air. The lumen of the thoracic esophagus generally opens with minimal insufflation. Pulsations of the aorta against the wall of the esophagus are seen at the level of the base of the heart.
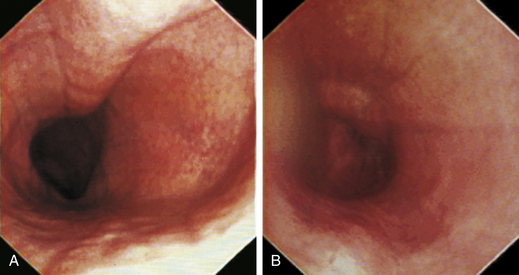
At the gastroesophageal junction the esophagus passes obliquely through the diaphragm to open into the stomach. The gastroesophageal sphincter (GES) is not a true anatomic sphincter but rather a high-pressure zone that keeps the distal esophagus closed between swallows (see Figure 3-3, A). To advance the endoscope through the slitlike opening of the GES and into the stomach, the endoscopist deflects the tip approximately 30 degrees to the left and slightly upward (see Figure 3-3, B). This can be done easily under direct visualization, and minimal or no resistance should be encountered as the endoscope advances through the GES.
A description of the normal appearance of the esophagus in dogs and cats and corresponding images are found in the “Atlas” section of this chapter (see Figures 3-1 through 3-10).
Sample Procurement
Esophageal biopsy is not usually required for diagnosis of most esophageal diseases. Visual inspection alone is generally adequate. The primary indications for mucosal biopsy of the esophagus include the presence of a mass or mucosal abnormalities indicative of esophagitis. It can be difficult to obtain adequate esophageal mucosal biopsy specimens with standard endoscopic pinch biopsy forceps, especially when the mucosa is normal. It is difficult to orient the forceps perpendicular to the wall of the esophagus, and the mucosa is often too tough to cut with the forceps, so the biopsy cups tend to slide off as they are closed. Rigid biopsy forceps with larger biopsy cups can be used with rigid endoscopy equipment to improve the quality of esophageal biopsies. Alternatively, a biopsy forceps with a central spike (bayonet type) can be used (see Figure 8-1). The spike is helpful for anchoring the biopsy forceps on the mucosa so that the “bite” is more perpendicular and slipping is prevented.
Postoperative Care and Complications
Most patients do not require medication after routine esophagoscopy. Postoperative medications to control pain and esophagitis are usually indicated after interventional esophagoscopy procedures such as esophageal foreign body removal and stricture dilation. Esophagitis is treated with a proton pump inhibitor for acid control, sucralfate as a mucosal protectant, and metoclopramide or cisapride as prokinetics to increase GES tone and prevent acid reflux. Analgesics are prescribed according to the anticipated level of discomfort from the procedure and associated mucosal injury.
Atlas Pages 46-94
Pigmented Esophageal Mucosa in Dogs
Normal Gastroesophageal Junction
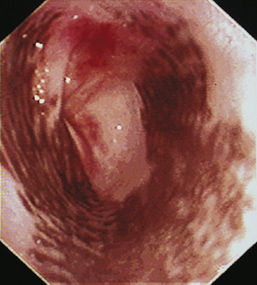
Gastroesophageal Intussusception
Atlas for Appearance of the Normal Esophagus
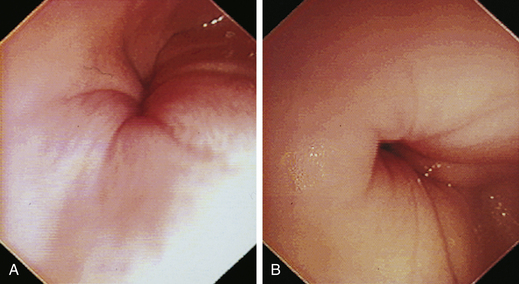
The normal esophagus in the fasted animal is empty or contains a minimal amount of clear fluid or foam (Figures 3-1 through 3-4). If the esophagus contains food residue, a large pool of fluid, or bilious fluid, then gastroesophageal reflux, hiatal hernia, motility dysfunction, or esophageal obstruction should be suspected. Because gastroduodenal contents may occasionally reflux into the esophagus during gastroduodenoscopy, the esophagus should be thoroughly assessed before advancing the scope into the stomach. In anesthetized animals the normal esophagus becomes flaccid and dilated, which makes the tubular lumen appear large when insufflated with air and allows the esophagus to drape over the trachea and mediastinal structures. Without other supportive clinical findings, this flaccid appearance should not be misinterpreted as megaesophagus. In some animals the head and neck need to be extended so that a redundant flexure of the normal esophagus at the thoracic inlet that can be mistaken for a diverticulum is eliminated. The cervical esophagus has pliable, longitudinal, mucosal folds, which are more pronounced in dogs than in cats. In the fully inflated cervical esophagus these longitudinal folds disappear, and the imprint of the tracheal rings is observed against the ventral wall of the flaccid esophagus (see Figure 3-2, B). As the esophagus passes over the base of the heart, the outline of the pulsating aorta against the esophageal wall forms a useful landmark (see Figure 3-4). Because the aorta is pulsatile, its imprint is easily distinguished from the imprints of other periesophageal structures or masses.
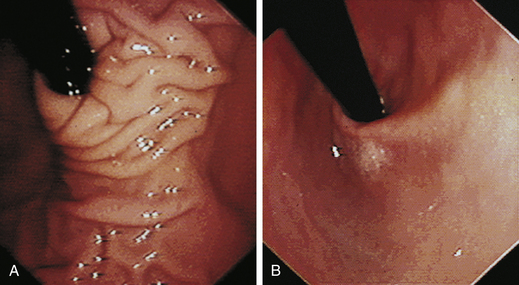
The feline esophagus is composed of striated muscle in the proximal two thirds and smooth muscle in the distal one third, whereas the canine esophagus is composed almost entirely of striated muscle. Longitudinal folds are found throughout the canine esophagus and in the cranial portion of the feline esophagus. In the cat, circumferential mucosal folds in the caudal esophagus form prominent annular ridges that have a herringbone pattern on contrast esophagrams and appear endoscopically as a pattern of circular rings (Figure 3-5). This ringlike appearance is not seen in the dog.
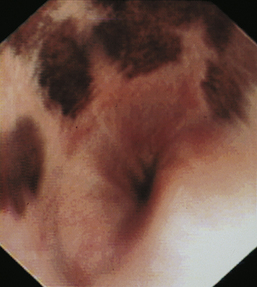
The normal esophageal mucosa in cats and dogs is smooth, glistening, and pale pink or grayish pink in color. It is noticeably less red than the gastric mucosa. Superficial submucosal vessels are normally visible in the feline esophagus (see Figure 3-5) but are not usually seen in the canine esophagus. In heavily pigmented dog breeds such as Chow Chow and Chinese Shar-Pei, the esophagus may contain variably sized confluent gray or black patches of pigmented mucosa (Figures 3-6 and 3-7).
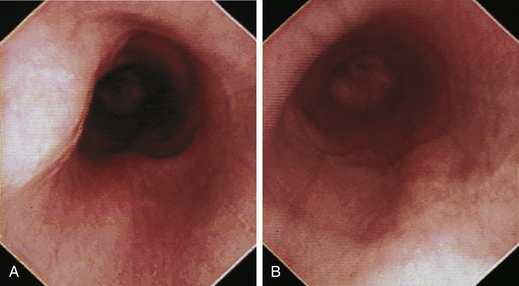
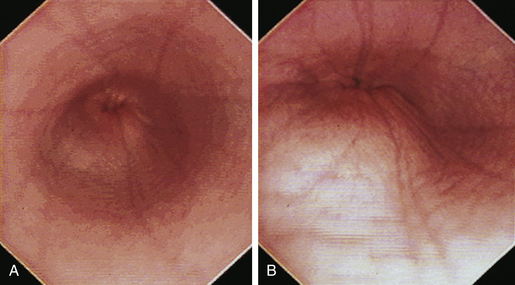
When the GES is first visualized, its configuration should be noted. The lumen of the GES usually forms a slitlike opening that is eccentrically located at the confluence of small radial folds configured in a rosette pattern (Figures 3-8 and 3-9). At the gastroesophageal junction the normal pale pink color of esophageal mucosa changes abruptly to the vivid pink or red color of normal gastric mucosa. In most normal dogs and cats, the GES is closed at the time of endoscopic examination, but this can be affected by anesthetic protocols that decrease the tone of the sphincter and by the degree of air insufflation. In some normal patients the GES can be gaping wide open; however, this is unusual and should raise the suspicion of GES dysfunction, gastroesophageal reflux, or hiatal hernia, especially when the open GES is accompanied by esophagitis and pooling of gastric contents (e.g., food, fluid, or bile) in the esophagus. The GES and cardia should also be examined from the stomach side with the scope in the retroflexed position within the stomach (Figure 3-10); see Chapter 4.
Atlas for Megaesophagus
Megaesophagus is a term that refers to a flaccid dilated esophagus resulting from diffuse hypomotility. Regurgitation associated with impaired esophageal transport of food is the most consistent clinical sign. Weight loss (or poor weight gain) and aspiration pneumonia may be complications. Primary megaesophagus is usually idiopathic and can be either congenital or acquired. Less frequently megaesophagus occurs secondary to other diseases. Congenital idiopathic megaesophagus is characterized by severe dilation of the esophagus and persistent regurgitation of food beginning shortly after weaning. Evidence suggests that the congenital form is inherited in several canine breeds and possibly in Siamese cats. Acquired adult-onset megaesophagus is usually idiopathic, but it also can occur secondary to various underlying neuromuscular diseases that impair esophageal motility, including myasthenia gravis, polymyositis, muscular dystrophies, other polymyopathies, peripheral neuropathies, central nervous system disease, dysautonomia, botulism, tick paralysis, tetanus, anticholinesterase toxicity, lead toxicity, hypoadrenocorticism, and hypothyroidism. Esophageal hypomotility can also occur secondary to esophagitis, hiatal hernia, and obstructing esophageal lesions, such as leiomyomas.
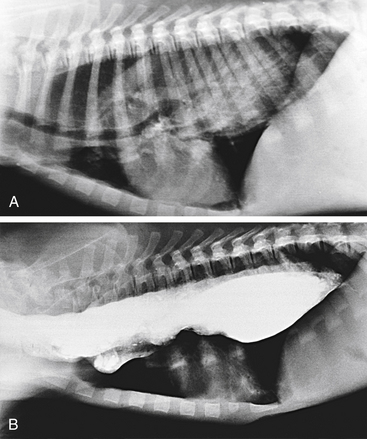
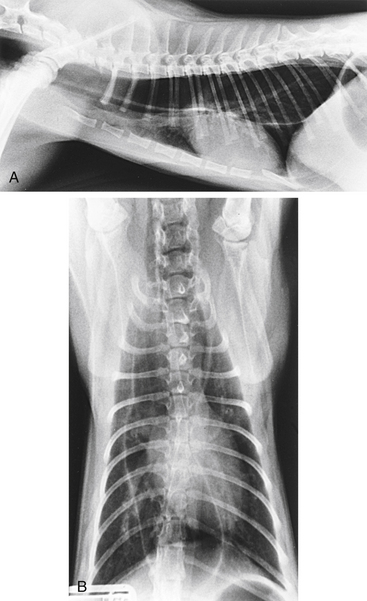
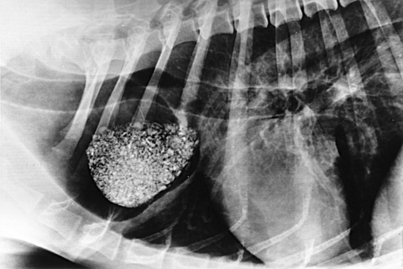
Survey radiography confirms the diagnosis of megaesophagus in most cases and is generally more reliable than endoscopy (Figures 3-11 and 3-12). In normal animals the esophagus is not usually visualized on survey thoracic radiographs, but in megaesophagus the enlarged esophagus distended with air, fluid, or food is readily identified. Disorders of esophageal motility without overt megaesophagus are best characterized by barium swallow videofluoroscopy.
The typical endoscopic appearance of megaesophagus is a markedly dilated, flaccid esophagus extending from the cranial cervical region to the GES, with variable amounts of froth, fluid, and fermenting food residue in the lumen (Figures 3-13 through 3-15). A motility or GES disorder should be suspected when the esophagus contains fluid and ingesta at the start of the examination. Dilation of the entire length of the esophagus distinguishes megaesophagus from segmental dilations or sacculations that develop proximal to luminal obstructions caused by vascular ring anomalies, strictures, tumors, or periesophageal masses. However, diverticulum of the cranial thoracic esophagus is an occasional complication of chronic megaesophagus. The esophageal mucosa in megaesophagus is usually normal in appearance, but secondary esophagitis (mucosal erythema, erosions, and friability) is occasionally observed.
Atlas for Diverticula
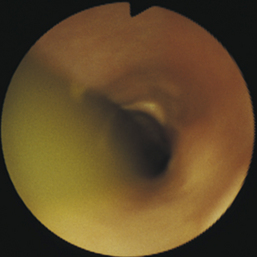
Diverticula can be diagnosed by radiography or endoscopy (Figures 3-16, 3-17, and 3-18). Survey thoracic radiographs show an air-, fluid-, or food-filled mass (pouch) adjacent to the esophagus, and contrast-enhanced radiographs demonstrate filling of the pouch with barium. Esophagoscopy reveals a saclike outpouching from the esophageal lumen, often with erosive esophagitis of the mucosa lining the diverticulum (see Figure 3-17, C). Food, fluid, or hair may have to be removed from the sac before the diverticulum can be adequately visualized (see Figure 3-18). Because of the thin, weakened wall of the diverticular sac, caution must be taken to avoid perforation. Without adequate air distension, the endoscope may enter a blind pouch and be inadvertently forced into the wall. If a diverticulum is small, the only obvious finding may be pooling of fluid. Redundancies in the esophagus that can be mistaken for diverticula are frequently found at the thoracic inlet in clinically normal brachycephalic and Chinese Shar-Pei dogs. Unlike true diverticula, these false diverticula lack associated impaction or esophagitis, and they may decrease or disappear with extension of the neck.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree