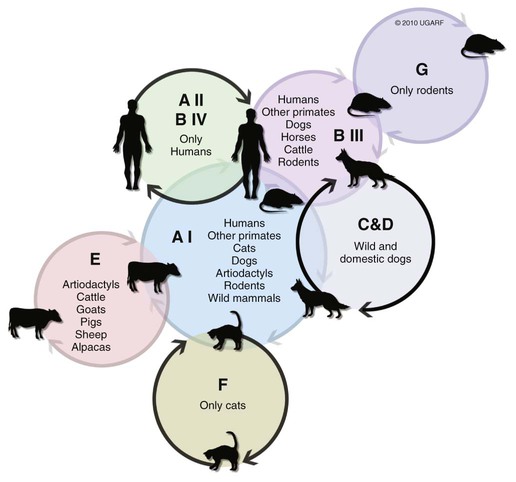
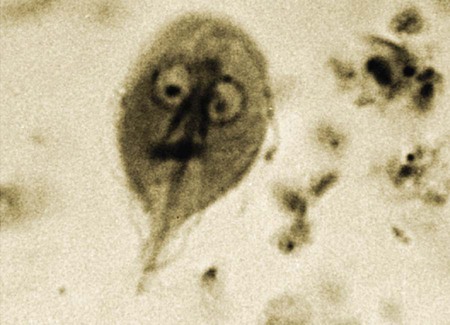
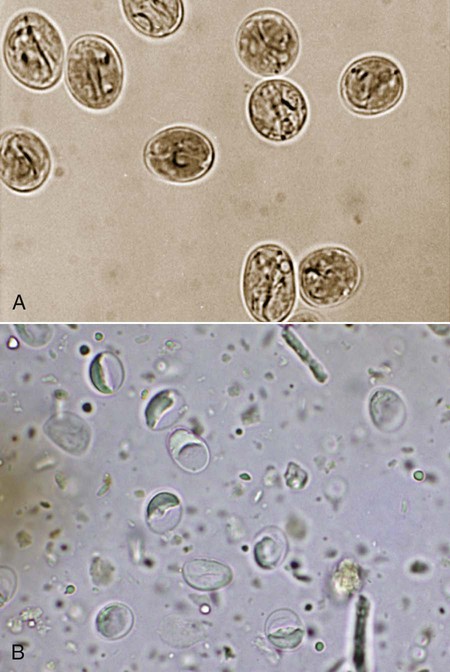
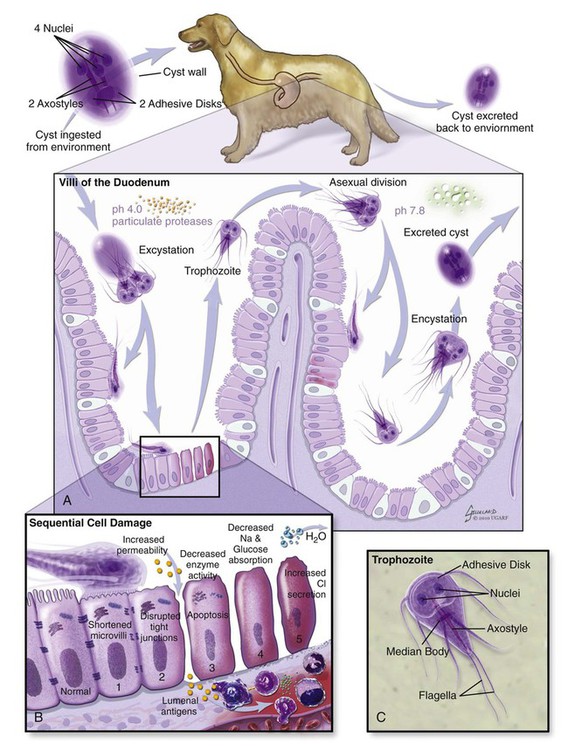
The enteric protozoa covered in this chapter are limited to four protozoan genera: Giardia, Tritrichomonas, and Entamoeba in the phylum Sarcomastigophora (having flagella or pseudopodia), and Balantidium in the phylum Ciliophora (having cilia). Salient characteristics of these organisms and the diseases they may cause are presented in Table 77-1. Enteric protozoa of the phylum Apicomplexa are discussed in Chapters 79 (Toxoplasmosis and Neosporosis), 80 (Enteric Coccidiosis), and 81 (Cryptosporidiosis and Cyclosporiasis). Detailed coverage of laboratory methods for diagnosis of enteric protozoan infections is presented in Chapter 70. TABLE 77-1 Comparison of Some Enteric Protozoa in Dogs and Cats C, Cat; Ca, cattle; Cy, cyst; D, dog; H, human being; NHP, nonhuman primate; P, pig; Tr, trophozoite. Valeria Scorza and Michael R. Lappin Giardia duodenalis is a protozoan parasite with worldwide distribution that infects a variety of mammals. Molecular genetic studies report that G. duodenalis is a species complex comprising seven assemblages (A to G). Some of the assemblages have been detected in animals and humans, but some others are species-specific. Dogs usually harbor the species-specific C-D assemblages, and cats usually harbor the cat-specific assemblage F (Table 77-2 and Fig. 77-1). In some situations, DNA of assemblages A and B have been amplified from feces of dogs and cats; these genotypes have mainly been detected in humans.13,161,161 Results of one morphologic study were that area, length, width and eccentricity of Giardia cysts were significantly different between assemblages, with B and F being most different from assemblages A and C or D.295 TABLE 77-2 Genotype/Assemblage and Host Range of Isolates Within the Giardia duodenalisa Giardia spp. infections in dogs and cats have been reported in many prevalence studies, case reports, and treatment studies (Web Tables 77-1 and 77-2). Giardia spp. occur in two forms, the trophozoite and the cyst form. The trophozoite is the active, motile form that is found in the intestinal tract. It is approximately 15 µm long and 8 µm wide and has a tear-drop shape (Fig. 77-2). The trophozoites can be identified under light microscopy by the smiling-face appearance, which is formed by the two nuclei in the anterior third forming the eyes, the axonemes passing longitudinally between the nuclei forming the nose, and the median bodies located transversely in the posterior third forming the mouth; four pairs of flagella complete the shape.15 Trophozoites are susceptible to inactivation by many environmental conditions, and they are usually not responsible for spread among animals. WEB TABLE 77-1 ELISA, Enzyme-linked immunosorbent assay; FA, fluorescent antibody; PCR, polymerase chain reaction. WEB TABLE 77-2 ELISA, Enzyme-linked immunosorbent assay; FA, fluorescent antibody; PCR, polymerase chain reaction. The cyst form (12 µm long and 7 µm wide) is the resistant stage mainly responsible for transmission. The cyst actually contains two incompletely separated but formed trophozoites, their axonemes, fragments of the ventral disks, and as many as four nuclei (Fig. 77-3, A and B). The cyst is resistant to some environmental conditions and can survive for several months outside the host in wet, cold conditions, but is susceptible to desiccation in dry and hot conditions. Giardia spp. are transmitted through the fecal-oral route by direct or indirect ingestion of contaminated water, food, or fomites. Transmission can also occur through carnivorism if the organisms are present in the intestines of the prey species.157 Many infected hosts shed fecal cysts for months, but infections may also be self-limited in 27 to 35 days in dogs and cats. The life cycle of Giardia within the host is summarized in Figure 77-4. After cysts are ingested by a susceptible host, excystation occurs in the duodenum after exposure to gastric acid and pancreatic enzymes. The two trophozoites contained in the cyst separate, mature quickly, and attach to the brush border of the villous epithelium (Fig. 77-5). The distribution of Giardia in the intestinal tract varies according to the host and its diet. In dogs, trophozoites are found from the duodenum to the ileum, and in cats, trophozoites predominate in the jejunum and ileum.152 Trophozoites multiply by binary fission in the intestinal tract and then encyst by an unknown mechanism.230a Cysts are passed in the feces within days after primary infection. Trophozoites can also be passed, especially in watery feces in cats, but rarely survive for a significant period outside the host.15 The prepatent period of giardiasis in cats ranges from 5 to 16 days (mean of approximately 10 days) and in dogs ranges from 4 to 12 days (mean of approximately 8 days). Shedding of Giardia cysts by cats may fluctuate from undetectable to concentrations greater than 1,000,000 cysts pre gram of feces.6 Peaks of cyst shedding occur sporadically rather than cyclically, and the duration between any two given peaks is generally from 2 to 7 days.152 Young dogs shed an average of 2000 cysts per gram of feces, and in one study the mean cyst count per gram of feces for all infected dogs was 705.8.267 The reported range in another study was between 26 and 114,486 cysts per gram of feces.27 Although the pathogenesis of Giardia is not completely understood, studies done in vitro and in vivo reveal that the mechanism producing clinical illness is multifactorial. It appears that a combination of intestinal malabsorption and hypersecretion are the primary mechanisms associate with Giardia diarrhea. The host-parasite interaction leads to the upregulation of genes involved in the apoptotic cascade and in the formation of reactive oxygen species within the intestinal cells.39 Results of studies indicate a novel biological mechanism in which a sodium-coupled-glucose-transporter-1 (SGLT-1) activation may rescue enterocytes from apoptosis by enhancing glucose uptake.39 This cellular response may be a nonspecific host defense mechanism to enhance cell survival against enteropathogens such as Giardia.39 The induction of apoptosis increases gastrointestinal (GI) permeability, allowing luminal antigens to activate host immune-dependent pathologic pathways. Loss of the epithelial barrier function may also result from disruption of F-actin and the tight junctional proteins (ZO-1). Giardia also alters epithelial claudin proteins that are components of sealing properties of the tight junctions.39 Despite these permeability changes, Giardia can cause clinical symptoms in the absence of light microscopic changes such as villous atrophy or mucosal injury.39 Diarrhea is caused by a combination of malabsorption and hypersecretion of electrolytes. Giardia infection stimulates chloride secretion and results in a diffuse loss of brush border microvillus length, leading to epithelial maldigestion and malabsorption of glucose, sodium, and water and to reduced disaccharidase activity. Increased numbers of intraepithelial lymphocytes can be observed microscopically in association with the sodium/glucose malabsorption. Mast cell hyperplasia may be in part responsible in the loss of epithelial barrier function. The loss of epithelial barrier function may lead to chronic intestinal disorders, but the mechanisms remain unclear.39 Giardia infection may elicit clinical signs similar to irritable bowel syndrome in humans, and giardiasis is associated with histopathologic changes similar to food allergies or inflammatory bowel disease. It is unknown how some humans and animals maintain chronic, subclinical infections. Although most of the cats and dogs shedding Giardia do not show clinical signs of disease, Giardia infection can induce illness in some animals. Younger, immunosuppressed animals and those living in crowded environments are at the highest risk of showing clinical disease.71a,131,150,153 The primary clinical signs of giardiasis include chronic diarrhea and weight loss. The diarrhea is usually mucoid, pale, and soft and has a strong odor; steatorrhea may be present as well.* The organism is not usually enteroinvasive, and so blood is uncommon. Most infected cats and dogs are afebrile, do not vomit, and have serum total protein concentration and complete blood count values within reference limits. Although some cats and dogs with giardiasis have only Giardia detected on diagnostic evaluation, co-infection with other parasites is common. Giardia and Cryptosporidium co-infection may often be found in association with diarrhea.216,236 Results of another study were combined Tritrichomonas foetus and Giardia infection in cats.106 It is unknown if the different assemblages vary in their disease-producing properties and induce respective range of severity of clinical signs in cats and dogs. Giardia is one of the most commonly misidentified (over- and underidentified) parasites.68 Pseudoparasites or yeasts are easily mistaken for Giardia, leading to false-positive fecal detection results. Giardia cysts are shed intermittently, and repeated fecal analysis may be needed to detect cysts. In addition, cysts can deteriorate in fecal flotation solutions, leading to false-negative results.68 A variety of different Giardia-detection tests have been evaluated for use in cats and dogs. Despite the improved specificity, there is no one test that can be performed on a single fecal sample that has 100% sensitivity. Even more problematic is the lack of an assay with high positive predictive value for clinical giardiasis. This problem arises from the high background prevalence of infection in clinically healthy animals. The following is a discussion of the advantages and disadvantages of available diagnostic methods. Giardia trophozoites can be observed in direct smears of unstained fecal specimens, using light microscopy. A very small amount of fresh diarrheic feces or fecal mucus is mixed with a drop of normal saline solution on a microscope slide and covered with a coverslip (wet mount examination, see Fig. 70-2, A). At 100×, trophozoites are recognized by their rapid motion, but their structural characteristics should be observed at 400×. Trophozoites are rarely found in formed feces. Because trophozoites may be entrapped in the mucus, the only visible motility may be the flagella.165 Although Giardia trophozoites can be confused with tritrichomonads that are similar in size, the latter may be differentiated by the presence of an undulating membrane, a rolling form of motility, the lack of a concave surface, and a single nucleus.165 Differences in linear mobility of these two organisms can also be used for distinction (see Diagnosis under Tritrichomoniasis, later). Further testing with Giardia antigen tests or fecal culture or polymerase chain reaction (PCR) for T. foetus (see later discussion) can be used to differentiate the organisms if cytology is inconclusive. Once Giardia is detected in a direct smear or wet mount by motility, further cytologic examination should be performed on a fresh sample. A refrigerated sample or one examined several hours after collection probably will contain no living organisms. Although they also inactivate the parasites, the application of Lugol’s solution, methylene blue, or acid-methyl green to the wet mount helps in the visualization of the internal structures of the trophozoites.158 A study of dogs that examined fresh feces on 3 different days detected 40% of the Giardia-infected dogs.305 In another study, the results were 30%;206a therefore the sensitivity of this method is low compared to the other methods discussed below. If trophozoites are not seen on the direct smear, examination for cysts should be performed.68 If fecal samples cannot be examined immediately, storage at 4° C for several days is acceptable, but samples should not be frozen. Giardia cysts, more likely to be found than trophozoites in more solid stool specimens, are best detected after fecal concentration techniques, such as Sheather’s sugar centrifugation and zinc-sulfate centrifugation technique (ZSCT) (see Fig. 70-2, B).158 Trophozoites are rarely detected after these procedures. If the feces contains much fat, formalin–ethyl acetate sedimentation is the best technique for detecting cysts. In addition to the identification of Giardia cysts, eggs of other common parasites can easily be identified after concentration procedures. ZSCT has also been superior to passive fecal flotation exam in detecting helminths and Giardia cysts. Flotation methods detected only 6.5% to 14.7% of samples with Giardia cysts, as compared to those found to contain cysts with the ZSCT.86,299 Giardia cysts can be confused with yeasts because of similar size, but with staining, Giardia should be easily recognized because of its distinct internal structure. Barium sulfate, several proprietary antidiarrheals, and enemas administered before the collection of the feces may interfere with the detection of the cysts.165 Because of the intermittent pattern of shedding, it is recommended that at least three samples be examined over a period of about 1 week before ruling out the presence of Giardia.68,158,165,305 In one study in dogs, ZSCT, in addition to fecal antigen kit testing, was required on three fecal samples over a period of 7 days to ensure a correct diagnosis. Endoscopic or peroral string sampling of duodenal secretions and then examination of the sediment for trophozoites was once considered the most sensitive technique for detection of Giardia spp. infection in dogs.165 In cats, because of the mid to distal small-bowel location of the parasite, this technique is unlikely to yield positive results. This technique is also cumbersome because it requires anesthesia for endoscopy or exploratory laparotomy, and immediate examination of the fluid for trophozoites. In one study, ZSCT and duodenal aspiration positive result rates were 39% and 89% of infected dogs, respectively.218 Findings in other studies contradicted these results.37,164 Because of the expense and uncertain accuracy, collection of intestinal contents is rarely done exclusively for the diagnosis of giardiasis, and if performed, it should be done before treatment is given. A monoclonal antibody-based direct fluorescent antibody (FA) assay (Merifluor Cryptosporidium/Giardia, Meridian Diagnostics, Cincinnati, OH) is used to detect Giardia spp. cysts and Cryptosporidium spp. oocysts in feces. The assay has been evaluated for detection of Giardia infections of dogs and cats in multiple studies.160,223,223 Giardia cysts from animal-associated assemblages C, D, and F are thought to be detected by this assay; however, this hypothesis has not been rigorously assessed using genotyped samples. Because the assay gives both immunologic confirmation (fluorescence) and morphologic evaluation (size and shape), results from this assay are sometimes used as the reference (gold) standard in test comparison studies.223 The direct FA assay was reported to be more sensitive and specific than zinc sulfate flotation but comparable to other antigen-detection techniques when used on refrigerated feline fecal samples and in fecal samples from shelter dogs.160,246 Cryptosporidium spp., which can co-infect with Giardia, can also be associated with small-bowel diarrhea. These infections are difficult to detect with many techniques, so the direct FA assay is used frequently as a confirmation for both infections (see Chapter 81). A fluorescent microscope is needed to read the direct FA slides; therefore, this assay can only be performed in properly equipped diagnostic laboratories. If the samples cannot be examined immediately or must be shipped, they can be stored at 4° C for a maximum of several days but should not be frozen. There are several point-of-care enzyme-linked immunosorbent assay (ELISA) kits available for detection of Giardia spp. antigen in human feces, and one kit is licensed for use with dog and cat feces (SNAP Giardia, IDEXX Laboratories, Portland, ME). Results vary among the specific ELISA methods, and inconsistent results have been reported with some Giardia antigen tests when compared to other non–ELISA-based techniques.16,10,53,54,215 For example, in one study in dogs, the immunoassay used (ProSpecT Giardia Rapid Assay, Remel, Lenexa, KS) had a false-negative result rate of 31.6% when compared to zinc-sulfate centrifugation results.215 Findings of another study comparing the zinc chloride–sodium chloride (ZnCl2-NaCl) flotation method and a commercial copro-antigen ELISA (ProSpecT Giardia Microplate Assay, Remel, Lenexa, KS) were prevalence rates of 9.5% (25 of 270) in dogs and 0% in cats (0 of 100 cats) by microscopic examination and 29.5% (79 of 270) in dogs and 22% (22 of 100) in cats by the fecal ELISA.53 The discrepancy of the results of this study with others that were performed using dog samples might be attributed to the use of ZnCl2-NaCl flotation instead of zinc sulfate, which might alter or destroy the cysts, thereby making detection difficult.53 The ELISA kit specifically licensed for use with canine or feline feces (SNAP Giardia antigen assay) has been evaluated in several studies. In cats, this assay had similar sensitivity and specificity when compared to fecal flotation, and when the results of the two techniques were combined, the overall sensitivity was 97.8%.179 In another study of dogs, using the direct FA as the gold standard test, at the prevalence rate of 10%, the Giardia antigen assay was estimated to have positive-predictive and negative-predictive values of 51.0% and 97.0%, respectively, which were similar to those of zinc-sulfate centrifugation.223 Although information is limited regarding detection of Giardia assemblages C, D, or F, in one study of 4 assemblage C isolates and 13 assemblage D isolates, all were positive by the SNAP Giardia antigen assay.54 The Companion Animal Parasite Council (CAPC) recommends that fecal antigen tests be used as an addition to fecal flotation only during the evaluation of dogs and cats with diarrhea, and not with clinically healthy pets, because the clinical and zoonotic impact of an antigen result-positive, cyst presence result-negative healthy pet is unknown.55 It is also unknown how long Giardia antigen assay results remain positive after resolution of diarrhea. Therefore, if a veterinarian chooses to assess the success of the treatment for giardiasis in cats and dogs, only fecal flotation is recommended for follow-up evaluation. PCR can be used to determine the Giardia spp. assemblage. A number of genes are used for the amplification of Giardia DNA by PCR.63 However, the results of studies showed that the assignment of isolates to specific G. duodenalis assemblages is not always consistent because alternate genes can give different results.41 Thus, some dog or cat isolates can be genotyped as “potentially zoonotic” by one gene but as “host specific” with another (see Web Tables 77-3 and 77-4).41 Another problem is that the available primers do not consistently amplify G. duodenalis DNA. Sometimes individual isolates can be amplified at one locus but not at another, whereas other isolates may have the opposite PCR pattern.41 Giardia PCR assay results can also be falsely negative because of the presence of PCR inhibitors in feces and so should not be used as the sole means of confirming Giardia spp. infection. Giardia spp. PCR testing could be considered for attempting to determine the G. duodenalis assemblage detected in feces of cats and dogs, particularly if the owner is concerned about zoonotic transmission of infection. Because of the inconsistent findings in single genes, use of multilocus genotyping is recommended.41,237 WEB TABLE 77-3a Distribution of Giardia Assemblages in Dogs aModified from Ref. 294. WEB TABLE 77-4a Distribution of Giardia Assemblages in Cats aModified from Ref. 294. In human medicine, a combination of nutritional intervention and phytotherapy is often the first line of approach for treating symptomatic giardiasis.118 In veterinary medicine, dietary manipulation is often combined with antiprotozoal chemotherapy. Although there is no licensed veterinary drug specific for Giardia, many are effective in reducing the level of infecting organisms in attempts to speed resolution of clinical signs (Table 77-3). TABLE 77-3 Drug Therapy for Giardiasis, Amoebiasis, Balantidiasis, Blastocystosis, and Trichomoniasis B, Both dog and cat; C, cat; D, dog; feb, febantel; PO, by mouth; praz, praziquantel; pyr, pyrantel. aSee the Drug Formulary in the Appendix for additional information on each drug. bDose per administration at specified interval. cDrontal Plus Tablets (Bayer Animal Health, USA). See Drug Formulary in the Appendix for tablet sizes. Feline dose for kittens is from Ref. 239. dDrontal Plus Flavour Tablets (Bayer Animal Health, Europe). Dose is approximately one tablet per 10 kg bodyweight. See Drug Formulary in the Appendix for tablet sizes. eMaximum recommended dosages are used for treatment of enteric protozoal infections. Neurotoxicity has been observed at levels of hydrochloride salt at ≥60 mg/kg/day. See Nitroimidazoles, Chapter 71 and the Drug Formulary in the Appendix. To facilitate the dosage to smaller animals, the 250- or 500-mg tablets may be ground, or smaller tablet size (50 to 100 mg) may be used. This may be placed in a palatable base. For cats, metronidazole benzoate formulations (62% metronidazole base) are better tolerated orally than hydrochloride formulations, but dosage needed would be 1.6 times listed. fIn drinking water and given ad libitum. gIn suspension, 200 mg/day maximum. Has been used in children and has the ability to inhibit cyst formation.117 Not available in some countries. Drugs in the nitroimidazole class have anti-Giardia effects. The suggested dosages of drugs listed here are summarized in Table 77-3. Although metronidazole has been widely used for the treatment of giardiasis in naturally and experimentally infected cats and dogs, controlled studies are lacking.* Unfortunately, central nervous system toxicity had been associated with the administration of metronidazole in some dogs67 and kittens.45 Neurologic toxicity was reported after either chronic therapy or acute high doses.45 The optimal dose and duration for metronidazole when used for the treatment of clinical giardiasis is unknown. The CAPC suggests a maximum dose of 25 mg/kg, orally (PO), twice daily for 5 days.55 Therapy longer than 7 days should never exceed 30 mg/kg/day (see the Drug Formulary in the Appendix). Metronidazole hydrochloride is the United States Pharmacopeia (USP) formulations in tablets, which causes cats to salivate, while the benzoate form available in some countries is less likely to cause this problem. However, products containing metronidazole benzoate are available in some countries, and the drug is available for formulation in the United States. When metronidazole benzoate was administered to cats with subclinical giardiasis in one study, cyst shedding ceased over the time period of the study, and signs of toxicity did not develop.235 Other nitroimidazoles including ipronidazole, ronidazole, and tinidazole had been used for the treatment of giardiasis in a small number of animals. Ipronidazole added to water was effective for the treatment of giardiasis in two dogs.5 Use of drugs in the nitroimidazole class also may have the added benefit of correcting bacterial overgrowth, which may also contribute to diarrhea, in some dogs and cats with clinical giardiasis. See Table 77-3 and the Drug Formulary in the Appendix for further details on each of these drugs. Fenbendazole has been evaluated for the treatment of giardiasis in a number of studies of dogs and cats.20,147,182,300 An additional benefit for the use of this drug is its effect against ascarid hookworms and Taenia infections that can occur as co-infections with Giardia spp. The CAPC recommends the administration of fenbendazole (50 mg/kg, once daily, for 5 days) to cats and dogs or a combination of this same dosage of fenbendazole and metronidazole (25 mg/kg, every 12 hours, for 5 days). However, when fenbendazole was administered to cats concurrently infected with Giardia and Cryptosporidium parvum, only 4 of 8 cats stopped shedding Giardia cysts.147 When fenbendazole was administered to naturally infected shelter dogs, the percent persistence of cyst counts per gram of feces were 84% and 78% when feces were rechecked 9 and 16 days, respectively, after treatment.182 In cats, fenbendazole was safe when administered to healthy, adult, nonpregnant cats at a dosage 5 times higher than the approved dosage.233 Other benzimidazoles used in some studies of giardiasis are albendazole, oxfendazole, and febantel. Albendazole was effective in one study involving dogs; however, because of bone marrow suppression, it is not recommended for use.18,263 Administration of oxfendazole when used in conjunction with other control measures led to resolution of diarrhea and cyst shedding in one study of kenneled dogs with giardiasis.288 A combination of febantel, pyrantel, and praziquantel (FPP) (Drontal Plus Tablets, Bayer, Shawnee Mission, KS), using a variety of dosage protocols, has had variable results in the treatment of giardiasis in dogs and cats.* Two different formulations of the drug exist (see Table 77-3), and dosage differences may be responsible for some of the discrepancies between results of efficacy studies. In one report involving seven healthy Giardia-infected dogs, organisms were not detected again in 3 days after 3 consecutive days of treatment with the Drontal Plus Tablets.34 However, 6 days after the last treatment, some dogs began to shed organisms again, and this was hypothesized as being from reinfection.34 Another study reported that the efficacy of the treatment of dogs naturally infected with Giardia spp. for 5 consecutive days with the European formulation was not significantly better than treatment for 3 consecutive days.189 Results of an additional study implied that when Drontal Plus Tablets were used to treat dogs with giardiasis, bathing the animal and changing its environment after treatment may be more important for preventing reinfection than duration of treatment.215 When Drontal Plus Tablets were administered at the empiric dosage of two small dog-tablets per cat, orally, for 5 days, to 6 cats, a significant decrease in cyst shedding was observed in the treated group in comparison to the untreated control group.239 In addition, 4 of the 6 treated cats had evidence of infection after two doses of repositol glucocorticoids at immunosuppressive levels.239 Azithromycin was used for the treatment of an 8-month-old dog that had persistent diarrhea after being treated with fenbendazole and metronidazole for giardiasis.309 Azithromycin was administered at a dose of 10 mg/kg once a day for 5 days, and after treatment the diarrhea stopped and no adverse reactions were observed. Giardia cysts and DNA were not detected after examination of fecal smears and PCR of fecal samples on days 2, 4, and 6 after therapy.309 However, azithromycin has failed to lessen Giardia attachment or growth in vivo, and so use of this drug for treatment of giardiasis in animals needs additional study.144 Other drugs used historically to treat giardiasis in dogs or cats included quinacrine and furazolidone; however, controlled efficacy studies are lacking. Other drugs with potential use for the treatment of giardiasis include paromomycin and nitazoxanide. See Chapter 70 and the Drug Formulary in the Appendix for a discussion of these drugs. Although controlled studied in dogs and cats are generally lacking, other adjunctive therapies, including fiber, silymarin, and probiotics, have been administered to some dogs or cats with giardiasis on the basis of work with other species. Addition of insoluble fiber in a Giardia model in gerbils was proposed to lessen trophozoite adherence and colonization to the microvillus.166 Silymarin was evaluated for the treatment of canine giardiasis.51 Although all dogs who received metronidazole, with or without silymarin, stopped shedding Giardia cysts, the addition of silymarin appeared to help to compensate for weight loss and increased the levels of serum total proteins and albumin when compared to dogs administered metronidazole alone.51 In a rodent model, the administration of the probiotic Enterococcus faecium strain SF68 lessened Giardia spp. shedding.26 However, positive effects on Giardia spp. cyst shedding, fecal antigen shedding, fecal IgA concentrations, or leukocyte phagocytic activity were not recognized in adult dogs with chronic giardiasis fed this probiotic.244 Further work is required before these adjunctive therapies can be routinely recommended.
Enteric Protozoal Infections
Organism
Stage
Average Size (µm)
Natural Hosts
Organ Parasitized
Pathogenic Mechanisms
Clinical Signs
Giardiaa
Tr
15 × 10 × 3
D, C, H, other mammals
Small intestine
Damage to glycocalyx and microvilli on intestinal epithelium; inhibition of some digestive enzymes; host elicits inflammatory response
None to chronic diarrhea, continuous or intermittent, malabsorption
Cy
10 × 8
Entamoeba histolytica
Tr
25 (diam)
D, C, H, NHP
Large intestine
Invades colonic wall, producing ulcers; may metastasize to extraintestinal sites
None to diarrhea; dysentery
Cy
12 (diam)
Balantidium coli
Tr
60 × 35
D, P, H, NHP
Large intestine
Invades colonic wall, producing ulcers, metastases (rare)
None to diarrhea; dysentery
Cy
50 (diam)
Pentatrichomonas hominis
Tr
8 × 5, five flagella
D, C, H, other mammals
Large intestine
Probably none; considered harmless commensal but may be opportunistic pathogen
None to diarrhea
Tritrichomonas foetus
Tr
8 × 5, three flagella
D, C, Ca, P
Genitalia (cattle)
Intestine, nasal (swine)
Large intestine (cat)
Epithelial adherent, elaboration of cytotoxins and enzymes; host elicits inflammatory response
Large bowel diarrhea in feline; infertility and fetal loss in cattle
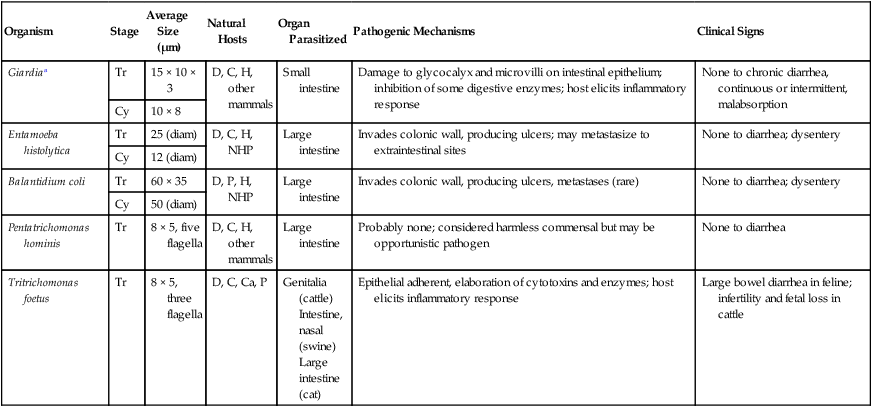
Giardiasis
Etiology and Epidemiology
Genotype/Assemblage
Host Range
Assemblage A
Humans, primates, cattle, cats, dogs, rodents, wild mammals
Assemblage B
Humans, primates, dogs, cattle, horses
Assemblages C, D
Dog
Assemblage E
Artiodactyls
Assemblage F
Cats
Assemblage G
Rodents
Location
Number Examined
Method
Prevalence (%)
Reference
Argentina
106
Microscopy
14.5
273
Brazil
166
Microscopy
31.33
131
Canada
70
Microscopy
7.1
242
Canada
1216
Fecal ELISA
7.2
138
Canada
102
Fecal ELISA
7
163
Czech Republic
458
Microscopy
2.5
265
Czech Republic
458
Serum
36.5
265
Germany
1281
Microscopy
2.3
71
India
101
PCR
20
280
Iran
147
Microscopy
0.68
241
Italy
105
Fecal ELISA
19
29
Italy
183
Fecal ELISA
55.2
212
Italy
143
PCR
30
213
Italy
127
PCR
20.5
232
Japan
1035
Microcopy
14.6
136
Japan
906
Microscopy
0.9
296
Poland
148
PCR
2.0
247
Scotland
101
Microscopy
13.9
243
Serbia
81
Microscopy
14.4
197
Spain
1161
Microscopy
7
182
Spain
505
Microscopy
6.1
108
Tasmania
55
Microscopy
14.5
181
The Netherlands
152
Fecal ELISA
15.2
210
United Kingdom
275
Microscopy
14.5
267
United States
1,199,293
Microscopy
4.0
172
United States
5959
Fecal ELISA
7.5
308
United States
16,114
Fecal ELISA
15.6
43
United States
49
Direct FA
26.5
139
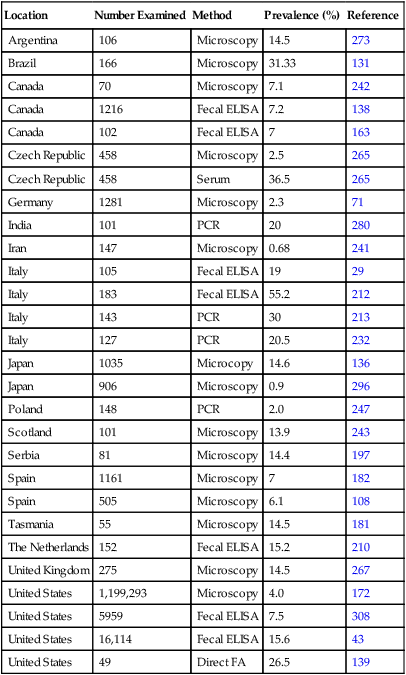
Location
Number Examined
Method
Prevalence (%)
Reference
Australia
40
Microscopy
5
178
Australia
1063
Microscopy
2
211
Australia
40
PCR
80
178
Brazil
131
Microscopy
6.1
21
Canada
41
Microscopy
2.4
242
Chile
230
Microscopy
19.1
173
Czech Republic
135
Microscopy
1
265
Czech Republic
135
Serum
57
265
Germany
3167
Microscopy
10
23
Italy
48
Fecal ELISA
4
29
Serbia
81
Microscopy
22
197
The Netherlands
305
Microscopy
1
224
The Netherlands
60
Fecal ELISA
13.6
210
United Kingdom
1355
Microscopy
6
283
United Kingdom
48
Fecal ELISA
15
283
United States (CO)
206
Microscopy
2.4
124
United States (MS)
250
Direct FA
13.6
287
United States (NY)
263
Microscopy
7.3
250
United States (Philadelphia, PA)
1566
Microscopy
2.3
86, 87
United States
344
Direct FA
9.8
179
United States
4978
Fecal ELISA
10.8
43
Pathogenesis
Clinical Findings
Diagnosis
Fecal Microscopy
Direct Smear
Concentration Techniques
Duodenal Aspirate Examination
Immunochemical Detection with Fluorescent Microscopy
Fecal Antigen Detection by Enzyme-Linked Immunosorbent Assays
Molecular Genetic Techniques
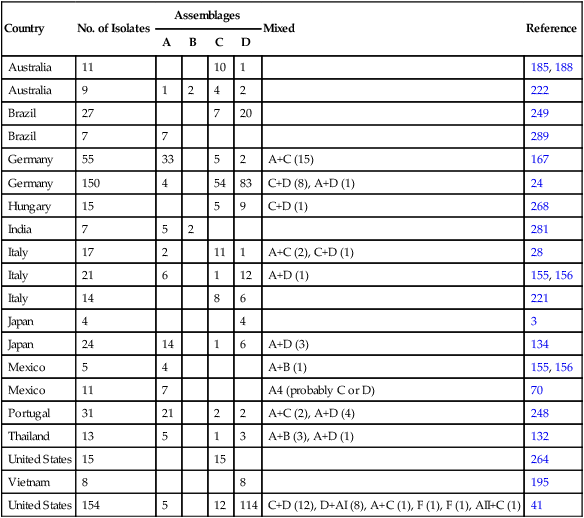
Country
No. of Isolates
Assemblages
Mixed
Reference
A
B
C
D
Australia
11
10
1
185, 188
Australia
9
1
2
4
2
222
Brazil
27
7
20
249
Brazil
7
7
289
Germany
55
33
5
2
A+C (15)
167
Germany
150
4
54
83
C+D (8), A+D (1)
24
Hungary
15
5
9
C+D (1)
268
India
7
5
2
281
Italy
17
2
11
1
A+C (2), C+D (1)
28
Italy
21
6
1
12
A+D (1)
155, 156
Italy
14
8
6
221
Japan
4
4
3
Japan
24
14
1
6
A+D (3)
134
Mexico
5
4
A+B (1)
155, 156
Mexico
11
7
A4 (probably C or D)
70
Portugal
31
21
2
2
A+C (2), A+D (4)
248
Thailand
13
5
1
3
A+B (3), A+D (1)
132
United States
15
15
264
Vietnam
8
8
195
United States
154
5
12
114
C+D (12), D+AI (8), A+C (1), F (1), F (1), AII+C (1)
41
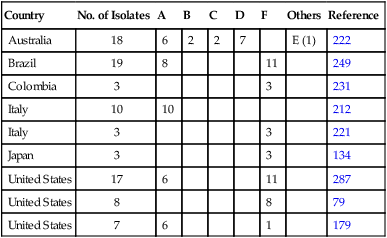
Country
No. of Isolates
A
B
C
D
F
Others
Reference
Australia
18
6
2
2
7
E (1)
222
Brazil
19
8
11
249
Colombia
3
3
231
Italy
10
10
212
Italy
3
3
221
Japan
3
3
134
United States
17
6
11
287
United States
8
8
79
United States
7
6
1
179
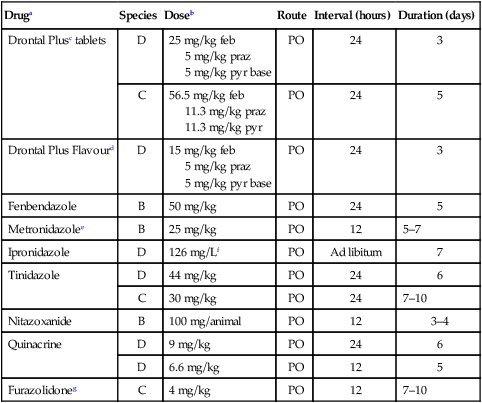
Therapy
Druga
Species
Doseb
Route
Interval (hours)
Duration (days)
Drontal Plusc tablets
D
25 mg/kg feb
5 mg/kg praz
5 mg/kg pyr base
PO
24
3
C
56.5 mg/kg feb
11.3 mg/kg praz
11.3 mg/kg pyr
PO
24
5
Drontal Plus Flavourd
D
15 mg/kg feb
5 mg/kg praz
5 mg/kg pyr base
PO
24
3
Fenbendazole
B
50 mg/kg
PO
24
5
Metronidazolee
B
25 mg/kg
PO
12
5–7
Ipronidazole
D
126 mg/Lf
PO
Ad libitum
7
Tinidazole
D
44 mg/kg
PO
24
6
C
30 mg/kg
PO
24
7–10
Nitazoxanide
B
100 mg/animal
PO
12
3–4
Quinacrine
D
9 mg/kg
PO
24
6
D
6.6 mg/kg
PO
12
5
Furazolidoneg
C
4 mg/kg
PO
12
7–10
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine