Chapter 5 Endoscopic Examination of the Small Intestine
One important decision facing clinicians using this technology is whether colonoscopy should be performed on all patients already being anesthetized for an endoscopic procedure geared toward the small intestines. There are clinicians and institutions where the combination of upper and lower GI endoscopy is standard protocol. The argument in favor of this approach is the potential for increased diagnostic yield (finding disease in both places), decreased likelihood of missing occult disease (finding neoplasia in the colon that is not present in the duodenum), and the greater area covered. As with all diagnostic tests the predictive value of the test result is affected by the prevalence of the disease; thus the decision to perform colonoscopy on a patient in the absence of signs of colonic disease becomes a difficult one to justify. In those patients seen because of signs consistent with mixed-bowel diarrhea, the most parsimonious assumption is that the same disease process is present in both the small and large bowel and endoscopic biopsy specimens from the duodenum are very likely to provide the same information as that from a colonic biopsy.
Indications
Routine baseline assessment is essential. An endoscopic biopsy of the small intestine can provide an intricate and detailed picture of a Giardia trophozoite on the mucosal surface (see Figure 5-67 in the “Atlas for Intestinal Parasites” section), but it is a very expensive way to diagnose giardiasis as the cause of diarrhea (the combination of fecal centrifugal flotation and Giardia antigen testing is far superior and much more practical). Endoscopy is the diagnostic tool of choice for actually visualizing ulceration of the proximal duodenum, but direct visualization is completely unable to distinguish between nonsteroidal antiinflammatory drugs (NSAIDs) and hypoadrenocorticism as possible causes of that ulceration. Endoscopic evaluation of the small intestine may reveal an unhealthy mucosa with blunted villi, but it will not reveal the absence of exocrine pancreatic tissue, nor will it determine that a patient’s diarrhea will only subside when an enzyme supplementation is instituted. The duodenal mucosa is not a practical place to perform an allergen-response test in search of the diagnosis of a food allergy.
Diet has long been implicated as an important cause for or treatment of GI signs, and an appropriate dietary trial is often a prudent diagnostic step before recommending endoscopy. Food allergy has to be ruled out for any dog with a combination of GI and dermatologic signs and requires an 8- to 12-week diet trial for diagnosis, although recent studies have highlighted the prevalence of food-responsive diarrhea in younger dogs in which a significant response can be seen after only 2 weeks. The importance and effectiveness of dietary intervention in cats with diarrhea have been illustrated by a number of recent studies and highlight the fact that several trials with a variety of different diets, each of 2 to 3 weeks’ duration, might be appropriate and beneficial in a number of cats. Finally, a number of endocrinopathies result in signs associated with the GI tract, and assessment of the thyroid and adrenal glands is often an important diagnostic undertaking before recommending endoscopy.
The most significant advantage offered by endoscopic examination and biopsy of the upper small intestine is the opportunity to diagnose inflammatory bowel disease relatively early, as clinical signs become evident, without having to procure tissue samples surgically. Fortunately, intestinal involvement is diffuse in the majority of dogs and cats with inflammatory bowel disease, and it is usually sufficient to obtain biopsy samples from the duodenum. Endoscopy also offers an alternative approach to surgery for obtaining small bowel biopsy samples in cases of protein-losing enteropathy. Multiple biopsy specimens can be obtained safely, even in patients in which hypoalbuminemia is severe enough to interfere with the healing of surgically obtained full-thickness biopsy samples. Although severe inflammatory bowel disease is a common cause of protein-losing enteropathy in dogs and cats, both lymphangiectasia and lymphoma are important differentials that can be addressed with endoscopic biopsies. Although a histopathologic diagnosis should never be made from gross observation, the mucosal surface of the small intestine in dogs with lymphangiectasia often has a characteristic gross appearance (see Figures 5-59 through 5-61 in the “Atlas for Intestinal Lymphangiectasia” section). Some degree of lacteal dilation can be secondary to intestinal inflammation, particularly in the villi, so it is important to try to obtain multiple biopsy samples from a single site (the “Pac man” approach) so that the likelihood of obtaining a submucosal tissue sample is increased. Obviously abnormalities on the minimum database may also be useful in rank-ordering inflammatory bowel disease and lymphangiectasia on the list of conditions to rule out. The same considerations and approach apply to cases in which neoplasia, particularly alimentary lymphoma, is a differential.
Ulceration of the small intestines occurs less commonly in animals than in humans. Drugs, specifically NSAIDs, remain a top differential for GI ulceration in both species, whereas other sources of mucosal erosions, ulceration, and GI bleeding in cats and dogs may include direct physical disruption, chronic inflammation, mast cell tumors, gastrinomas and other neoplastic conditions, metabolic and systemic diseases such as renal or hepatic failure or hypoadrenocorticism, coagulopathies and severe thrombocytopenia, acute pancreatitis, shock, stress, and disseminated intravascular coagulation. Appropriate diagnostic testing can rule out a number of secondary GI conditions, and abdominal ultrasound can identify masses, enlarged intraabdominal lymph nodes, focal thickening of the GI wall, or loss of the normal layered architecture of the wall of the intestine. Endoscopy then gives the clinician the ability to directly visualize and sample the lesion(s) and the surrounding tissue. It is important to obtain multiple biopsy specimens from both abnormal and normal-appearing areas to help delineate the extent of the disease as well as avoid a sample that only reveals the necrotic tissue that would be common to any ulcerated mucosa, regardless of the underlying cause.
Inflammatory bowel disease is much more common than alimentary lymphoma in dogs and cats, but the ability to accurately distinguish and diagnose the two conditions remains a tremendously important and challenging task for veterinarians. Although there is some overlap in the treatment of the two diseases (corticosteroids), there is usually minimal overlap in the prognosis or progression of the two conditions. A pathologist would consider multiple full-thickness biopsies from various different areas of the small bowel the ideal sample type for making that distinction. The strength of endoscopy is the relative cost, time, effort, and minimal morbidity associated with obtaining multiple biopsy samples from the stomach and upper portion of the small intestine compared with surgery and the relative wealth of information obtained from histopathologic analysis compared with having no biopsy samples at all. The weaknesses of endoscopy include the limitations on the length of the small intestine that can be evaluated and the fact that the samples obtained are not full-thickness biopsy specimens. Recent studies have shown that histopathologic evaluation of endoscopic biopsy specimens can result in both false-negative and false-positive results in the diagnosis of alimentary lymphosarcoma. Studies also show that the clinician can affect the likelihood of a correct diagnosis by obtaining a sufficient number of samples (approximately eight from each area of interest) and doing everything possible to ensure that the biopsy samples are of the highest quality (i.e., correct angle, depth, and amount of tissue). Working closely and consistently with an experienced pathologist is important because, not surprisingly, it has been shown that different pathologists will arrive at different conclusions even when evaluating the same slide. Provide the pathologist with an accurate and detailed history of the case. As always, use clinical judgment to guide diagnostic decisions, and if there is a discordance between the clinical diagnosis and a pathologist’s report, discuss that disparity with the pathologist. The use of special stains, flow cytometry, and immunohistochemical analysis are a few of the tools being developed that are making endoscopy an even more powerful diagnostic tool (see Chapter 8 for further discussion on special testing for lymphoma).
Instrumentation
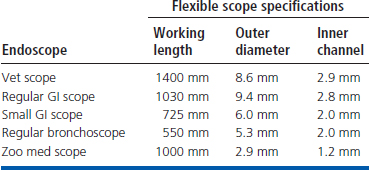
The endoscope specifications for small bowel examinations are the same as those described in Chapters 2 and 4. An endoscope diameter as large as 9.8 mm can be used in most dogs and some cats, but the success rate for advancing through the pylorus to the duodenum is estimated to be only 50% to 60% when this size endoscope is used in cats. (This success rate is higher for very experienced operators.) The duodenum can be reliably entered when a 7.8- to 9-mm-diameter endoscope is used in cats and small dogs. The smaller diameter scopes routinely allow duodenoscopy to be performed in canine and feline patients as small as 1.4 kg (3 lb). The ideal size for the working length of an endoscope in small animal practice is 140 cm. This provides sufficient length to reach the duodenum in the largest of dogs. A 100-cm scope is too short to reach the duodenum of some dogs of the larger breeds. This could include any dog weighing more than 27 kg (60 lbs). A 100-cm scope can actually be long enough to reach the duodenum of some dogs weighing 35 to 40 kg (77 to 90 lbs), but in dogs of certain breeds that weigh less, the length simply may be insufficient. There are significant differences among breeds; thus for versatility, especially in a general practice where only one endoscope will be available, the longer scope is best. Table 5-1 shows the range of endoscopes available at a tertiary referral facility such as Colorado State University, where one of the contributors (Craig Webb) practices. All are videoscopes, and although the range of capabilities is useful (especially considering the variety of species undergoing endoscopic procedures), the standard length and diameter endoscope is still used in the majority of cases.
Table 5-1 Description of the Flexible Endoscopes Currently Available to Clinicians at Colorado State University
Having a variety of high-quality sharp-edged biopsy forceps available is imperative for obtaining biopsy samples of sufficient size and quality for evaluation. The size of the biopsy sample obtained will be directly proportional to the size of biopsy forceps used. The actual cup of the biopsy instrument can incorporate a variety of modifications beyond the basic round or oval shape, such as a bayonet pin or serrated edges that may increase the tissue yield (see Chapter 8 for details on biopsy forceps). Brush cytology and duodenal juice aspiration are performed infrequently but are easily accomplished with relatively simple instrumentation.
Patient Preparation
Patient preparation for upper small intestinal examination is the same as for gastroscopy (see Chapter 4). No food should be given for 12 to 18 hours before the examination, and water should be withheld 4 or more hours. Intravenous fluids should be administered as needed in patients with vomiting and/or diarrhea to prevent dehydration. If a barium contrast radiographic examination has been done, the small bowel should be relatively empty before an endoscope is introduced. Pools of barium should not be suctioned through the instrument channel because residue is likely to adhere to the channel walls. Small flecks of barium residue along the mucosal lining do not need to be suctioned, however, and pose no hindrance to completing a thorough examination (see Figure 5-23 in the “Atlas for Barium Residue” section). Ileoscopy requires that the patient be prepared for complete colonoscopy. This is discussed in detail in the section on patient preparation for colonoscopy in Chapter 6.
Restraint
As with any endoscopic procedure of the upper GI tract, general anesthesia is required for enteroscopy. The guidelines recommended for gastroscopy should be followed (see Chapter 4). Both duodenoscopy and ileoscopy are performed with the patient positioned in left lateral recumbency.
Procedure
To successfully maneuver an endoscope into the duodenum, the endoscopist must first become proficient at gastroscopy. Techniques for maneuvering the endoscope around the incisura angularis (angulus) of the stomach, evaluating the antrum and pylorus, and advancing the endoscope through the pyloric canal are described in detail in Chapter 4. Techniques for traversing the pylorus are reviewed in this chapter, which also discusses in detail the examination of the duodenum and ileum, as well as techniques for obtaining biopsy samples from the small intestine.
Pylorus to Proximal Duodenum
As the endoscope is advanced into the actual tissue, the pyloric orifice often appears as a small dark hazy slit in an otherwise blurred field. The ability to continue to maintain the central position of the pyloric orifice is an essential skill for being able to predictably enter the duodenum. Both the pressure from the tip of the endoscope as well as GI motility will create conditions where the pylorus is moving in various directions within the same plane as the endoscope tip (or behaving as if it were an elevated green in golf), with the endoscope tip sliding off and away from the target. At this point, retract the endoscope to a position where the pylorus can be fully visualized at a distance that allows full focus of its surroundings. Because the movement of the pylorus will often be stereotypical and therefore something the endoscopist can anticipate, you should learn from each attempt. As described in Chapter 2, it is highly desirable to learn to hold the handset and operate both controllers with the same hand (left hand), leaving the right hand on the insertion tube of the scope. This allows several things to happen simultaneously. First, the right hand maintains a dynamic tension on the insertion tube, which is translated to the pyloric orifice as a force that either helps to maintain its position or actually helps to open the orifice. Second, the right hand can exert a surprising range of motion on the tip of the endoscope independent of the headset controllers simply by gently rotating the insertion tube either clockwise or counterclockwise (torque). Third, it is much easier for a single brain to coordinate the required movements (tip deflections, right-hand rotation, and forward advancement) than it is to coordinate a similar effort through two brains.
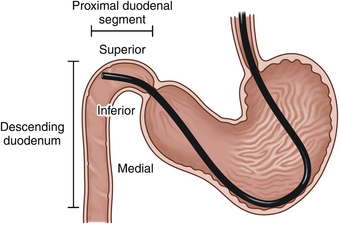
If the pylorus is closed, several fairly predictable maneuvers can facilitate entrance to and advancement through the pyloric canal. Based on the endoscope that is used, the following recommendations may vary slightly. Often the endoscope tip can be directed into the pyloric canal by application of leftward tip deflection (turn outer control knob in a counterclockwise direction) alone or, in some cases, along with slight to moderate upward tip deflection (turn inner control knob counterclockwise) while the scope is gradually advanced. Once the tip of the endoscope is in the pyloric canal, only a blurred image is seen because the pyloric walls are usually pressed in around the tip of the scope. Turning both control knobs in a clockwise direction as the endoscope is advanced provides a downward and right tip deflection change that facilitates entry into the proximal duodenal segment, a small area immediately beyond the pylorus. Although the first part of the duodenum is not expanded to form a pronounced duodenal bulb or cap (as occurs in humans), its functional independence is retained. The initial portion of the duodenum continues from the pylorus and passes toward the right body wall before being deflected caudally to become the descending duodenum. A convex curve is formed by the junction of the antrum and descending duodenum; this area, along with the pylorus, provides some resistance to passage of the endoscope. In large dogs it is sometimes possible to clearly visualize the mucosa along this curved area, but in most cases the image remains blurred because the endoscope tip is still tightly confined. Despite the lack of a clear view, the endoscopist is usually aware that the proximal segment of the duodenum has been reached because a distinct sensation is felt as the pyloric resistance is passed. In addition, the color of the mucosa usually changes from cream (antrum and pylorus) to pinkish red (duodenum). The color change is quite subtle in some cases. In a particularly tight pyloric canal, the color change provides an important landmark that determines the point at which directional changes are made to help guide the endoscope tip to the proximal duodenal segment (both control knobs forward). In cats and most dogs the duodenum still is not viewed clearly at this point because the endoscope is wedged against or is close to the wall of the proximal duodenal segment (Figure 5-1). One final maneuver is performed to direct the tip into the descending duodenal lumen (the second duodenal segment). Excessive force should never be used in this area. As the tip lies against the superior wall of the proximal duodenal segment (see Figure 5-1), it is angled acutely up (turn inner control knob counterclockwise) and in some cases also to the left (turn outer control knob counterclockwise). Further gentle advance usually provides a tunnel view of the descending duodenum (Figure 5-2). Air insufflation is usually continued to distend the walls. In some cases, minor forward or backward movements of the entire insertion tube (using the right hand placed close to the patient’s mouth) are also necessary to help free the endoscope tip. If a clear view cannot be obtained after these collective maneuvers have been attempted, the endoscope should be retracted a short distance rather than forced forward against resistance. If in doubt, it is almost always best to retract and insufflate to regain one’s bearings. Once the technique is mastered, an endoscope can usually be advanced from the antrum to the proximal descending duodenum in a matter of seconds. When a patient is small or the pyloric canal and proximal duodenal areas are narrow, successful passage to the duodenum can usually be accomplished with the maneuvers described here in sequential order along with observation of important color change landmarks. As described in Chapter 4, occasionally (especially in patients with pyloric mucosal hypertrophy) even a pediatric endoscope cannot be advanced through the pylorus. The patient’s heart rate and respiratory rate and character should always be monitored carefully during difficult passages in which the pyloroantral and proximal duodenal areas can be stretched considerably or distorted. Although significant cardiopulmonary changes as a result of an increase in vagal tone occur only occasionally, the endoscopy team should remain alert for these changes because they may require pharmacologic intervention. Chapter 4 provides further information on the potential complications of pyloral passage in cats.
Examination of Descending Duodenum
Varying degrees of insufflation are usually necessary to maintain a clear view of the lumen of the small intestine as the endoscope is advanced. This is easily accomplished by using the air insufflation button on the control housing. As in gastroscopy, great care must be taken to monitor the degree of gastric distension, especially in cats and small dogs. Air insufflated to the intestine may reflux into the stomach and cause significant gastric dilation, which may subsequently cause respiratory compromise. Moderate gastric distension usually is not a significant problem, except in some obese patients whose conformation may potentiate respiratory difficulty under general anesthesia. Marked gastric distension should be quickly relieved because it can significantly compromise respiration. To relieve gastric distension, the endoscopist withdraws the tip of the scope to the stomach so that the air can be effectively suctioned. Traversing the pylorus again usually is not difficult after it has already been done one or more times.
The intestine should be carefully examined as the endoscope is advanced and as it is retracted after the farthest point of advance has been reached. It is usually easier to view parts of the proximal duodenal segment during retraction than on entry. The major limiting factor of endoscopic examination of the small intestine is that only a limited length of intestine can be visualized with the use of standard endoscopes. A minor limitation is that certain portions of the duodenum, including the area immediately beyond the pylorus and parts of the medial wall of the descending segment, are sometimes difficult to view other than tangentially. A special effort is made to obtain full circumferential views by rotation of the endoscope tip in various directions during retraction. Under the different conditions of motility and organ shapes determined by distension and endoscope position during retraction, areas previously seen only tangentially may be brought into direct view. This minimizes blind areas that cannot be examined thoroughly on entry. Examination on entry is most important for recognizing general mucosal appearance and obvious lesions. In small patients the endoscope itself sometimes causes minor mucosal trauma, which usually appears as streaks of erythema or mild superficial hemorrhage (see Figures 5-64 and 5-65 in the “Atlas for Endoscope-Induced Mucosal Trauma” section). Comparison of the examination findings during advance and retraction can confirm whether lesions are iatrogenic or were present before the evaluation was undertaken.
Normal Appearance of the Upper Small Intestine
The normal duodenal and jejunal mucosa appears reddish pink to yellow-red in dogs and cream to slightly reddish pink in cats. Because of the presence of villi the mucosa has a velvety or shaggy appearance, especially on close visualization. Aggregated lymphatic nodules (Peyer’s patches) can routinely be identified in the descending duodenum of dogs. The nodules, some as large as 2 cm × 1.5 cm, are found on the lateral wall. A shallow central crater effect (approximately 2 to 3 mm deep) is associated with these nodules. Although aggregated lymphoid nodules also occur in the cat, they are not readily identified on endoscopic examination.
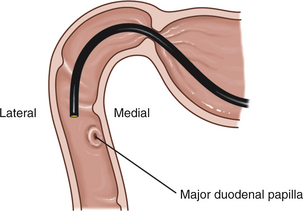
With newer endoscopes that have wide-angle illumination, one or both duodenal papillae of dogs can often be visualized. The major duodenal papilla is located along the medial wall approximately 5 cm from the pylorus. This papilla represents the common opening for the bile duct and the pancreatic duct (the duct of Wirsung). The appearance of the papilla varies from a flattened white disk to a small thickened projection from the medial wall. Occasionally an active bile discharge can be seen emanating from the papilla. A second papilla, the minor papilla, is located on the upper wall, approximately 2 cm distal to the major papilla. The accessory pancreatic duct (the duct of Santorini) opens onto the minor duodenal papilla. This papilla is generally flattened and appears as a small (1.5 to 2 mm in diameter) white, round to oval disk against the reddish pink of the surrounding mucosa. Cats have only one papilla (the major duodenal papilla) for the pancreatic and biliary ducts. As a result of its location close to the convex curve formed by the antrum, pylorus, and proximal duodenum, this papilla is sometimes difficult to visualize endoscopically. See Figures 5-33 through 5-38 in the “Atlas for Duodenal Papillae” section for various normal appearances of the duodenal papillae.
Examination of the Terminal Ileum
For ileoscopy the colon must be thoroughly cleansed. Retained fecal material prevents thorough mucosal evaluation and makes it extremely difficult to see clearly enough to maneuver through the curves of the colon. Therefore when ileoscopy is contemplated, every effort must be made to ensure that the colon is completely evacuated. Procedures for cleansing the colon and examining this organ to the level of the ileocolic junction are described in Chapter 6.
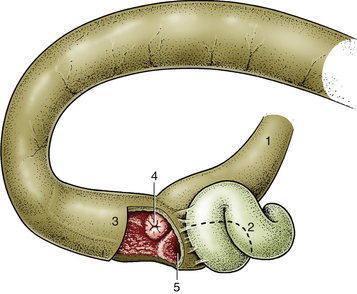
The ileal orifice usually protrudes into the lumen of the terminal ascending colon as a papillary form and as such is easily recognized (Figure 5-3, and see Figures 5-39 through 5-42 in the “Atlas for Canine Ileocecocolic Junction and Ileum” section). A close-up view of the orifice reveals either a narrow opening or, more commonly, a closed opening represented only by a small depression in the center of the raised valve structure. In contrast, the cecocolic junction is often open to some degree and is flattened. In some cases the ileocolic orifice is difficult to identify either because it also is both flattened and closed or because it is tucked behind a fold. In the former instance, the ileocolic valve may be correctly identified after observation of intestinal contents issuing from the valve. It may be necessary to suction pooled fluid from the area at various times during the procedure to improve visualization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree