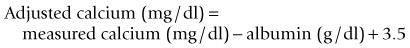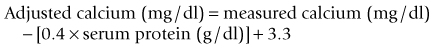8 Endocrine, Metabolic, and Lipid Disorders
Calcium
Analysis
Calcium can be measured in serum (preferred), heparinized plasma, and urine by photometric assays that use colorimetric reactions or potentiometry using ion-specific calcium electrodes. Oxalate, citrate, and ethylenediaminetetraacetic acid (EDTA) anticoagulants should not be used, because calcium is bound to these chemicals and becomes unavailable for analysis. Most automated and in-house serum chemistry analyzers measure total serum calcium concentration, which consists of biologically active, ionized calcium (55%), protein-bound calcium (35%), and calcium complexes (10%). In dogs, alterations in the plasma protein concentration may alter the total serum calcium concentration, yet the ionized calcium levels remain normal. Simple quantitative changes in the albumin and total plasma proteins do not cause hypocalcemia or hypercalcemia in dogs, even though the total serum calcium levels may “appear” low or high on the biochemistry panel. The following formulas can be used to determine the corrected total serum calcium concentration22:
The formula based on albumin is preferred because of the stronger relationship between serum albumin and total calcium concentrations. The formulas should not be used in dogs less than 24 weeks of age because high values may be obtained, nor are they used in cats because there is no linear relationship between serum total calcium and serum albumin and total protein concentrations in cats.11 These formulas yield a rough estimate of the corrected total serum calcium concentration and were developed without verification by serum ionized calcium measurements. Unfortunately, the correlation between “corrected” serum calcium concentration and ionized calcium concentration is weak,24 suggesting that corrected total serum calcium concentrations may not be reliable indicators of calcium homeostasis.
Artifacts
Serum Ca may be falsely decreased by increased bilirubin concentrations or laboratory error. Serum Ca may be falsely increased by laboratory error, dehydration (mild increase), and lipemia (see Chapter 1).
Causes of Hypercalcemia
In dogs, nonparathyroid malignancy (i.e., humoral hypercalcemia of malignancy [HHM]), most notably lymphosarcoma, is the most common cause of hypercalcemia (Box 8-1). Other hemolymphatic malignant tumors (i.e., lymphocytic leukemia, multiple myeloma, myeloproliferative diseases), anal sac apocrine gland carcinoma, and soft tissue tumors metastasizing to bone (e.g., mammary gland adenocarcinoma) may also cause hypercalcemia. Less frequent causes include primary hyperparathyroidism, chronic renal failure, hypoadrenocorticism, and hypervitaminosis D (i.e., cholecalciferol rodenticide toxicity, ingestion of toxic plants containing glycosides of calcitriol). In the cat, idiopathic hypercalcemia, hypercalcemia of malignancy (especially lymphoma and squamous cell carcinoma), chronic renal failure, and primary hyperparathyroidism are the most common diagnoses. Calcium oxalate urolithiasis and consumption of acidifying diets are commonly identified in cats with hypercalcemia; however, their role, if any, in causing hypercalcemia is unknown.
Box 8-1 Causes of Hypercalcemia in Dogs and Cats
Hypercalcemia of malignancy (very common)
Carcinoma (squamous cell, mammary, bronchogenic, prostate, thyroid, nasal cavity)
Primary hyperparathyroidism (uncommon but important)
Hypoadrenocorticism (uncommon but important)
Chronic and acute renal failure (common)
Hypervitaminosis D (uncommon but important)
Plants containing glycosides of calcitriol (e.g., jasmine)
Idiopathic hypercalcemia of cats (common)
Excessive calcium supplementation
Excessive oral phosphate buffers
Doronex ointment (calcipotriene)
Young dog (<6 months), large or giant breed
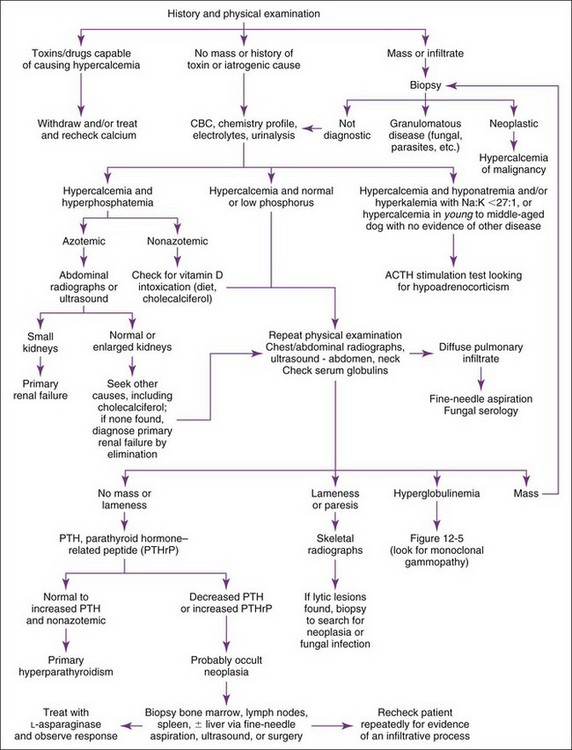
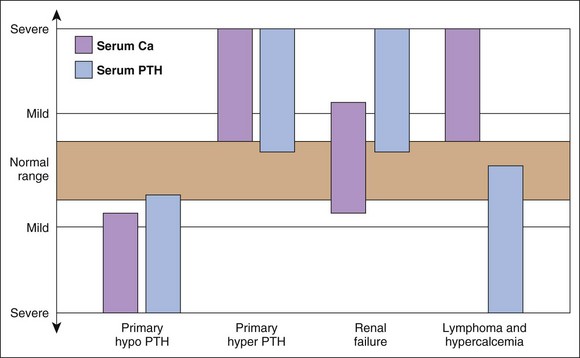
Hypercalcemia should always be reconfirmed, preferably from a nonlipemic blood sample obtained from the dog or cat after a 12-hour fast, before embarking on an extensive diagnostic evaluation. Results of a complete blood count (CBC), serum biochemistry panel, and urinalysis, in conjunction with the history and physical examination findings often provide clues to the diagnosis (Figure 8-1). Special attention should be paid to the serum electrolytes and renal parameters. Hypoadrenocorticism-induced hypercalcemia occurs in conjunction with mineralocorticoid deficiency; hyponatremia, hyperkalemia, and prerenal azotemia should usually be present. The serum phosphorus concentration is in the lower half of the normal range or low with HHM and primary hyperparathyroidism (Figure 8-2). If the serum phosphorus concentration is increased and renal function is normal, hypervitaminosis D or bone osteolysis from metastatic or primary bone neoplasia are the primary differentials. Measurement of serum ionized calcium concentration may help identify dogs and cats with renal failure–induced hypercalcemia; serum ionized calcium concentrations are typically normal or decreased in renal failure and increased in hypercalcemia caused by other disorders.
Sternal and hilar lymphadenopathy is common with lymphoma-induced hypercalcemia and can be readily identified with thoracic radiographs. Radiographs of the thorax and abdomen can also be used to evaluate bones; discrete lytic lesions in the vertebrae or long bones suggest multiple myeloma. Hyperproteinemia, proteinuria, and plasma cell infiltration in the bone marrow suggest multiple myeloma (see also Ehrlichia, Chapter 15). Cytologic evaluation of peripheral lymph node, bone marrow, and splenic aspirates can be helpful in identifying lymphoma; involvement of the peripheral lymph nodes or spleen by lymphoma can be present without causing their enlargement. Ideally the largest lymph node should be evaluated. Normal lymph node, bone marrow, and splenic aspirates do not rule out lymphoma.
Measurement of serum ionized calcium, PTH, and PTHrP from the same blood sample is helpful in differentiating primary hyperparathyroidism from HHM (Figure 8-3). Excessive secretion of biologically active PTHrP plays a central role in the pathogenesis of hypercalcemia in most forms of HHM. Increased serum ionized calcium concentration, detectable serum PTHrP concentration, and nondetectable serum PTH concentration is diagnostic for HHM. Lymphoma is the most common cause for detectable PTHrP concentrations, but other tumors, including apocrine gland adenocarcinoma and various carcinomas (e.g., mammary gland, squamous cell, bronchogenic), can also cause hypercalcemia by this mechanism. In contrast, increased serum ionized calcium, normal-to-increased serum PTH, and nondetectable PTHrP concentrations are diagnostic of primary hyperparathyroidism. Ultrasonographic examination of the thyro-parathyroid complex may reveal enlargement of one or more parathyroid glands. Most parathyroid adenomas measure 4 to 8 mm in diameter, although some can exceed 2 cm.10 In contrast, the parathyroid glands will be small (<2 mm in diameter) or undetectable with HHM. Evaluation of the change in serum calcium concentration after l-asparaginase administration should be considered for the patient with hypercalcemia of undetermined cause to rule out occult lymphoma. A marked reduction in serum calcium within 48 hours, usually into the normal range, is strongly suggestive of occult lymphoma.
Idiopathic hypercalcemia is an increasingly common diagnosis in young to middle-aged cats.23 Hypercalcemia is usually mild (<13 mg/dl) and asymptomatic. Serum phosphorus concentration and renal parameters are normal. The cause is unknown. Results of a complete diagnostic evaluation as described previously are unremarkable. Serum PTH concentrations are in the normal range or low; primary hyperparathyroidism has not been confirmed in any of these cats. Excessive serum PTHrP, 25-hydroxyvitamin D, or calcitriol concentrations have not been identified. Nephrocalcinosis and urolithiasis may develop, presumably secondary to increased urinary calcium excretion. Effective treatment has not been identified, primarily because the pathogenesis of this problem remains unknown. Serum calcium concentrations have decreased in some cats following a dietary change to a high-fiber diet, a diet designed for renal failure, or a diet designed to prevent calcium oxalate urolithiasis, or after prednisone treatment (initial dose, 5 mg q24hr) was initiated, but the response has been unpredictable and often short lived.
Causes of Hypocalcemia
The most common causes of hypocalcemia in dogs and cats are puerperal tetany, acute and chronic renal failure, malassimilation syndromes, and primary hypoparathyroidism (especially after thyroidectomy in hyperthyroid cats (Box 8-2).
Box 8-2 Causes of Hypocalcemia in Dogs and Cats
Primary hypoparathyroidism (uncommon but important)
Post-thyroidectomy (bilateral)
Puerperal tetany (eclampsia) (uncommon but important)
Acute and chronic renal failure (more common in acute)
Critical illness (e.g., systemic inflammatory response syndrome, sepsis)
Intestinal malabsorption syndromes
Hypoproteinemia or hypoalbuminemia (common)
Nutritional secondary hyperparathyroidism (rare)
Sodium bicarbonate administration
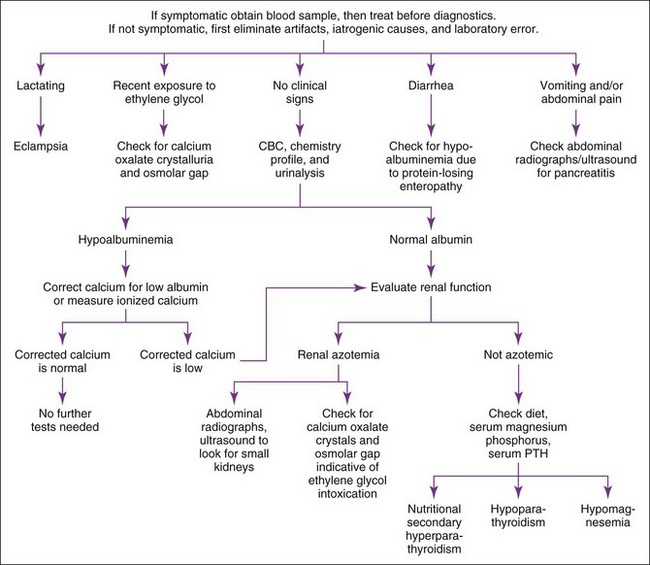
Hypocalcemia should be confirmed before initiating diagnostic tests to identify the cause. The list of differential diagnoses for hypocalcemia is relatively short, and the history, physical examination findings, CBC, serum biochemistry panel, and urinalysis usually provide the clues necessary to establish the diagnosis (Figure 8-4). Measurement of serum pancreatic lipase immunoreactivity and evaluation of an abdominal ultrasound should be done if pancreatitis is suspected (see Chapter 9). Primary hypoparathyroidism is the most likely diagnosis in the nonazotemic, nonlactating dog or cat with clinical signs of hypocalcemia. Documentation of a low baseline serum PTH concentration confirms this diagnosis (see Figure 8-3).
Phosphorus
Artifacts
Serum phosphorus may be falsely increased: by postprandial protein intake (mild change), hemolysis, hyperlipidemia, hyperproteinemia, and thrombocytosis. The effect is dependent on the methodology used to measure phosphorus. Serum phosphorus may be falsely decreased: by postprandial carbohydrate intake (mild change; see Chapter 1).
Causes of Hyperphosphatemia
Hyperphosphatemia can result from increased intestinal phosphate absorption, decreased phosphate excretion in urine, or a shift in phosphate from the intracellular to the extracellular compartment. Translocation of phosphate between the intracellular and extracellular compartments is similar to that of potassium. The most common cause for hyperphosphatemia is decreased renal excretion secondary to renal failure (Box 8-3). History, physical examination, and routine clinical pathologic assessment (e.g., CBC, serum biochemical panel, urinalysis) usually enable the clinician to identify the cause. Azotemic patients may require additional tests to distinguish between prerenal, renal, and postrenal azotemia (see Chapter 7). (Evaluation for primary hypoparathyroidism is discussed under Causes of Hypocalcemia earlier in this chapter.) Serum thyroxine concentration should be determined in the nonazotemic cat with signs of hyperthyroidism (i.e., weight loss, polyphagia, restlessness). Survey skeletal radiographs may identify osseous neoplasia.
Box 8-3 Causes of Altered Serum Phosphorus in Dogs and Cats
| HYPERPHOSPHATEMIA | HYPOPHOSPHATEMIA |
|---|---|
| Young growing animal (common) | Decreased intestinal absorption |
| Renal failure (common) | Decreased dietary intake |
| Prerenal and postrenal azotemia (common) | Malabsorption, steatorrhea |
| Endocrine | Vomiting, diarrhea |
| Primary hypoparathyroidism | Phosphate-binding antacids |
| Nutritional secondary hyperparathyroidism | Hypovitaminosis D |
| Hyperthyroidism (cats) | Increased urinary excretion |
| Acromegaly | Primary hyperparathyroidism (uncommon but important) |
| Hyperadrenocorticism | Humoral hypercalcemia of malignancy (important) |
| Hypervitaminosis D | Diabetic ketoacidosis |
| Excess supplementation | Eclampsia |
| Cholecalciferol rodenticides | Fanconi’s syndrome |
| Jasmine ingestion | Diuretics |
| Osteolytic bone lesions (neoplasia) | Transcellular shifts |
| Rhabdomyolysis (rare) | Hypothermia recovery |
| Trauma | Aggressive parenteral fluid therapy |
| Necrosis | Insulin administration, esp. for DKA (common and important) |
| Tumor cell lysis syndrome (rare) | Sodium bicarbonate administration |
| Metabolic acidosis | Parenteral glucose administration |
| Hemolysis | Hyperalimentation |
| Drugs—see text | Respiratory, metabolic acidosis |
| Iatrogenic | Laboratory error |
| Intravenous phosphorus supplementation | |
| Phosphate-containing enemas | |
| Diuretics | |
| Laboratory error |
Causes of Hypophosphatemia
Hypophosphatemia results from decreased phosphate absorption in the intestinal tract, increased urinary phosphate excretion, or a shift from the extracellular to the intracellular compartment. Hypophosphatemia is commonly associated with HHM (i.e., lymphosarcoma), primary hyperparathyroidism, and aggressive therapy for diabetic ketoacidosis (DKA; see Box 8-3).10 Translocation of phosphate between the intracellular and extracellular compartments is similar to that of potassium. Factors that promote a shift of potassium into the intracellular compartment (e.g., alkalosis, insulin, glucose infusion) also promote a similar shift in phosphate.
When evaluating hypophosphatemia, one should eliminate artifacts and iatrogenic causes first. Mild hypophosphatemia (i.e., >2.0 mg/dl) without hypercalcemia is often ignored unless the animal is ketoacidotic (see Causes of Hyperglycemia later in this chapter). If concurrent hypercalcemia is present, diagnostic evaluation for HHM and primary hyperparathyroidism should be performed (see Causes of Hypercalcemia earlier in this chapter).
Magnesium
Occasionally Indicated
Serum magnesium should be measured in dogs and cats with disorders and predisposing factors associated with hypomagnesemia and hypermagnesemia (Box 8-4), especially those with unexplained hypocalcemia (hypomagnesemia may inhibit the secretion and actions of PTH and promote calcium uptake into bone); hypokalemia resistant to parenteral supplementation (hypomagnesemia may cause potassium-losing nephropathy); DKA (hypomagnesemia may develop during the initial 24 hours of therapy); cardiac arrhythmias refractory to conventional therapy; and unexplained muscle weakness (including dysphagia and dyspnea), muscle fasciculations, or seizures.
Box 8-4 Causes of Altered Serum Magnesium in Dogs and Cats
Hypomagnesemia
Gastrointestinal Causes (common)
Drug-induced tubular injury (e.g., aminoglycosides, cisplatin)
Prolonged intravenous fluid therapy
Diabetic ketoacidosis (common)
Acute administration of insulin, glucose, amino acids
Peritoneal dialysis, hemodialysis
Causes of Hypomagnesemia
Hypomagnesemia results from decreased oral intake or gastrointestinal absorption of magnesium (e.g., small intestinal disease causing malabsorption), increased gastrointestinal loss (e.g., protracted vomiting, diarrhea), increased urinary magnesium excretion (e.g., interstitial nephritis, diuretics), or a shift of the cation from the extracellular to the intracellular compartment. The most common causes of clinically significant hypomagnesemia include disorders causing small intestinal malassimilation, renal disorders with high urine output, osmotic diuresis of DKA, and shift of potassium, phosphate, and magnesium from the extracellular to the intracellular compartment occurring within the first 24 hours of therapy for DKA (see Box 8-4). Magnesium is predominately an intracellular cation. Translocation of magnesium between the intracellular and extracellular compartments is similar to that of potassium. Factors that promote a shift of potassium into the intracellular compartment (e.g., alkalosis, insulin, glucose infusion) also promote a similar shift of magnesium. During therapy for DKA, serum magnesium concentration can severely decline (i.e., <1 mg/dl) because of the dilutional effects of fluid therapy and intracellular shift of magnesium after initiation of insulin and bicarbonate therapy.
Identifying hypomagnesemia is problematic because no simple, rapid, and accurate laboratory test is available to identify total body magnesium status. Serum total magnesium represents 1% of the body’s magnesium stores, and serum ionized magnesium represents 0.2% to 0.3% of total body magnesium stores. As a result, serum total and ionized magnesium concentrations do not always reflect total body magnesium status. A normal serum magnesium concentration can occur despite total body magnesium deficiency. A low serum magnesium concentration, however, supports a total body magnesium deficiency, especially when clinical signs or concurrent electrolyte abnormalities are consistent with hypomagnesemia. A serum-ionized magnesium concentration determined using an ion-selective electrode more accurately assesses total body magnesium content than measurement of serum total magnesium and is recommended.27 In animals with low serum magnesium concentration, a review of history, physical examination, and routine clinical pathologic assessment (e.g., CBC, serum biochemistry panel, urinalysis) usually provides clues to the underlying cause (see Box 8-4).
Causes of Hypermagnesemia
Hypermagnesemia occurs in dogs and cats with renal failure and postrenal obstruction (see Box 8-4) or is iatrogenically induced after excessive magnesium intake (e.g., IV administration, antacids, laxatives). Because the healthy kidney rapidly excretes excess magnesium, iatrogenically induced hypermagnesemia usually reflects underlying renal insufficiency. Hypermagnesemia has also been reported in cats with thoracic neoplasia and pleural effusion, although the mechanism involved with the development of hypermagnesemia in these cats is unknown.42 Measurement of serum magnesium concentration identifies hypermagnesemia. Evaluation of history, physical examination, and routine clinical pathologic assessments (e.g., CBC, serum biochemistry panel, urinalysis) usually identifies the cause of hypermagnesemia.
Parathyroid Hormone
Analysis
PTH concentration is measured in serum by immunoradiometric and chemiluminescent immunoassays.12 The sample should be centrifuged as soon as possible after clotting, frozen, and shipped frozen to the laboratory. Different PTH assays measure different parts of the PTH molecule and may give different results in the same patient. The “two-site” PTH assay system uses two different polyclonal antibodies to measure midregion and C-terminal 39 to 84 amino acids and N-terminal 1 to 34 amino acids of PTH simultaneously, is valid for measurement of dog and cat PTH, and is currently used by most veterinary laboratories.
Artifacts
Prolonged storage or transport at temperatures above freezing may produce erroneous results.
Drug Treatment That May Alter PTH Concentrations
Any drug therapy affecting serum calcium concentration can affect serum PTH concentration (see Drug Therapy That May Alter Serum Calcium Concentration earlier in this chapter). Drugs that decrease serum calcium may increase serum PTH concentration and vice versa.
Causes of Increased Serum PTH Concentration
Disorders that cause increased serum PTH concentration include primary hyperparathyroidism, secondary renal hyperparathyroidism, secondary nutritional hyperparathyroidism, secondary adrenal hyperparathyroidism31,39 and nonparathyroid causes of hypocalcemia (see Causes of Hypocalcemia earlier in this chapter). A midnormal or increased serum PTH concentration in a hypercalcemic patient with normal renal function strongly suggests primary hyperparathyroidism (see Figure 8-3).10 Animals with nonparathyroid-induced hypercalcemia have low to undetectable serum PTH concentrations. Serum PTH concentrations can be increased in animals with renal failure because of concurrent secondary renal hyperparathyroidism. Serum calcium concentration in these animals is usually in the normal range but may be decreased or, less commonly, increased with chronic end-stage renal failure. Serum ionized calcium concentration is usually normal in dogs and cats with secondary renal hyperparathyroidism. Serum calcium is in the low-normal or low range in animals with secondary nutritional hyperparathyroidism.
Causes of Decreased Serum PTH Concentration
Nondetectable serum PTH concentration in a hypocalcemic animal strongly suggests primary hypoparathyroidism. Patients with nonparathyroid-induced hypocalcemia should have normal or increased serum PTH concentrations. Nonparathyroid disorders causing hypercalcemia (see Causes of Hypercalcemia earlier in this chapter) also have low to undetectable serum PTH concentrations. The notable exception is hypercalcemia of chronic renal failure.
Glucose
Analysis
Glucose can be measured in whole blood, serum, or plasma (heparin, sodium fluoride, or EDTA). One can measure blood glucose in two main ways: (1) reagent strips and a reflectance meter (portable blood glucose meter [PBGM]) and (2) standard laboratory methods. The latter usually entail photometric assays that use glucose oxidase or hexokinase to initiate colorimetric reactions. PBGMs use whole blood, and are quick, simple, inexpensive, and readily available. Correlation between results obtained with PBGMs versus standard laboratory methods varies widely.5
Differences between these methods are more pronounced at higher glucose concentrations (i.e., >300 mg/dl). Blood glucose concentrations measured by PBGMs are usually lower than corresponding results with standard laboratory methods. The exception is a PBGM designed for use in dogs and cats (AlphaTrak; Abbott Laboratories, Abbott Park, IL), with which results may be higher or lower than corresponding results with standard laboratory methods.5
Causes of Hypoglycemia
Hypoglycemia is typically the result of excessive glucose use by normal (e.g., with hyperinsulinism) or neoplastic cells, impaired hepatic gluconeogenesis and glycogenolysis (e.g., hepatic insufficiency), a deficiency in diabetogenic hormones (e.g., hypocortisolism), inadequate dietary intake of glucose and other substrates required for hepatic gluconeogenesis (e.g., starvation in neonates), or a combination of these mechanisms (e.g., sepsis; Box 8-5). Iatrogenic hypoglycemia is a common problem with overzealous insulin administration to diabetics.
Box 8-5 Causes of Altered Blood Glucose in Dogs and Cats
| HYPOGLYCEMIA | HYPERGLYCEMIA |
|---|---|
| Beta cell tumor (insulinoma) (uncommon but important) | Diabetes mellitus (common) |
| Extrapancreatic neoplasia | “Stress,” aggression, excitement, fear (esp. cats) (common) |
| Hepatocellular carcinoma, hepatoma | Postprandial (propylene glycol, corn syrup, xylitol sweetener) |
| Leiomyosarcoma, leiomyoma | Hyperadrenocorticism |
| Hemangiosarcoma | Acromegaly (cat) |
| Hepatic insufficiency (uncommon but important) | Diestrus (bitch) |
| Portocaval shunts | Pheochromocytoma (dog) |
| Chronic fibrosis, cirrhosis | Pancreatitis |
| Sepsis (important) | Exocrine pancreatic neoplasia |
| Hypoadrenocorticism (uncommon but important) | Renal insufficiency |
| Hypopituitarism | Head trauma |
| Idiopathic hypoglycemia | Drug therapy |
| Neonatal hypoglycemia | Glucocorticoids |
| Juvenile hypoglycemia (esp. toy breeds) | Progestagens |
| “Hunting dog hypoglycemia” | Megestrol acetate |
| Renal failure (uncommon) | Thiazide diuretics |
| Exocrine pancreatic neoplasia | Parenteral nutrition solutions |
| Glycogen storage diseases (rare) | Dextrose-containing fluids (common) |
| Severe polycythemia | |
| Prolonged starvation | |
| Prolonged sample storage (common) | |
| Iatogenic | |
| Insulin therapy (common) | |
| Sulfonylurea therapy | |
| Ethanol | |
| Ethylene glycol | |
| Artifact | |
| Portable blood glucose meters | |
| Laboratory error |
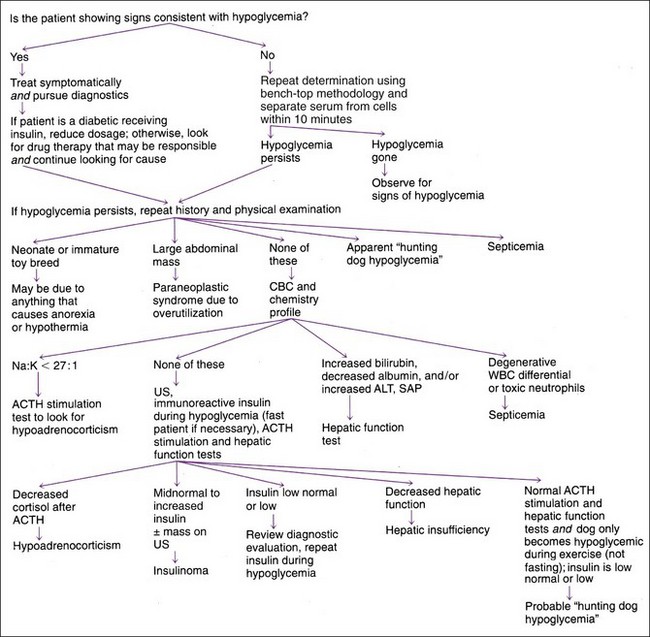
Hypoglycemia should always be confirmed before beginning diagnostics to identify the cause. Careful evaluation of history, physical findings, and routine clinical pathologic assessments (e.g., CBC, serum biochemical panel, urinalysis) usually provides clues to the underlying cause (Figure 8-5). Starvation, hepatic insufficiency (i.e., portosystemic shunt), sepsis, or idiopathic hypoglycemia are the usual causes in the puppy or kitten. In young adults, hepatic insufficiency, hypoadrenocorticism, or sepsis usually causes hypoglycemia. In older animals, hepatic insufficiency, beta cell neoplasia, extrapancreatic neoplasia, hypoadrenocorticism, and sepsis are the most common causes.
Hypoglycemia tends to be mild (i.e., >45 mg/dl) and is often an incidental finding in animals with hypoadrenocorticism and hepatic insufficiency. Additional alterations in clinical pathologic assessments (e.g., hyponatremia or hyperkalemia [hypoadrenocorticism], increased alanine aminotransferase [ALT] activity, hypoalbuminemia [hepatic insufficiency]) are usually present. An adrenocorticotropic hormone (ACTH) stimulation test or hepatic function test (see Chapter 9) may be required to confirm the diagnosis. Severe hypoglycemia (i.e., <35 mg/dl) may develop in neonates and juvenile kittens and puppies (especially toy breeds) and with sepsis, beta cell neoplasia, and extrapancreatic neoplasia (especially hepatic adenocarcinoma and leiomyosarcoma). Sepsis is readily identified by physical findings and CBC abnormalities, including a neutrophilic leukocytosis (typically >30,000/µl), a shift toward immaturity, neutropenia, and/or toxic neutrophils (see Chapter 4). Extrapancreatic neoplasia can usually be identified on physical examination or on abdominal or thoracic imaging. Dogs with beta cell neoplasia typically have normal physical examination results and lack abnormalities except hypoglycemia. Measurement of baseline serum insulin concentration when blood glucose is less than 60 mg/dl (preferably <50 mg/dl) is necessary to confirm a beta cell tumor (see next section on Insulin).
Causes of Hyperglycemia
Hyperglycemia results from insulin deficiency, impairment of insulin’s action in peripheral tissues (i.e., decreased glucose use), increased hepatic gluconeogenesis and glycogenolysis, or a combination of these (see Box 8-5). Iatrogenic causes of hyperglycemia include IV infusion of dextrose-containing fluids and parenteral nutritional solutions, and administration of diabetogenic drugs (e.g., glucocorticoids, megestrol acetate). Infusion of fluids containing as little as 2.5% dextrose may cause hyperglycemia, depending on infusion rate and concurrent disorders interfering with carbohydrate tolerance. Severe hyperglycemia (typically without glucosuria) occurs commonly in “stressed” cats, presumably from epinephrine secretion. Many diseases also cause carbohydrate intolerance and mild hyperglycemia, primarily by interfering with insulin action in peripheral tissues.
Hyperglycemia between 130 and 180 mg/dl does not cause glucosuria, polyuria, or polydipsia. Hyperglycemia in this range is clinically silent and often an unsuspected finding. If the patient with mild hyperglycemia (i.e., <180 mg/dl) also has polyuria-polydipsia, a disorder other than insulin-requiring diabetes mellitus should be sought. Mild hyperglycemia can occur in some animals shortly after consumption of foods containing large amounts of monosaccharides, disaccharides, or xylitol; in stressed cats and dogs; with administration of diabetogenic medications; in early stages of diabetes mellitus; and with disorders decreasing insulin’s effectiveness (see Box 8-5). A diagnostic evaluation for disorders causing insulin ineffectiveness is indicated if mild hyperglycemia (i.e., <180 mg/dl) persists in the fasted, unstressed animal in which diabetogenic medications have been discontinued.
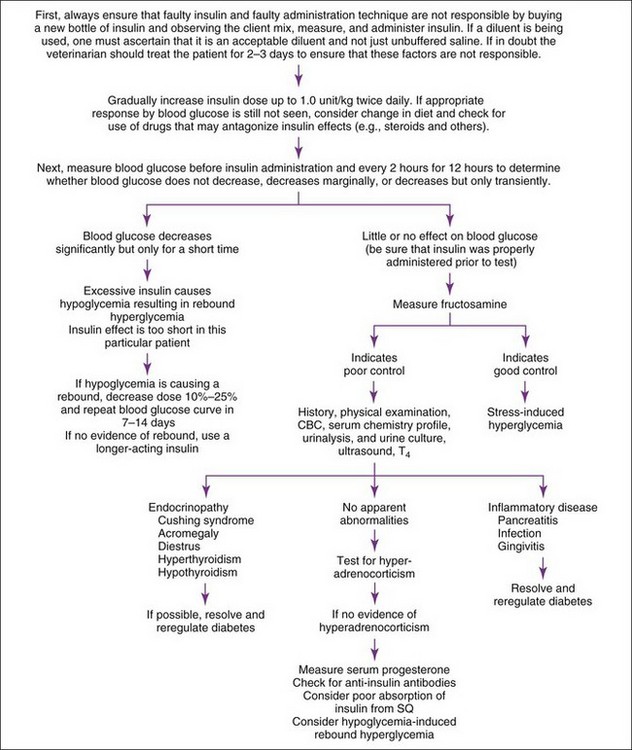
All hyperglycemic animals should be checked for glycosuria. Persistent hyperglycemia and glucosuria plus polyuria-polydipsia are diagnostic of diabetes mellitus. Persistence of diabetes mellitus depends, in part, on pathologic findings in the pancreatic islets, functional status of beta cells, and reversibility of concurrent diabetogenic diseases. Diabetogenic drugs (see Drug Therapy That May Alter Blood Glucose Concentration earlier in this section) should be stopped or adjusted; concurrent inflammatory, infectious, hormonal, or neoplastic disorders controlled or eliminated; diets designed for treatment of diabetes initiated; and effects on blood glucose re-evaluated in animals with suspected diabetes mellitus. Concurrent diabetogenic disorders are usually suggested from the history, physical examination, evaluation of routine clinical pathologic assessments (e.g., CBC, serum biochemical panel, urinalysis), and ease of glycemic regulation with insulin therapy. Concurrent diabetogenic disorders should always be suspected when glycemic control is difficult to attain with insulin therapy. The most common disorders interfering with glycemic regulation in dogs are obesity; chronic inflammation such as chronic pancreatitis, gingivitis, and inflammatory bowel disease; hyperadrenocorticism; diestrus; and concurrent infection. In cats, the most common are obesity, chronic inflammation (especially pancreatitis), hyperadrenocorticism, acromegaly, occult hyperthyroidism, and infection (Figure 8-6). Additional diagnostic tests (e.g., plasma lipase immunoreactivity, low-dose dexamethasone suppression test (LDDS), serum thyroxine concentration) may be necessary. Problems with insulin therapy itself (e.g., poor absorption, Somogyi response, short or prolonged duration of insulin effect) should also be considered in the animal with poorly regulated diabetes. Assessment of insulin therapy by measuring blood glucose concentrations at the time of and every 2 hours for 10 to 12 hours after insulin administration is the first diagnostic step in identifying problems with insulin therapy.
Insulin
Artifacts and Effect of Drugs on Insulin Concentration
Serum insulin concentration is increased for several hours after a meal. In addition, many drugs and disorders affecting blood glucose concentration also affect serum insulin concentration. Insulin derived from an insulin injection may be measured in blood samples for up to 24 hours after the insulin injection. Chronic exogenous insulin therapy in diabetics may cause circulating insulin-binding antibody formation, which can interfere with some RIAs and enzyme-linked immunosorbent assays (ELISAs), causing spuriously increased (i.e., >400 µU/ml) or nondetectable values.6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree