Direct contact transmission is probably the most frequent and important means of spread of infection. This transmission involves direct physical contact or close approximation between the reservoir host and the susceptible individual. Venereal transmission of Brucella canis between dogs and bite transfer of feline immunodeficiency virus (FIV) between cats are examples of direct physical contact transmission. Airborne droplets from respiratory, fecal, or genitourinary secretions of dogs and cats generally do not travel farther than 4 or 5 feet unless there are wind currents. Therefore, droplet spread can be considered a form of direct transmission. The spread of infection under such circumstances can usually be limited, as long as fomite transmission is prevented, by ensuring adequate distance between affected and susceptible animals. Aerosol transmission, a subset of airborne transmission, is spread of very small (less than 100 µm diameter) airborne particles that remain aloft, depending on their respective particle size, with smaller particles traveling longer distances. Freshly aerosolized particles containing microbes rarely remain airborne for more than 1 minute unless they are smaller than 5 µm in diameter. Droplet nuclei, which are desiccated aerosolized particles containing resistant microbes, may also be carried alone or on dust particles by air currents for extended periods and distances. Following inhalation, smaller particles also are able to travel to, and become deposited in, lower portions of the respiratory tract than larger particles. Resistant respiratory pathogens such as Mycobacterium tuberculosis and Histoplasma capsulatum are commonly spread by this means. In human hospitals, nosocomial infections with Bordetella pertussis, Staphylococcus aureus, and Streptococcus pyogenes can be spread by these means, although direct contact with secretions and fomites (see later discussion) is usually more common for the latter two agents.341 S. aureus infections have been a concern because antimicrobial-resistant strains of S. aureus have been transmitted from humans to dogs and cats (see Chapters 34 and 99).182,387 Vector-borne disease may be considered a specialized form of vehicular or indirect contact spread whereby invertebrate animals transmit infectious agents. Vectors are generally arthropods that transmit infection from the infected host or its excreta to a susceptible individual, to its food and water, or to another source of immediate contact. Vectors such as flies may transfer organisms externally, or mechanically, on their feet or internally within their intestinal tracts. The ability of organisms to survive in the vector without further propagation has been demonstrated with Shigella and Salmonella infections. Propagative transmission means that the infectious agent multiplies in or on the vector before transfer. Transmission of the plague bacillus, Yersinia pestis, by fleas occurs in this manner (see Chapter 45). Transovarial transmission results when the vector transfers the organism to its progeny, as in the case of ticks transmitting Rickettsia rickettsii, the agent of Rocky Mountain spotted fever (see Chapter 27). Transstadial transmission, the transfer of infection only between molting stages in the life cycle of the vector, occurs in canine ehrlichiosis (see Chapter 26). True biologic (developmental or cyclopropagative) transmission by arthropod vectors involves an obligate developmental stage in the life cycle of the vector. Some of the protozoal pathogens of the dog and cat (e.g., Trypanosoma, Leishmania, and Hepatozoon) have a developmental life cycle in the vector (see Chapters 72, 73, and 74, respectively). Vertebrate vectors, such as rodents, may also transmit disease, both in the community and in veterinary hospitals. The health of both humans and domestic animals depends on the ability to control microorganisms that cause or have the potential to cause disease. Destruction of the organisms occurs when the microenvironment is changed adversely by physical or chemical means. Several levels of microbial disinfection (low, intermediate, or high) are recognized (Box 93-1). Good decontamination always requires initial cleaning to remove organic residues and debris. With prior cleaning, most of the organisms are removed and disinfectants are more effective. Protozoal cysts, mycobacteria, and bacterial spores are highly resistant to disinfection and sanitation. Nonenveloped viruses also tend to be resistant to disinfection, depending on the methods used. Vegetative bacteria are relatively susceptible to most disinfectants; however, resistance to specific disinfectants can be present in certain microorganisms, either inherently or through acquisition of resistance genes. Enveloped viruses are quite susceptible to disinfectants. Prions, the proteinaceous agents that cause transmissible degenerative (spongiform) encephalopathies (see Chapter 82), are the most resistant infectious agents known. Some loss of infectivity of prions occurs at 100° C; however 130° C for 30 to 60 minutes is required for their inactivation. Prions are not affected by sterilizing levels of radiation, formalin, nonpolar organic solvents, burial for years, or passage through 0.1-µm filters. Their infectivity is destroyed by 1 M sodium hydroxide at 55° C or sodium hypochlorite (household bleach) diluted 1:1, or stronger (Table 93-1). TABLE 93-1 Recommendations and Concentrations for Use of Bleach Solutions in Disinfectiona 1 ppm = 1 mg/L= 1 µg/mL; 1% bleach = ∼10,000 ppm solution; 0.1% bleach = ∼1000 ppm solution; 1 cup = 236.5 mL; 1 gallon = 3785.4 mL; 1 quart = 946.4 mL; 1 ounce = 30 mL; 1 tablespoon = 15 mL; 1 teaspoon = 5 mL. Household bleach ranges in concentration from 5.25% to 6.15% sodium hypochlorite depending on the manufacturer. 5.25% bleach = 5.25 g/100mL = 52.5 g/L = ∼52,500 ppm solution. This concentration is used in dilutions noted in the table. Bleach solutions stored at room temperature in opaque containers lose 50% activity over 1 month. Double strength concentrations should be used where diluted bleach solutions are stored before use. Use unscented 5.25% solution (household bleach). Never mix with other cleaners. Wear protective rubber gloves and eye protection when using undiluted or handling >50 ppm solutions. Respiratory protection may be needed if fumes are detected. d Can also use concentrated sodium hydroxide solutions. aInformation adapted from Centers for Disease Control,54,55,55 and McGlynn W. Food Technology Fact Sheet, Oklahoma State University, www.fapc.okstate.edu. Accessed 2/6/11. bMinimum concentration of disinfection. For general washing, residual concentrations of 2–7 ppm are used. Municipal water systems have concentrations of 0.25–2 ppm. cMaximum concentration for food contact implements. Higher concentrations require rinsing off chlorine solutions before use. This concentration has been used for decontamination of fruits and vegetables for at least 1 minute, but must be washed off with potable water thereafter. Greater levels may cause bad odors or taste. Carcinogenic trihalomethanes form if higher concentrations are used. Ionizing or high-energy radiation can be produced by radioactive elements, which are sources of γ-rays, or by a cathode-ray tube that produces x-rays. γ-Rays and x-ray radiation induce ionization of the vital cell components, especially nuclear DNA. Because of the cost and dangers of handling this equipment, this type of microbial control has found practical application chiefly in the industrial field. Pharmaceuticals, plastic disposables, and suture materials are generally sterilized by the manufacturer by means of ionizing radiation. Foodstuffs can be sterilized or disinfected of pathogenic microbes by using ionizing radiation. γ-Irradiation is used for sterilization of foods intended for specific-pathogen free or germ-free animals.51 Although bacterial sterilization can be achieved, vitamin A levels can be reduced and peroxide levels may increase. Pasteurization similarly reduced vitamin A levels but had no effect on peroxide concentration. Despite its safety, use of any irradiation method has met with resistance from the public. An unfounded misconception exists concerning residual radioactivity in treated foods. Nonionizing, or low-energy, radiation in the form of ultraviolet (UV) light has found practical application in the destruction of airborne organisms. Because low-energy rays do not penetrate well, they are used primarily as surface-active agents. The bactericidal range of UV light is 240 to 280 nm. UV lamps usually produce radiation in the range of 254 nm and work at maximal efficiency at temperatures of 27° C to 40° C. They depend on air convection currents to circulate airborne organisms. Germicidal lamps must be positioned above eye level to prevent retinal burns. For best efficiency, the lamps can also be placed in air conditioning or heating ducts. (See Airborne Contaminants, under Prevention of Nosocomial Infections.) Biocides (germicides) denote chemical agents having antiseptic, disinfectant, or preservative properties. In addition to the environmental control of infection, many of these agents are used to preserve food, pharmaceuticals, and medical supplies. Typically, biocides lack selective toxicity to microorganisms. Viruses in a dried state are less susceptible to disinfectant, and their survival is enhanced by the presence of plasma protein.369 Moisturizing surfaces before application of disinfectants may improve their efficacy. The antimicrobial properties of various chemical disinfectants are summarized in Table 93-2. Table 93-3 describes the uses of the compounds to disinfect hospital equipment. It is difficult to achieve a high level of disinfection for thermometers. At exposure times of 10 to 12 hours to various chemicals, Salmonella has been isolated from contaminated thermometers.391 Therefore, it is recommended that disposable thermometer covers be used. TABLE 93-2 Antimicrobial Properties of Common Classes of Chemical Disinfectants quats, Quaternary ammonium compounds; +, effective; ±, somewhat effective; -, not effective; ?, effectiveness not known. TABLE 93-3 Treatment Time Required for Chemical Disinfection of Hospital Equipment aH, High-level disinfection (free of all microorganisms; equivalent to sterilization); I, intermediate-level disinfection (free of all vegetative bacteria, fungi bacilli, and most viruses); L, low-level disinfection (free of vegetative bacteria, fungi, and most enveloped viruses). b1, 70%–90% ethyl or isopropyl alcohol; 2, 70%–90% ethyl alcohol; 3, hypochlorite (1000 ppm); 4, hypochlorite (100 ppm); 5, 0.2% iodine + alcohol; 6, iodophors (500 ppm); 7, iodophors (100 ppm); 8, 20% formalin + alcohol; 9, 20% formalin aqueous; 10, 2% activated glutaraldehyde aqueous; 11, 0.13% activated glutaraldehyde + phenate complex; 12, 2% phenolic aqueous; 13, 1% phenolic aqueous; 14, quaternary ammonium compounds; 15, amphoterics; 16, chlorhexidine; 17, ethylene oxide. Ethyl and isopropyl alcohol are rapidly bactericidal against vegetative bacteria but have little effect against spores. Alcohols can be virucidal, provided that exposure time is adequate. Ethyl alcohol is slightly more effective against the nonenveloped viruses than is isopropyl alcohol, whereas the reverse is true for the enveloped viruses. Ethyl alcohol is effective against Proteus and Pseudomonas, whereas isopropyl alcohol has a broader antibacterial spectrum. A concentration of 70% alcohol was sufficient for inactivation of caliciviruses after 1-minute exposure time.85a Absolute (100%) alcohol has no disinfecting qualities. Water is essential for the antimicrobial action of alcohols. Concentrations found to be most bactericidal are between 50% and 95% by volume. The two most widely employed concentrations are 70% and 85%. The alcohols are inactivated by organic soil, and they are ineffective if diluted to less than 50%. Effects of alcohol-containing disinfectants have been extensively studied for feline calicivirus (FCV), a surrogate for human norovirus disinfection. Ethanol (75% by volume), or a commercial hand gel containing at least 62% ethanol, was more effective in reducing the transfer rate of FCV by human hands than handwashing with water or no cleansing measures.28 However, in other studies, the efficacy paralleled higher concentrations (99.5% best) of ethanol being the best and corresponding concentrations of isopropyl alcohol being less effective.201 In the same study, hand sanitizers containing 60% ethanol or lower were much less effective. For these reasons, the authors of this chapter (CEG et al) recommend products with at least 70% ethanol. Alcohol-based sanitizers with added disinfectants have shown more efficacy.196,215 These compounds are ineffective or unstable in the presence of organic material, soap, or hard water. Halogens are active against a wide variety of viruses and resistant bacteria, such as Proteus and Pseudomonas. Table 93-4 summarizes the disinfectant activity of chlorine and iodine, which are described in greater detail next. TABLE 93-4 Disinfectant Activity of Chlorine and Iodine16,397 aFor further information on chlorine bleach, see Table 93-1. Household bleach, a 5.25% to 6.15% sodium hypochlorite solution diluted to a minimum concentration of 50 ppm (vol/vol), is a common form of chlorine for disinfection of inanimate objects (see Table 93-1). Bleach is sold at a pH of 12 to prolong its shelf life. To increase its germicidal activity, especially against spores, the bleach is diluted with water to increase the available chlorine to change the pH of the solution to 7.0.320 Increasing the temperature of the solution decreases the exposure time needed. Precautions should be taken, because increasing temperature and decreasing pH of bleach can result in the release of chlorine gas, which is toxic. Chlorine bleach loses its effect in the presence of oil, dirt, or organic debris. Other than aldehydes, peroxymonosulfates, and chlorine dioxide, sodium hypochlorite solutions are one of the few chemicals that will inactivate parvoviruses231 (see Chapters 8 and 9) and kill clostridial spores. For inanimate surfaces, sodium hypochlorite at a concentration of 1000 ppm for 1 minute was effective in inactivating FCV.396 Dilution and acidifying bleach solutions reduces their stability and increases their corrosiveness, and bleach is also deactivated by light. Therefore, bleach should be kept in opaque containers and diluted fresh daily. Because diluted bleach in opaque plastic can lose 50% of its activity over 1 month, tightly closed brown-colored bottles should be used after its dilution. Bleach is corrosive to metals and will discolor fabrics. Because it causes severe tissue damage, bleach solutions are not routinely used for antisepsis. It can be used for emergency antisepsis, but only in very dilute (less than 50 ppm) solutions. Chlorine dioxide (Endimal, DuPont, USA), a halogen, is superior to chlorine in the destruction of bacteria, including spores and viruses.321a It has high solubility and no odor, and it is unaffected by pH in the range of 4 to 10 and is nonreactive with ammonia compounds. Unlike bleach, chlorine dioxide is noncorrosive to metals even at high chlorine concentrations and is hypoallergenic and nontoxic to living tissues. It must be generated from on-site synthesis or can be ordered in a stabilized form. Chlorine dioxide has high penetration of environmentally resistant bacterial biofilms and has been used to treat air conditioning cooling systems to remove pathogenic bacteria. Iodophors have an advantage over iodine because they are nonstaining and produce minimal tissue damage. Povidone-iodine is a complex of polyvinylpyrrolidone and iodine (Betadine solution, Purdue Frederick, Norwalk, CT). Such iodine compounds have been used for presurgical preparation, topical wound therapy, and joint or body cavity lavage. Solutions of 10% (undiluted) to 1% (1:9) povidone-iodine have been applied for skin and wound disinfection. Dilutions (1:4 to 1:100) of 10% stock solution result in increased bactericidal activity owing to increased concentrations of free iodine compared to the undiluted stock solution. A 1:50 dilution of povidone-iodine is recommended as an ocular surface disinfectant in presurgical situations.267,268 A 7.5% scrub containing an anionic detergent damages tissues and should be used only on intact skin. Bacterial concentrations are reduced on canine skin for up to 1 hour after scrub application.267 Polyhydroxydine is a potent iodine-containing wound and skin antiseptic (Xenodine, Squibb Animal Health Division, Princeton, NJ). It has been effective in treating canine wounds when used undiluted (100%) or as a 1:9 dilution (10%). As long as the iodophor solution maintains its color, it is effective. A concentration of 0.2% povidone-iodine was highly effective in killing greater than 99% of Mycobacterium tuberculosis isolates within 60 seconds.304 Systemic absorption of iodine may result in transient reduction in serum thyroxine or bicarbonate concentrations. Contact dermatitis that persists for several hours may occur in dogs.267–269,306 The skin irritation may lead to inactivation of the iodine through weeping proteins and increased postsurgical infection. Iodophors are also damaging to deeper tissue fibroblasts and must be diluted to 0.001% to be applied as wound or body cavity rinses.206 Concentrations of 0.5% to 1% have been effective but may be too strong for lavage of contaminated peritoneal cavities. Peritoneal lavage with 10% povidone-iodine can be fatal to dogs if 8 mL/kg is infused with intact peritoneum or 2 mL/kg with peritonitis. Concentrations of greater than 0.1% should not be used in joint irrigation. Glutaraldehyde is chemically related to formaldehyde but is more reactive, even in the presence of organic materials, soaps, and hard water. A 2% aqueous alkaline solution is equivalent to 20% formalin in alcohol in biocidal activity. The alkaline solution is much more biocidal but less stable. Stability is maintained for approximately 2 weeks at pH 7.5 to 8.5. At the dilution at which it is used, glutaraldehyde is slightly irritating to the skin and mucous membranes and very irritating to the eyes. Both glutaraldehyde and formalin are high-level disinfectants for cold sterilization of instruments that are unable to withstand steam or EO gas, including lens instruments, such as endoscopes, and plastic tubing and catheters. After disinfection, items should be rinsed thoroughly with sterile distilled water. A glutaraldehyde-phenate complex (Sporicidin, Sporicidin International, Washington, DC) has been shown to be as effective at 1 : 16 dilution as is undiluted glutaraldehyde and as stable and less irritating. Aldehydes are often used for high levels of chemical disinfection. For example, in the absence of disinfection and moisture, FCV can survive for 3 days on inanimate objects, although greater than 90% of the virus was inactivated by 4 hours.59 Metricide (Metrex, Orange, CA), a 2.5% buffered glutaraldehyde solution, had 100% efficacy in inactivating FCV on all carpets in 10 minutes contact time.219–221 Sterilization time was 10 hours at 22° C. In the presence of organic material, glutaraldehyde was not as effective.291 A stabilized 0.3% glutaraldehyde solution, with or without alcohol, was as effective as 4% chlorhexidine gluconate and alcohol solution in skin disinfection for dogs undergoing ovariohysterectomy.202 Reductions of colony counts of Staphylococcus pseudintermedius cultured from the skin were similar with both of these preparations, as were mild cutaneous reactions. Diphenol hydroxybenzene complex (Citricidal, Nutribiotic, Lakeport, CA), a quaternary compound from grapefruit seed bioflavonoids, is a nontoxic, biodegradable, noncorrosive disinfectant for inanimate surfaces. This compound comes as a liquid or powder concentrate and was virucidal for enveloped viruses such as feline herpesvirus but was not effective against FCV or feline parvovirus.107 At a concentration of 5%, or greater, at room temperature, sodium bicarbonate was effective in inactivating greater than 99% of FCV, with a contact time of 1 minute.220 This effect was enhance when it was combined with aldehydes or hydrogen peroxide. Ready availability, low cost, and minimal toxicity make it an effective way to clean food surfaces.
Environmental Factors in Infectious Disease
Means of Transmission
Environmental Control of Microbes
Use (Final Bleach Concentration)
Bleach Amount
Water Amount
Details
Susceptible Organisms (Concentration Needed to Kill)
Drinking water decontamination (50 ppm)b
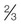 teaspoon or 3.75 mL—depending on clarity
teaspoon or 3.75 mL—depending on clarity
1 gallon, or 3.79 liters
Let stand 30 min before use
Mycoplasma (25 ppm)
Vegetative bacteria (<5 ppm)
Food handling equipment, bleach left on as residual (200 ppm)c
1 tablespoon, or 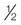 oz, or 15 mL
oz, or 15 mL
1 gallon, or 3.79 liters
Rinse surfaces after cleaning. Apply bleach and let sit for at least 5 min, then air dry
Bacillus spores (5 min, 100 ppm)
Mycotic agents (1 hr, 100 ppm)
25 unenveloped viruses (10 min)
Salmonella, Pseudomonas (10 min, 100 ppm)
Nonabsorbable surface disinfection (400 ppm)
2 tablespoons, or 1 oz, or 30 mL
1 gallon, or 3.79 liters
Rinse surfaces after cleaning. Apply bleach and let sit for at least 5 min, then air dry
Dermatophytes
Nonabsorbable surface disinfection (640 ppm)
1 cup or 8 oz or 237 mL
5 gallons, or 18.9 liters
Clean surface soap and water. Disinfect with bleach solution. Air dry.
Disinfect yeasts, (e.g., Candida [30 sec, 500 ppm])
Food or water utensils, bleach rinsed off after disinfection (1000 ppm)
5 tablespoons or 60 mL
1 cup or 237 mL
Wash with soap and water. Rinse, then sanitize with bleach solution. Air dry.
Mycobacterium tuberculosis
Calicivirus or Norovirus (1 min)
Nonabsorbable surface disinfection (1750 ppm)
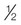 cup or 4 oz or 120 mL
cup or 4 oz or 120 mL
1 gallon, or 3.79 liters
Wash with soap and water. Rinse, then sanitize with bleach solution. Air dry.
Unenveloped viruses, (e.g., parvoviruses, adenoviruses without organic debris [10 min])
Giardia cysts
Fungal growth on impervious surfaces (3200 ppm)
1 cup or 237 mL
1 gallon, or 3.79 liters
Wash with bleach. Scrub with brush. Rinse with water and air dry.
Clostridium difficile spores (10 min)
Retard fungal growth
High-level disinfection in presence of organic debris or 10% plasma (5250 ppm, 10% solution)
1.5 cups or 25 tablespoons or 300 mL
1 gallon, or 3.79 liters
Clean surface, if possible, with water and soak organic debris. Apply bleach solution liberally.
Unenveloped viruses in organic debris (10 min)
Highest level disinfection (26,250 ppm)
1 gallon or 3.79 liters
1 gallon, or 3.79 liters
Wash and soak in bleach. Scrub with brush. Rinse with water and air dry.
Prions (1 hr)
Physical Agents
Heat
Radiation
Chemical Agents
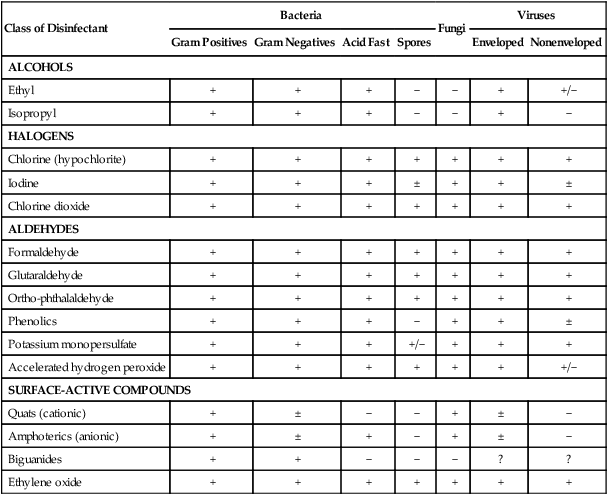
Class of Disinfectant
Bacteria
Fungi
Viruses
Gram Positives
Gram Negatives
Acid Fast
Spores
Enveloped
Nonenveloped
ALCOHOLS
Ethyl
+
+
+
−
−
+
+/−
Isopropyl
+
+
+
−
−
+
−
HALOGENS
Chlorine (hypochlorite)
+
+
+
+
+
+
+
Iodine
+
+
+
±
+
+
±
Chlorine dioxide
+
+
+
+
+
+
+
ALDEHYDES
Formaldehyde
+
+
+
+
+
+
+
Glutaraldehyde
+
+
+
+
+
+
+
Ortho-phthalaldehyde
+
+
+
+
+
+
+
Phenolics
+
+
+
−
+
+
±
Potassium monopersulfate
+
+
+
+/−
+
+
+
Accelerated hydrogen peroxide
+
+
+
+
+
+
+/−
SURFACE-ACTIVE COMPOUNDS
Quats (cationic)
+
±
−
−
+
±
−
Amphoterics (anionic)
+
±
+
−
+
±
−
Biguanides
+
+
−
−
−
?
?
Ethylene oxide
+
+
+
+
+
+
+
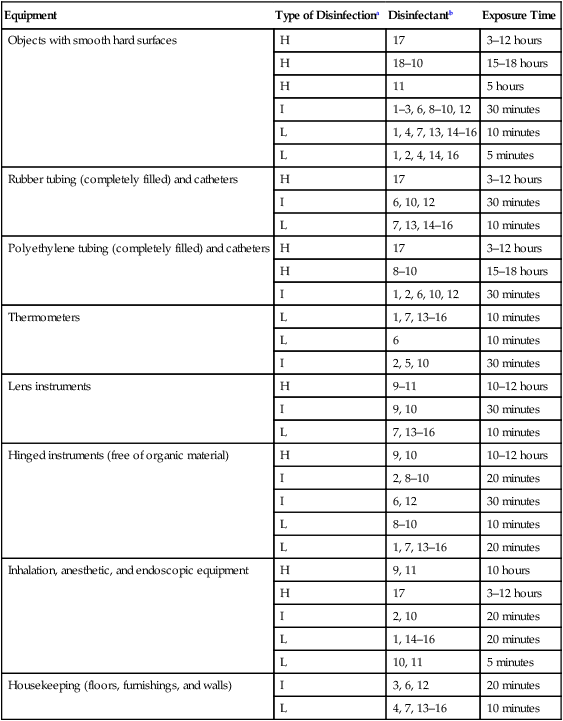
Equipment
Type of Disinfectiona
Disinfectantb
Exposure Time
Objects with smooth hard surfaces
H
17
3–12 hours
H
18–10
15–18 hours
H
11
5 hours
I
1–3, 6, 8–10, 12
30 minutes
L
1, 4, 7, 13, 14–16
10 minutes
L
1, 2, 4, 14, 16
5 minutes
Rubber tubing (completely filled) and catheters
H
17
3–12 hours
I
6, 10, 12
30 minutes
L
7, 13, 14–16
10 minutes
Polyethylene tubing (completely filled) and catheters
H
17
3–12 hours
H
8–10
15–18 hours
I
1, 2, 6, 10, 12
30 minutes
Thermometers
L
1, 7, 13–16
10 minutes
L
6
10 minutes
I
2, 5, 10
30 minutes
Lens instruments
H
9–11
10–12 hours
I
9, 10
30 minutes
L
7, 13–16
10 minutes
Hinged instruments (free of organic material)
H
9, 10
10–12 hours
I
2, 8–10
20 minutes
I
6, 12
30 minutes
L
8–10
10 minutes
L
1, 7, 13–16
20 minutes
Inhalation, anesthetic, and endoscopic equipment
H
9, 11
10 hours
H
17
3–12 hours
I
2, 10
20 minutes
L
1, 14–16
20 minutes
L
10, 11
5 minutes
Housekeeping (floors, furnishings, and walls)
I
3, 6, 12
20 minutes
L
4, 7, 13–16
10 minutes
Alcohols
Antisepsis
Halogens
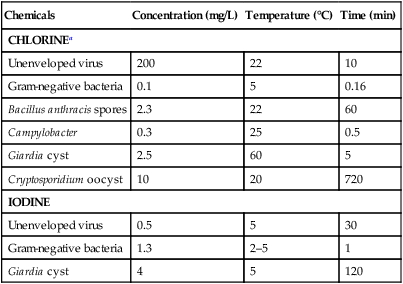
Chemicals
Concentration (mg/L)
Temperature (°C)
Time (min)
CHLORINEa
Unenveloped virus
200
22
10
Gram-negative bacteria
0.1
5
0.16
Bacillus anthracis spores
2.3
22
60
Campylobacter
0.3
25
0.5
Giardia cyst
2.5
60
5
Cryptosporidium oocyst
10
20
720
IODINE
Unenveloped virus
0.5
5
30
Gram-negative bacteria
1.3
2–5
1
Giardia cyst
4
5
120
Disinfection
Antisepsis
Aldehydes
Phenolics
Antisepsis
Sodium Bicarbonate
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Environmental Factors in Infectious Disease
