, Daniele Caprioli1, Arianna Testa1, Maria Teresa De Luca1 and Michele Celentano1
(1)
Department of Physiology and Pharmacology, Sapienza University of Rome, Rome, Italy
Abstract
A variety of animal models have been developed to mimic the interactions between drugs and environment that are thought to play a crucial role in human addiction. A history of exposure to stress, for example, facilitates the development of drug addiction and drug relapse. Furthermore, there is solid evidence that drug-related contextual cues (i.e., environmental stimuli paired with drug taking that have acquired conditioned stimulus properties) can precipitate drug seeking in both humans and animals, indicating the importance of associative learning processes. Finally, there is some evidence (mostly of anecdotal nature) that the circumstances immediately surrounding drug taking can modulate drug intake in ways that are not easily reducible to conditioning or stress. In the past few years some effort has been made to investigate this latter type of drug-environment interaction using animal models. Most importantly, we have recently shown that the context can modulate the reinforcing effects of addictive drugs independently of its physical characteristics. In these studies, some animals were transferred to the test cages immediately before the treatment (Non Resident group), whereas other animals were kept at all times in the test cages (Resident group). Some studies were conducted using a single drug, whereas others employed a polydrug taking procedure. In the present chapter, we will review not only the results obtained using this animal model but also those yielded by translational studies conducted in human addicts. Finally, we will discuss the implications of these findings for the study of drug addiction in humans and animals.
Key words
ConditioningStimulus propertiesContextual learningAssociative learningRelapseOpiateStimulantPolydrug use1 Introduction
In the past 2 decades, a variety of animal models have been developed to mimic the interactions between drugs and environment that are thought to play a crucial role in human addiction (1). A history of exposure to stress, for example, is known to facilitate the development of drug addiction and drug relapse (2–7). Furthermore, there is solid evidence that drug-related contextual cues (i.e., environmental stimuli paired with drug taking that have acquired conditioned stimulus properties) can precipitate drug seeking in both humans and animals (8–12), indicating the importance of associative learning processes. Finally, there is some evidence (mostly of anecdotal nature) that the circumstances immediately surrounding drug taking can modulate drug intake in ways that are not easily reducible to conditioning or stress (13–18). In the past few years, some effort has been made to investigate this latter type of drug-environment interaction using animal models. It has been shown, for example, that drug taking in rodents can be altered by changes in the physical characteristics of the drug environment, as indicated by reports that exposure to novel objects can reduce amphetamine intake (19) and that heat can enhance MDMA intake (20). Most importantly, we have recently shown that the context can modulate the reinforcing effects of addictive drugs independently of its physical characteristics. In these studies, some animals were transferred to the test cages immediately before the treatment (Non Resident group), whereas other animals were kept at all times in the test cages (Resident group). Some studies were conducted using a single drug whereas others employed a polydrug taking procedure. In the present chapter, we will review not only the results obtained using this animal model but also those yielded by translational studies conducted in human addicts. Finally, we will discuss the implications of these findings for the study of drug addiction in humans and animals.
2 Environmental Modulation of Cocaine, Amphetamine, and Heroin Self-Administration in Rats
An initial series of studies was devoted to investigating the effect of environmental context on cocaine, amphetamine, and heroin self-administration (21, 22). In these experiments, male Sprague–Dawley rats were implanted with a catheter in their right jugular vein and 1 week after the surgery were assigned to one of two conditions: Resident or Non Resident. The rats in the Resident groups were housed in the self-administration chambers (each equipped with two retractable levers) where they remained for the entire duration of the experiment. In contrast, Non Resident rats were transferred to the self-administration chambers only for the experimental session.
All experiments included 13 experimental sessions (3 h each). At the start of each session, the two levers were extended and remained extended for the entire duration of the session, except during the time-out periods. Only one of the two levers produced a drug infusion (active lever), whereas the other lever had no direct consequences on drug infusion but did reset the counter of the active lever. Independent groups were trained with different drug doses (delivered in a 40-μl volume over a 3-s period): 200, 400, or 800 μg/kg per infusion for cocaine; and 12.5, 25.0, or 50.0 μg/kg per infusion for amphetamine and heroin. Some rats were also tested for saline self-administration.
During sessions 1–7, the fixed ratio (FR), i.e., the number of consecutive lever presses required to obtain a single infusion, was raised from FR1 (sessions 1–2) to FR2 (sessions 3–5) and then to FR5 (sessions 6–7). Upon completion of the task, both levers retracted and were extended again after a time-out period of 40 s. During the subsequent sessions, the rats were allowed to self-administer additional drug doses and were then tested in a progressive-ratio procedure during which the number of responses required to obtain a single infusion was increased within the session according to the following progression: 5, 10, 20, 30, 50, 70, 100, 150, 200, 300, 500, and so on. The number of lever presses emitted during this session was used as an index of the motivation for heroin taking.
The following is a summary of the characteristics of the Resident versus the Non Resident condition.
(1) Both Resident and Non Resident rats were single-housed after surgery. (2) The self-administration environment was physically identical for all rats, but for some animals this was also the home environment (Resident group), whereas for other animals it represented a distinct and, at least initially, novel environment (Non-Resident group). (3) During testing, the self-administration chambers contained no food or water. The rest of time the animals had free access to food and water. (4) The distance traveled by the Non Resident rats during the transfer to the self-administration chamber was about 1 m (all animals were kept in the same dedicated testing rooms for the entire duration of the experiments, and therefore there was no transport from one room to another). (5) Immediately before the start of each session, the Resident rats were briefly handled to remove food and water from the chamber. When necessary, both Resident and Non Resident rats were briefly handled to deliver priming infusions. (6) All testing took place in the dark phase (between 13:00 and 17:00 h) 7 days a week. (7) All other husbandry routines were identical in the two groups.
Figure 1 summarizes the results of the data concerning the acquisition of drug self-administration. In particular, it shows the number of lever presses on the last training session conducted on an FR5 schedule of reinforcement (session 7). It can be seen that cocaine and amphetamine self-administration was greater in the Non Resident rats than in the Resident rats. Indeed, it appears that the dose–effect curve for the acquisition of cocaine and amphetamine self-administration was shifted to the right in the Resident versus the Non Resident condition. In contrast, heroin self-administration was greater in the Resident rats than in the Non Resident rats, with an upward shift of the dose–effect curve. The results of the progressive ratio sessions confirmed that the motivation for heroin was greater in Resident than in Non Resident rats, whereas the motivation for cocaine was greater in Non Resident than in Resident rats (21).
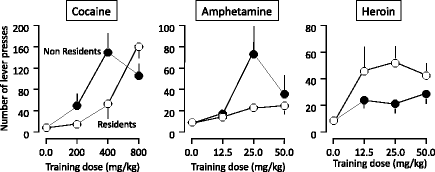

Fig. 1.
Number of lever presses (means ± SEMs) on the active lever in animals self-administering different doses of cocaine, amphetamine, or heroin on the 7th training session (conducted on an FR5 schedule of reinforcement). The rats were either transferred to the test cages immediately before the treatment (Non Resident group) or kept at all times in the test cages (Resident group). Each group was trained with only one dose of each drug (From (I) and (22). With kind permission).
These findings were quite surprising for at least two reasons. First, they were partly at odds with previous studies, conducted by us and other authors, concerning drug-induced psychomotor sensitization (a phenomenon consisting of a progressive increase in locomotor activity following repeated administrations of drugs, such as cocaine, amphetamine, heroin, etc.). In particular, it had been had reported that Non Resident rats exhibit greater sensitization than Resident rats when repeatedly treated with cocaine (23, 24), amphetamine (25, 26), morphine (27, 28), or heroin (29). The existence of a close relationship between the psychomotor and the rewarding effects of addictive drugs is widely accepted in the literature (30). It has even been hypothesized that the neuroadaptations associated with psychomotor sensitization are somewhat similar to those responsible for the development of drug addiction (31). Thus, it would be expected that any manipulation capable of facilitating drug-induced psychomotor sensitization would also facilitate drug self-administration. Indeed, this is what we found with cocaine and amphetamine. By contrast, heroin reward was substantially greater in Resident rats, relative to Non Resident rats, indicating unforeseen dissociations between opioid and psychostimulant reward and between opioid reward and opioid-induced activity.
The second reason the findings summarized above were surprising is represented by the unforeseen dissociation in the effects of drug taking context on psychostimulant versus opioid reward. Indeed, research in the last 2 decades has stressed the existence of shared neural substrates for the rewarding effects of addictive drugs (32). Also from a theoretical point of view, the current trend is to emphasize the similarities among various types of natural and pharmacological rewards. Thus, the importance of better characterizing our animal model became obvious. To this end, we employed polydrug taking procedures rarely used in the experimental setting.
3 Environment and Polydrug Taking
Drug abuse is rarely limited to a single substance, polydrug abuse being the norm rather than the exception. Most heroin addicts, for example, also abuse psychostimulants, especially cocaine (33, 34), and vice versa. The pattern of drug taking and drug preference is widely thought to be a function of local availability, street price, lifestyle, and other sociocultural factors (35–37). As a consequence, very little laboratory research has been devoted to this type of issue. However, the findings described in the previous section suggested that the environmental context could differentially modulate the intake of opioid and psychostimulant drugs in a much more basic manner. In the following sections we will discuss the results obtained using three different procedures in which the same rats were given the opportunity to self-administer both heroin and a psychostimulant drug (amphetamine or cocaine).
3.1 Sequential Amphetamine and Heroin Self-Administration
In this study, Resident and Non Resident male Sprague–Dawley rats that had been trained to self-administer amphetamine were given the opportunity to self-administer heroin (22). The experimental design of the first part of this study was similar to that described for the experiments in the previous section, but after session 12, the rats were given a 7-day period of rest (during which they were kept in their respective home cages) and were then challenged with a noncontingent i.v. infusion of 100.0 μg/kg of heroin (session 13) to assess their psychomotor response over 60 min. On the following days (sessions 14–25) the rats were switched to heroin self-administration (25.0 μg/kg per infusion) following procedures similar to those used for amphetamine in sessions 1–12.
As shown in Fig. 2, environmental context modulated heroin and amphetamine self-administration in opposite directions. Thus, the results illustrated in Fig. 1 could be reproduced even with a within-subject design. We also found a dissociation in the effects of environment on heroin self-administration versus heroin-induced locomotor activity; that is, the psychomotor response to heroin was greater in Non Resident than in Resident rats (22).
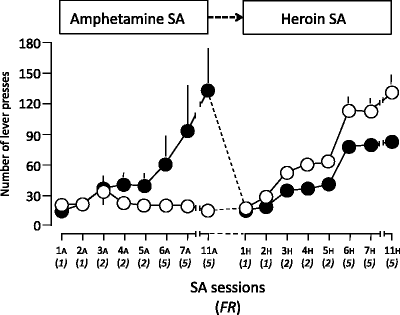

Fig. 2.
Number of lever presses (means ± SEMs) on the active lever in animals trained first to self-administer amphetamine (acquisition dose: 25 μg/kg per infusion) and then heroin (acquisition dose: 25 μg/kg per infusion) at a progressively higher schedule of reinforcement. The rats were either transferred to the test cages immediately before the treatment (Non Resident group) or kept at all times in the test cages (Resident group) (Data from (42). With kind permission).
3.2 Alternating Cocaine and Heroin Self-Administration
In this study, we used an experimental procedure in which Resident and Non Resident male Sprague–Dawley rats were given the opportunity to self-administer both cocaine and heroin on alternate days, under different schedules of reinforcement (38). Although we did expect that the acquisition of cocaine self-administration would affect that of heroin (and vice versa), somewhat blunting the effect of environment, we predicted that Non Resident rats would take more cocaine relative to heroin than Resident rats. More specifically, we predicted that the ratio of cocaine to heroin taking in individual rats would be greater in Non Resident than in Resident rats.
Throughout the experiment, heroin (25.0 μg/kg) and cocaine (400.0 μg/kg) were made available to the rats on alternate days, the starting drug being counterbalanced within groups. These doses were chosen on the basis of the results illustrated in Fig. 1. Given that the half-life of both cocaine and heroin is shorter than 1 h (39–41), it was safe to assume that the 21 h separating consecutive sessions were sufficient to achieve the complete clearance of each drug. Right and left levers were paired to either heroin or cocaine, in a counterbalanced manner. The FR was raised from FR1 (sessions 1–4) to FR2 (sessions 5–10) and then to FR5 (sessions 11–14). At the end of session 14, the rats were assigned to two different experimental procedures. Some rats underwent, on sessions 15 and 16, a progressive-ratio procedure, for heroin on 1 day and cocaine on the other, in a counterbalanced manner. The other rats continued to alternate heroin and cocaine self-administration for 12 additional sessions (sessions 15–26), during which the FR was raised from FR10 (sessions 15–16) to FR20 (sessions 17–18), FR 30 (sessions 19–20), FR50 (sessions 21–22), FR70 (sessions 23–24), and, finally, FR100 (sessions 25–26).
Figure 3 illustrates the data concerning the rats that were tested over 26 sessions. It can be seen that Resident rats took much more cocaine relative to heroin than Non Resident rats and that these differences became larger by increasing the FR. For example, on FR50, Resident rats took on average about 4 times more infusions of cocaine than of heroin while Non Resident rats took on average 18 times more cocaine than heroin. The results of the progressive ratio session confirmed that the reinforcing effects of cocaine and heroin are a function of the context of drug taking (38).
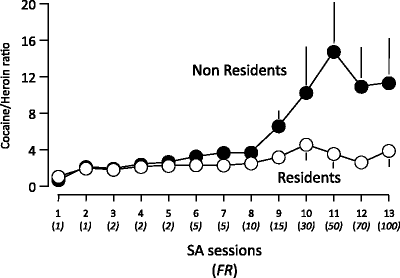

Fig. 3.
Ratios (means ± SEMs) of cocaine infusions (400 μg/kg per infusion) over heroin infusions (25 μg/kg per infusion) for rats trained to self-administer these drugs on alternate days at a progressively higher schedule of reinforcement. The rats were either transferred to the test cages immediately before the treatment (Non Resident group) or kept at all times in the test cages (Resident group) (Data from (38). With kind permission).
3.3 Choosing Heroin Versus Cocaine
Although the combined abuse of cocaine and heroin by addicts is the rule rather than the exception, very little is known about the variables that regulate cocaine versus heroin taking. As discussed above, rats adjust their intake of these two drugs as a function of the environment of drug taking, Non Resident rats taking more cocaine relative to heroin than Resident rats. Thus, we wondered whether environmental context could exert its modulatory influence even on drug preference, that is, on drug choice when the two drugs are made available simultaneously (42).
To test this hypothesis, male Sprague–Dawley rats with a double-lumen catheter were trained in ten consecutive daily 3-h sessions to self-administer cocaine (400 μg/kg/infusion) and heroin (25 μg/kg) on alternate days (i.e., there were five sessions for each drug). Cocaine and heroin were each paired with one of the two retractable levers and one of two cue lights (red or green). The assignment of levers and cues was counterbalanced for heroin and cocaine. At the start of each session, only the lever associated with the drug to be self-administered on that session was extended and the appropriate cue light was turned on. As in the studies described above, the number of consecutive lever presses required to obtain a single infusion was raised from FR1 to FR5 across test sessions. After each infusion, the cue light turned off and the lever retracted. The cue light turned on and the lever extended again after 40 s. The rats were then given simultaneous access to both cocaine and heroin for seven consecutive daily sessions. We predicted that a larger proportion of Non Resident rats would choose cocaine over heroine relative to Resident rats, and vice versa.
Indeed, as shown in Fig. 4, 76% of rats exhibited a preference for either cocaine or heroin (as indicated by bootstrapping analysis, P < 0.001) but with important differences between the Resident versus the Non Resident condition. The rats that preferred heroin to cocaine were six times more numerous in the Resident group than in the Non Resident group (47% vs 8%), whereas the rats that preferred cocaine to heroin were two times more numerous in the Non Resident group than in the Resident group (67% vs 33%).
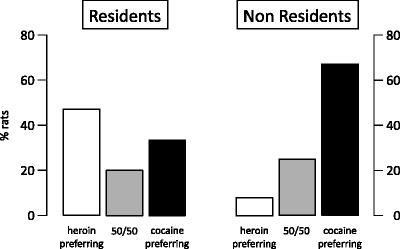

Fig. 4.
Proportion of rats expressing a preference for cocaine versus heroin (based on the results of bootstrapping analysis) after being trained for both cocaine (400 μg/kg per infusion) and heroin (25 μg/kg per infusion) self-administration and then given the opportunity to choose between the two drugs over seven consecutive choice sessions, during which both drugs were made simultaneously available. The 50/50 label refers to the rats that self-administered about the same amount of the two drugs. The rats were either transferred to the test cages immediately before the treatment (Non Resident group) or kept at all times in the test cages (Resident group) (Data from (42). With kind permission).
Interestingly, we found no correlation between the amounts of cocaine and heroin self-administered during the training phase and the amounts of cocaine and heroin self-administered during the choice phase, as indicated by linear regression analysis.
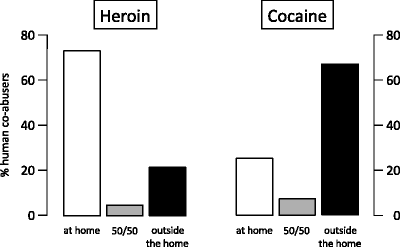
4 Cocaine and Heroin Taking as a Function of Environmental Context in Human Addicts
As discussed above, rats that were given the choice between cocaine and heroin self-administration expressed different drug preferences depending on environmental context: cocaine being preferred by most rats not residing in the self-administration chambers and heroin by most rats living in the chambers. The possible implications of these results for human addiction were obvious but, to the best of our knowledge, we could find no reports on the context of heroin versus cocaine taking in human co-abusers. Thus, we decided to adopt a translational approach to investigate the ambience selected by intravenous drug users to inject heroin and cocaine intravenously (42). We predicted that heroin would be injected more often at home than outside and vice versa for cocaine.
The participants in the study were recruited among the outpatients of Villa Maraini Therapeutic Community (an addiction clinic affiliated with the Italian Red Cross) who had a fixed address and met DSM-IVR (43) Drug Dependence criteria for cocaine and/or heroin but were not affected by other major psychiatric disorders. One hundred and four individuals completed the interview. Seventy-nine of the participants reported using both heroin and cocaine whereas 21 reported using only heroin and 4 using only cocaine. During the interview, the participants were asked where they had usually taken heroin and/or cocaine in the last 3 months. Follow-up questions were aimed at assessing, for each drug, whether it was taken: (1) always at home, (2) mostly at home, (3) sometimes at home sometimes outside (50/50), (4) mostly outside, and (5) always outside. The participants were also asked whether the context of drug taking represented a real preference or was the result of constraints.
Figure 5 illustrates the setting of cocaine versus heroin taking for the 79 individuals who co-abused the two drugs, 24 of whom used both drugs intravenously and 13 both drugs intranasally. Approximately 74% of all co-abusers preferred to inject heroin at home (60.8% always and 12.7% mostly) whereas about 22% preferred to take it outside (16.5% always and 5.1% mostly). The opposite was true for cocaine: approximately 25% preferred to take it at home (20.3% always and 5.1% mostly) versus 67% outside (58.2% always and 8.9% mostly). Similar results were obtained when the analysis was limited to the individuals taking both cocaine and heroin intravenously or intranasally. The pattern of drug taking for the 13 individuals who reported occasionally injecting a combination of heroin and cocaine (speedball) was identical to that of heroin alone: 70% of them preferred to inject the speedball exclusively at home whereas 23% preferred to take it always outside. It is important to note that none of the demographic variables (including employment status, age, amount of drug taking, replacement treatments, etc.) had any significant influence on setting preference for either cocaine or heroin (42).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


