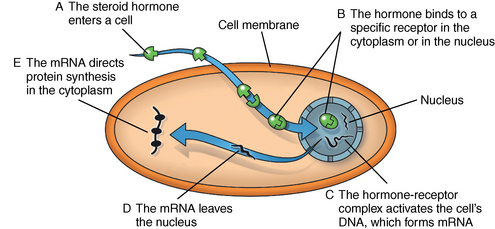
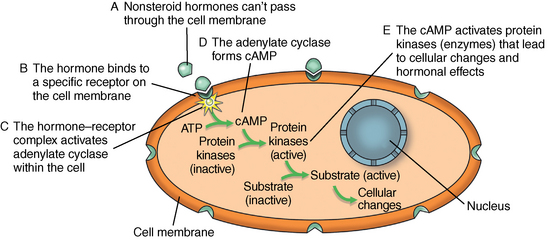
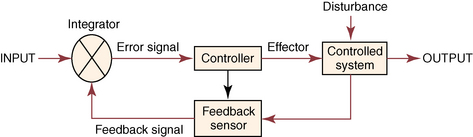
CHAPTER 7 Most horses spend the majority of their day eating, standing, and occasionally exercising. Physical activity can range from running up and down the fence line (in anticipation of the feed truck) to athletic training for a variety of competitive endeavors. Under resting conditions, the horse has a relatively easy job of maintaining the internal environment. However, work or exercise has the potential to be a major physiologic and homeostatic challenge that invokes an integrative response from multiple organ systems (McKeever, 2002). The response to exercise requires the transport of oxygen from the atmosphere to the cells in the working muscles, where it is utilized in metabolic pathways generating adenosine triphosphate (ATP) for fuel utilization (see Chapter 3). In reality, though, the adjustments to acute exercise require the coordination of several systems, including the respiratory, cardiovascular, muscular, integumentary, renal, hepatic, and digestive systems. Each tissue or organ called upon to facilitate activity must function in coordination with others in a variety of classic feedforward and feedback loops. To accomplish this, multiple layers of control exist in the body. The first muscle contractions associated with work will alter the mechanisms of autoregulation, causing changes in local control that are sensed peripherally. Longer or progressively more intense work causes systemwide alterations, which require integrated whole body responses that involve neural and endocrine mediation (Dickson, 1970; McKeever, 2002; McKeever and Hinchcliff, 1995; Rowell, 1993; Willmore and Costill, 1994). The most rapid mechanisms used to facilitate a coordinated response to exercise involve an integration of signals in the periphery that are communicated via the nervous system to the central command centers, where adjustments are made to the respiratory and cardiovascular systems (McKeever and Hinchcliff, 1995; Rowell, 1993; Willmore and Costill, 1994). Initial rapid adjustments in cardiopulmonary function at the onset of exercise can be accomplished primarily via a shift in the autonomic tone, with an initial withdrawal of parasympathetic tone followed by an increased sympathetic drive with increasing intensity of activity. However, as exercise progresses beyond a few seconds, more sophisticated mechanisms are called upon to finetune the initial response to the disturbance of exercise. Fine–tuning of the response to exercise that lasts longer than a few seconds is reliant on the regulation of several key variables governing the cardiopulmonary, vascular, and metabolic adjustments. Regulation allows the internal environment to be maintained within relatively narrow limits so as to maintain optimal function. This type of classic regulation involves a multiple system response where some variables are controlled so that those that are the most vital to the defense of the internal environment can be regulated. There is also a certain degree of redundancy in the systems which provides for more effective adjustments and tighter control. This type of integrative response is slower than a neural response because it requires communication between systems that relies on the secretion of substances by one tissue or organ that are transported remotely to other tissues or organs to evoke a response to adjust to the disturbance (McKeever and Hinchcliff, 1995; Rowell, 1993; Willmore and Costill, 1994). By definition, a hormone is a signaling molecule that regulates and coordinates physiologic and metabolic functions by acting on receptors located on or in target tissues (Dickson, 1970; Willmore and Costill, 1994). These chemical messengers can be endocrine, if the target tissues are remote from the secreting organ; paracrine, if the hormone is acting locally; or autocrine, if the hormone is acting on the tissue or cell it was secreted from. Most hormones fall into two major categories: (1) the steroid hormones and (2) the amine or peptide hormones. Steroid hormones include cortisol, aldosterone, and the reproductive hormones, testosterone, estrogen, progesterone. Steroid hormones have a classic ring structure and are lipid soluble, a characteristic that allows them to diffuse across cell membranes. Mechanistically, steroid hormones exert their effect through direct gene activation that occurs after diffusion into the cell (Figure 7-1). Once across the cell membrane, they bind to receptors in either the cytoplasm or in the nucleus. The complex formed by the steroid hormone and receptor induces the deoxyribonucleic acid (DNA) in the cell to produce messenger ribonucleic acid (mRNA), which, when it enters the cytoplasm, is transcribed, resulting in the production of proteins. The resultant protein (e.g., enzymes) leads to the physiologic action of the hormone on cellular function (Dickson, 1970; Willmore and Costill, 1994). The amine/peptide hormones are hydrophilic rather than lipophilic and, thus, cannot pass through the cell membrane. Instead, these hormones bind in a lock and key fashion to very specific receptors on the surface of the cell membrane and serve as first messengers (Figure 7-2). The hormone–receptor complex remains in the membrane but is still able to activate the enzyme adenylate cyclase within the cytoplasm of the cell. Activation starts a cascade that results in the active formation of the enzyme cyclic 3’, 5’ adenosine monophosphate (cAMP) through the combination of adenylate cyclase and ATP (Dickson, 1970; Willmore and Costill, 1994). The enzyme cAMP serves as a second messenger and activates specific inactive protein kinases, which cause the conversion of inactive substrates into active substrates that have the capability to induce changes in cellular form or cellular function (Dickson, 1970; Willmore and Costill, 1994). With the exception of the feedforward type of secretion of some hormones in immediate response to an emergency or stressor (i.e., onset of intense exercise), release of most hormones is part of a controlled negative feedback system that keeps a specific variable within narrow limits (Figure 7-3). The typical negative feedback system is composed of a variable that has an input or set point that is read or sensed by an integrator. For the system to be in a state of balance or homeostasis, the input and output signals must be the same. If the input variable is changed by a disturbance such as exercise, the integrator senses the mismatch and sends an error signal, usually via nerves, to the controller. The controller then alters the system via an effector. In many cases, the effector is a nervous signal. However, in some cases, the effector is another hormone. The effector causes a change in the controlled system, at which point a feedback sensor sends a signal to the integrator, which then determines if the input matches the output of the system. The more rapid responses used to maintain homeostasis are usually mediated by a neural control mechanism, with the less rapid responses usually mediated by endocrine mechanisms. As an example of the complexity of systemic control, maintenance of mean arterial pressure (MAP) around a narrow set point is critical to cardiovascular performance (Rowell, 1993). Multiple systems are controlled to ensure that MAP is sufficient to allow perfusion of all the working muscles as well as obligate tissues during exercise. At the onset of exercise, there is a decrease in total peripheral resistance (TPR) that results from the opening of blood vessels in the working muscles, which allows for the increase in blood flow to those vascular beds. However, there is an almost simultaneous increase in cardiac output (McKeever, 2002; Rowell, 1993). This rapid increase in central cardiac function comes about through the matching of the input and output signals sensed by volume and baroreceptors placed at strategic points in the cardiovascular system. Matching of the input and output signals (see Figure 7–3) by the vasomotor center of the medulla (the integrator) results in an error signal via the autonomic nervous system (the controller). A simultaneous withdrawal of vagal tone and increase in sympathetic drive results in the local release of norepinephrine (effector), which causes a rapid increase in heart rate and force of contraction that increase cardiac output (the controlled system) enough to maintain MAP. Higher-intensity exercise usually requires more dramatic responses, including a decrease in blood flow to nonobligate tissues such as the splanchnic vascular beds. The initial part of this response is mediated by neural mechanisms affecting the arterioles in those vascular beds. However, higher–intensity exercise also requires the added influence of endocrine effectors such as vasopressin and angiotensin II to cause sufficient vasoconstriction in nonobligate tissues to facilitate the rise in MAP needed for optimal cardiovascular function. Longer-term control of MAP can be affected by the duration of exercise which results in multiple strategies to control blood volume, defend cardiac filling pressure, cardiac output, and MAP (McKeever, 2002; Rowell, 1993). The pituitary, often referred to as the “master gland,” is found at the base of the brain and is divided into three lobes: (1) the anterior, (2) the intermediate, and (3) the posterior. Hormones of importance during exercise are produced and released by the anterior and posterior lobes. The pituitary is linked to the hypothalamus, an area of the brain with many very specific neural tracts that act as feedback sensors. The hypothalamus also acts as the integrator in the system exerting control over the pituitary through neural and endocrine mechanisms. The endocrine mechanisms include various releasing hormones, inhibitory hormones, and other biochemical substances. The central location of this hypothalamic–pituitary axis makes it ideal for centrally mediating the control of a wide variety of functions. For example, the hypothalamic–pituitary–adrenal axis (HPAA) is intricately involved in the stress response, fuel mobilization, and adaptation to exercise. The hypothalamic–pituitary–gonadal axis (HPGA) directly controls hormones in males and females linked to hypertrophy, macronutrient storage and mobilization, and reproductive status (Dickson, 1970; Willmore and Costill, 1994). Hormones produced by the anterior lobe of the pituitary include growth hormone or somatotropin (GH, ST), thyrotropin (thyroid–stimulating hormone, or TSH), adrenocorticotropin (ACTH), endorphins (EN), enkephalins, dynorphins, follicle–stimulating hormone (FSH), luteinizing hormone (LH), and prolactin (Dickson, 1970; Willmore and Costill, 1994). The first three play an important role in growth and development in the young animal and in metabolism in the adult animal. The endorphins, enkephalins, and dynorphins are opiate-like peptide hormones that modulate analgesia and immune function and interact with the neural pathways of the hypothalamic–pituitary axis to influence releasing and inhibiting hormones (McLaren et al., 1989; Mehl et al., 2000). FSH, LH, and prolactin are considered hormones essential for normal reproduction and lactation. Research in species other than horses has demonstrated that severe or prolonged exertion can alter their release and, thus, normal reproductive cycles (Dickson, 1970; Willmore and Costill, 1994). Furthermore, prolactin appears to play an important role in the response to severe stress, interacting with many of the metabolic hormones. Though FSH and LH are technically anterior pituitary hormones, their function will be discussed later in the chapter in relation to the gonadal hormones which they influence. GH affects all the cells in the body stimulating development and growth in younger animals. In mature animals, GH plays a vital role in muscle metabolism through its effects on protein synthesis and its role in fat and carbohydrate utilization (Dickson, 1970; Willmore and Costill, 1994). The importance of GH in maintenance of normal physiologic function and its possible role in slowing, or possibly even reversing, the effects of aging can be seen in some younger adult humans, where GH deficiency results in changes in appearance, decreased lean body mass, impaired immune function, and other “sequelae” of aging. Treatment of these individuals with recombinant human GH (hGH) results in increased lean body mass, decreased body fat, and increased muscle mass (Murray et al., 2001; Yarasheski, 1994). Chronic hGH administration also appears to increase strength and the ability to perform a battery of weight-lifting exercises in older male humans. These effects have led to increased doping with hGH in human athletes, partly because of improved repair and recovery as well as lack of valid tests for exogenous hGH until recently. Although there have been no reported effects of GH on aerobic capacity, the increase in muscle mass and strength have been shown to benefit the quality of life in humans by increasing the ability to do daily tasks such as maintaining balance while walking and climbing stairs. Those human experiments served as part of the justification for several recent studies of the efficacy of recombinant GH treatment in preventing or retarding functional decline in geriatric humans (Murray et al., 2001; Yarasheski, 1994) and in geriatric horses (Horohov et al., 1999; Malinowski et al., 1997; McKeever et al., 1997). Researchers conducting equine studies have asked “quality of life” questions similar to those posed in experiments using older humans. Those studies demonstrated that GH therapy increases nitrogen retention and improves appearance in geriatric horses, but they did not demonstrate any effect of chronic GH administration on body weight or the dimensions of several key muscles measured using ultrasonography (Malinowski et al., 1997). Functionally, chronic GH administration did not alter aerobic capacity or several commonly used indices of exercise performance, at least not in unfit old mares (McKeever et al., 1997). Furthermore, data from unfit geriatric horses indicated that exogenous GH did not alter lactate tolerance or cause an increase in maximal power that one would associate with an increase in muscular strength, which is a logical observation, since there was no evidence of an increase in muscle mass (McKeever et al., 1997). It is possible that the lack of significant findings in the horse may be due to insufficient dosage of GH, as was common in many of the early studies on GH and anabolic steroids in humans. Interestingly, studies of geriatric male humans have shown that recombinant GH therapy results in increased muscle mass and, in some cases, increased strength as measured in standardized tests performed with weightlifting equipment. However, although GH therapy does appear to increase strength in humans, there are no data to show that GH therapy alters maximal aerobic capacity or enhances endurance performance (Murray et al., 2001; Yarasheski, 1994). Some studies of younger horses have demonstrated that administration of GH prevents some of the bone demineralization that occurs in the first months of intense training. Other studies found that there was minimal or no therapeutic benefit of administering GH to prevent tendon or cartilage injuries or to promote wound healing (Gerard et al., 2001; Gerard et al., 2002). It has also been demonstrated that GH does not alter aerobic capacity or markers of performance in young (∼2 yr) Thoroughbreds (Gerard et al., 2001; Gerard et al., 2002). However, as with the studies of older horses, the experiments performed on younger animals had no way to evaluate the effects of GH administration on muscular strength. Data are clearly needed to determine if GH alters strength and power, with particular attention also paid to dose–response issues. Release of TSH is stimulated by thyroid-releasing hormone (TRH), which is produced in the hypothalamus (Dickson, 1970; Willmore and Costill, 1994). Studies of humans and other species have demonstrated that acute exercise elicits mixed effects on TSH release. The release of TSH appears to be linked to exercise intensity, as mild and moderate exertions do not appear to have an effect on TSH release. However, there appears to be a threshold for stimulating TSH release, as plasma concentrations of this hormone increase with work intensity only when exercise intensity exceeds 50% of maximal oxygen uptake ( The release of ACTH is stimulated by corticotropin–releasing hormone (CRH), which is secreted by the hypothalamus (Dickson, 1970; Willmore and Costill, 1994). A number of published papers have demonstrated that exercise causes an increase in ACTH in the horse; however, most of those only report postexercise, rather than during-exercise, values (Elsaesser et al., 2001; McCarthy et al., 1991). The major conclusion of most exercise studies has been that both high-intensity and long-duration endurance exercises cause an increase in ACTH and subsequent increase in cortisol. If one looks at the role of ACTH and glucocorticoids in substrate mobilization and utilization during exertion, it is apparent that the ACTH or cortisol response is an appropriate and adaptive response to physical exertion, which itself is a stressor (Elsaesser et al., 2001; McCarthy et al., 1991; Caloni et al., 1999). In fact, mobilization of the “stress response” provides for remarkable regulation of homeostasis. As the intensity of exercise increases (and, hence, the threat to homeostasis increases), the activation of the HPAA exponentially increases to handle the stress of exercise (Nagata et al., 1999). The ACTH response appears to be highly correlated to the catecholamine and lactate responses to incremental exercise, all of which also increased in a curvilinear fashion (Nagata et al., 1999). When horses were exercised at 80% or 110% These peptides are released from the pituitary in response to stress, pain, or pleasure. Although some classify these substances as hormones, others classify them as neurotransmitters (McLaren et al., 1989; Mehl et al., 2000). Nevertheless, these substances are naturally occurring opiat-like analgesics primarily derived from the pre–prohormone proopiomelanocortin (POMC) that may allow a horse to tolerate higher-intensity exercise (Art et al., 1994; Caloni et al., 1999; Li and Chen, 1987; McCarthy et al., 1991; McLaren et al., 1989; Mehl et al., 2000). The endorphins, enkephalins, and dynorphins play a role in the response to physiologic and psychological stress and appear to modulate pain perception as well as immune responses (McLaren et al., 1989; Mehl et al., 2000). Given that an overload of duration, intensity, or resistance is needed for exercise training to elicit an adaptive response, the endorphins may play an important role in allowing a horse to better tolerate the progressive increases in exercise intensities or longer durations needed to improve fitness and performance. Mehl and coworkers (McLaren et al., 1989; Mehl et al., 2000) have demonstrated that a threshold exists for evoking an increase in plasma beta (β)-endorphin concentration. This threshold appears to correspond to approximately 60% Hormones released from the posterior lobe of the pituitary, which technically comprises neural tissue rather than glandular tissue, include arginine vasopressin (AVP) and oxytocin. These two protein hormones are actually produced in the hypothalamus by specialized bundles of nerves (Dickson, 1970; Willmore and Costill, 1994). Arginine vasopressin is synthesized in cells of supraoptic and paraventricular nuclei and stored in vesicles in the nerve endings located in the posterior pituitary. Oxytocin causes smooth muscle contraction in the epididymis of males and the uterus of females, and it also acts on mammary tissue causing milk let-down in lactating mares. However, oxytocin’s role in the response to exercise is unclear. Arginine vasopressin (AVP), also known as antidiuretic hormone (ADH), is associated with acute as well as chronic defense of blood pressure, plasma volume, and fluid and electrolyte balance (Dickson, 1970; McKeever, 2002; McKeever and Hinchcliff, 1995; Wade, 1984; Zambraski, 1990). The physiologic actions of AVP include vasoconstriction and decreased free water clearance (McKeever and Hinchcliff, 1995; Wade, 1984; Willmore and Costill, 1994; Zambraski, 1990). AVP also plays a major role in the short-term and long-term control of cardiovascular function during and following exercise (Dickson, 1970; Willmore and Costill, 1994). Additionally, AVP is a critical stress hormone and serves as a secretagogue of ACTH. In fact, during acute exercise and in response to other noxious acute stressors (i.e., surgery, shock), AVP appears to be an even more potent stimulus for ACTH secretion than is CRH (Schmidtt et al., 1996). The mechanism for the release of AVP primarily involves osmoreceptors in the supraoptic and paraventricular nuclei of the hypothalamus and cardiopulmonary baroreceptors in the atria of the heart (Dickson, 1970; McKeever and Hinchcliff, 1995; Wade, 1984; Zambraski, 1990). Data from rats and other species have shown that a very small 1% to 2% decrease in cell volume in the hypothalamus or change in extracellular osmolality of 2 to 4 milliosmole per kilogram (mOsm/kg) will stimulate secretion of AVP and intake of water (Wade, 1984). Exercise causes an increase in plasma AVP that is correlated with both duration and intensity (Convertino, 1991; Convertino et al., 1983; Wade, 1984). Comparative data show that AVP is secreted during exercise in concentrations well above the threshold level associated with its antidiuretic effects, suggesting that its extrarenal actions are more important during acute exercise (McKeever and Hinchcliff, 1995; McKeever et al., 1991a; McKeever et al., 1993; McKeever et al., 1991; McKeever et al., 1992b; Wade, 1984). Extrarenal actions include AVP’s action as a powerful vasoconstrictor, an ACTH stimulus, and an important component in the control of blood pressure during exercise and its action on splenic blood vessels to prevent resequestration of the splenic reserve in the horse (Davies and Withrington, 1973; McKeever and Hinchcliff, 1995). Interestingly, some studies suggest that drinking water, especially cold hypotonic water, during exercise may suppress AVP and thirst, leading to dehydration (Zambraski, 1990). However, sustained elevations in AVP stimulate thirst and drinking water after exercise, cause a decrease in free water clearance by the kidneys, and may influence the uptake of sodium and water from the colon (McKeever and Hinchcliff, 1995; Zambraski, 1990). In exercising horses, plasma AVP concentration was recently reported to increase from approximately 4.0 picogram per milliliter (pg/mL) at rest to about 95 pg/mL at a speed of 10 meters per second (m/s). It was also reported that the relationship between AVP concentration and exercise intensity was curvilinear and did not plateau at speeds producing maximal heart rate (McKeever et al., 1992b). Another paper reported that AVP increases during steady-state submaximal exercise in horses without a change in free water clearance (McKeever et al., 1991a). However, the increase does not become significant until between 20 and 40 minutes of exertion (McKeever et al., 1991a). Two possible explanations have been given for the delay in AVP secretion in submaximally exercised horses (McKeever et al., 1991a). First, there appears to be a suppression of AVP secretion due to the volume overload sensed by the neural pathways associated with the atrial baroreceptors and the hypothalamus (McKeever and Hinchcliff, 1995; McKeever et al., 1991a). Second, AVP release is inhibited by the increase in atrial natriuretic peptide (ANP) concentrations at the beginning of exercise (McKeever and Hinchcliff, 1995; McKeever et al., 1991). Nevertheless, an increase in AVP concentration has been seen with prolonged exercise that appears to be related to sweat losses and decreases in body water that altered plasma osmolality and blood pressure (McKeever and Hinchcliff, 1995; McKeever et al., 1991; McKeever et al., 1992b). Studies of humans have demonstrated that training alters the slope of the AVP response to acute exercise, suggesting a change in the sensitivity to the exercise challenge (Freund et al., 1991; Convertino et al., 1983; Convertino, 1991). No studies on the effect of training on the AVP response to acute exertion in the horse have been published. The thyroid is located in the neck close to the larynx region. It plays a major role in the control of the basal metabolic rate, which has led some to refer to it as the “body’s thermostat” (Dickson, 1970; Willmore and Costill, 1994). The two iodine–containing hormones produced by the thyroid, triiodothyronine (T3) and thyroxine (T4), act on all cells in the body affecting metabolic rate and, subsequently, energy metabolism. The cells of the thyroid have three major actions when it comes to the synthesis and secretion of T3 and T4: (1) collection and transport of iodine, (2) synthesis and secretion of the glycoprotein thyroglobulin into the intracellular colloid, and (3) removal of T3 and T4 from thyroglobulin and secretion into the bloodstream. Thyroid hormones circulate in plasma in both a free form and a protein-bound form, with the bound form accounting for 99.98% of the circulating hormone and the unbound form being the active hormone able to influence cellular metabolism. The thyroid also produces calcitonin, an important hormone in the control of calcium metabolism with potent effects on bone mineral density (Dickson, 1970; Willmore and Costill, 1994). The release of these hormones is stimulated by TSH which, as previously mentioned, is released during exercise. As with TSH, the release of T3 and T4 is associated both with the intensity and duration of exercise in humans and horses (Gonzalez et al., 1998; Willmore and Costill, 1994). Irvine (1967) demonstrated that training increases both the secretion rate of T3 and T4 by approximately 65%. This would indirectly support the suggestion that TSH, which increases with training in humans and other species, is also increased with training in the horse. Training also increases the turnover rate of the thyroid hormones, though not necessarily in a clinically relevant manner that would suggest hyperthyroid function (Willmore and Costill, 1994). In addition to its control of metabolic rate, the thyroid is vital to calcium homeostasis. For this function, the thyroid synthesizes and produces calcitonin (Dickson, 1970; Willmore and Costill, 1994). Calcitonin plays a role in calcium homeostasis either by inhibiting osteoclast activity in bone or through its action on the kidney tubules to cause an increase in calcium loss by actively inhibiting tubular reabsorption. New bone is formed by osteoblasts and reabsorbed by osteoclasts. In young growing horses, both osteoclasts and osteoblasts are active; however, the activity of osteoblasts outpaces the activity of osteoclasts, allowing for bone growth and development (Dickson, 1970). To this end, calcitonin appears to be more important in the young growing animal through its inhibitory action on the osteoclasts. Calcitonin is also important in the healing of fractures (Dickson, 1970). Chiba and coworkers (Chiba et al., 2000) documented substantially elevated plasma calcitonin concentrations in racehorses with various fractures. Studies have also demonstrated that there is a period of bone demineralization in young Thoroughbreds in the first few months of training. Growing and adult humans who exercise regularly have increased bone density (Willmore and Costill, 1994). Although a great deal of work has examined markers of bone turnover, more data are needed to determine if acute and chronic exertion affect plasma calcitonin concentrations (Chiba et al., 2000; Geor et al., 1995; Murray et al., 2001). The parathyroid glands, which are located close to the thyroid gland, regulate calcium homeostasis by synthesizing and secreting parathyroid hormone or parathormone (PTH) in response to a change in plasma calcium (Ca++) concentration (Dickson, 1970; Willmore and Costill, 1994). PTH has receptors in the intestinal tract, in the osteoclasts in bones, and the tubules of the kidneys. The action of PTH to stimulate osteoclast activity is antagonistic to calcitonin’s inhibitory action. The resultant effect is a net bone reabsorption and the release of calcium and phosphate into the bloodstream. Actions on bone are relatively slower compared with PTH’s ability to alter both the uptake and excretion sides of the homeostatic balance equation by acting on the intestine and the kidney tubule (Dickson, 1970). PTH has a profound ability to enhance the enzymatic pathway that mediates increases in intestinal absorption of calcium and phosphate (Dickson, 1970; Willmore and Costill, 1994). At the same time, PTH can act on the kidney tubules where it enhances calcium reabsorption and phosphate excretion (Dickson, 1970; Willmore and Costill, 1994). As with calcitonin, most equine research has focused on effects of repeated exercise on markers of bone turnover (Chiba et al., 2000; Geor et al., 1995; Murray et al., 2001). It is well recognized that nutritional influences can alter calcium and phosphate balance and bone metabolism. Thus, more work is needed to determine if exercise intensity, duration, or both are factors affecting PTH concentrations. Furthermore, data are needed to determine if exercise alters synthesis and secretion rates, receptor numbers and sensitivity, and general interplay of PTH and calcitonin in bone metabolism. The adrenal glands are multilayered organs that sit atop the kidneys. Functionally, the primary layers are the adrenal medulla and the adrenal cortex (Dickson, 1970). The medullary portion of the adrenal produces the catecholamines, epinephrine (E), and norepinephrine (NE), which have the potential to affect most cells in the body (Dickson, 1970; Willmore and Costill, 1994). In general, E potentiates the response to exercise causing profound effects on central cardiovascular and respiratory function. It can cause increases in muscle blood flow and can mobilize glycogen and free fatty acids to fuel exertion. The adrenal cortex contains three specialized zones: (1) the zona glomerulosa, (2) the zona fasciculata, and (3) the zona reticularis. The cortex produces a multitude of steroid hormones that fall into three major categories: (1) the mineralocorticoids (aldosterone), (2) the glucocorticoids (cortisol), and (3) the onadocorticoids (androgens and estrogens) (Dickson, 1970; Willmore and Costill, 1994). The release of the catecholamines has its origin in the fight-or-flight response. This “stress” response involves the local release of NE from the sympathetic nerve endings (acting as a neurotransmitter) and a systemic release of E and NE from the adrenal medulla. Receptors for the catecholamines are specialized and are divided into two primary categories, referred to as alpha (α)- and beta (β)-adrenergic receptors. These two major categories are divided into subcategories, namely, α1, α2, β1, β2, and β3 receptors (McKeever, 1993). Sympathetic nervous activity increases with intensity and duration of exercise, but notable changes in plasma catecholamine concentrations are not always apparent below 50% of maximal aerobic capacity (McKeever, 1993). Plasma catecholamine levels increase in a curvilinear fashion with increasing exercise intensity and are highly correlated with plasma lactate concentrations (Gonzalez et al., 1998; Jimenez et al., 1993; Nagata et al., 1999). The measurable increase appears to coincide with the intensity at which one would expect complete parasympathetic withdrawal (Freund et al., 1991; Jimenez et al., 1993; Nagata et al., 1999). These increases in the catecholamines, particularly E, enhance the increase in heart rate (a chronotropic effect), force of cardiac contraction (an inotropic effect), and cardiac output (McKeever, 1993; McKeever and Hinchcliff, 1995). The catecholamines also play a role inducing splenic contraction and the delivery of 6 to 12 liters (L) of blood into the central circulation at the onset of exercise in horses (McKeever, 1993; Persson, 1967). Even with this mobilization of reserve blood volume, the demands of exercise may exceed central cardiovascular capacity in the horse. Thus, during high-intensity exercise or long-duration exercise, the catecholamines contribute to the vasoconstriction that decreases blood flow to nonobligate tissues (McKeever, 1993; McKeever and Hinchcliff, 1995; Rowell, 1993). The catecholamines are also linked with the respiratory response to exercise (Jimenez et al., 1993; McKeever, 1993; McKeever and Hinchcliff, 1995; Plummer et al., 1991; Sexton and Erickson, 1986; Snow and Rose, 1981). At the onset of exercise the β2-adrenergic receptor action relaxes tracheal and bronchial smooth muscle, increasing airway diameter, decreasing airway resistance, and, thus, facilitating movement of greater amounts of air into and out of the lungs. Although the sympathetic system is not directly responsible for the control of ventilation during exercise, the increase in ventilatory drive associated with activation of the motor cortex can be enhanced during exercise by catecholamine secretion and augmentation of the sensitivity of chemoreceptors in the carotid bodies. During high-intensity exercise, ventilation may be further affected by catecholamine release as part of the stress response (McKeever, 1993). The catecholamines also have major effects on metabolic pathways associated with substrate utilization during exercise (McKeever, 1993; Willmore and Costill, 1994). Increases in sympathetic activity result in an increase in hormone-sensitive lipase and, subsequently, an increase in circulating free fatty acids. Exercise-induced and anticipatory secretion of catecholamines also cause an increase in glycogen breakdown resulting in increased glucose availability. It has been suggested that one way that warmup exercises benefit the athlete is through activation of these metabolic pathways, which allows for elevated blood concentrations of glucose and free fatty acids prior to race-induced increases in utilization, thus facilitating delivery of substrate to tissues without a significant lag time (McKeever, 1993; Willmore and Costill, 1994). The above-mentioned responses have been well documented during acute exercise. Recent data also suggest that exercise training alters adrenergic receptor numbers and sensitivity in selected tissues. For example, β-adrenergic receptor numbers are unchanged in cardiac muscle with training, whereas α-adrenergic and muscarinic receptor numbers are reduced. Both β-adrenergic receptor numbers and sensitivity are increased in skeletal muscle and in vascular and bronchial smooth muscle (McKeever, 1993; Willmore and Costill, 1994). Changes in receptor number and sensitivity with training may be important with respect to adjustment of drug doses for the animal that has been trained extensively versus an animal that is at the beginning of a training program or one that is being reconditioned following removal from training (McKeever, 1993; Willmore and Costill, 1994). Some sources suggest that more than 30 structurally distinct steroid hormones are secreted by the adrenal cortex. Secretion of the mineralocorticoid aldosterone and the glucocorticoid cortisol is, however, the most important to the physiologic response to exercise (Dickson, 1970; Willmore and Costill, 1994). Aldosterone plays an important role in electrolyte homeostasis, particularly sodium and potassium balance (Dickson, 1970; McKeever, 1998; McKeever and Hinchcliff, 1995; Willmore and Costill, 1994). It is well recognized that aldosterone acts on the kidneys to enhance sodium (and chloride) reabsorption and potassium excretion. Aldosterone also acts on the intestines to facilitate the uptake of electrolytes and water. Aldosterone release can be stimulated by decreases in plasma [Na+] or by increases in plasma [H+], plasma [K+], plasma ACTH, by increased renin (McKeever and Hinchcliff, 1995; McKeever, 1998), or by both. However, the most potent of these stimuli is an increase in plasma [K+] (McKeever, 1998; McKeever and Hinchcliff, 1995). Studies of horses have attempted to identify factors that may stimulate the release of aldosterone during exercise (Cooley et al., 1994; McKeever et al., 1987; McKeever, 1998; McKeever et al., 1991a; McKeever et al., 1992b; McKeever et al., 1997; ). In one study, plasma [Na+] was not significantly affected by exercise and thus, a decrease in plasma [Na+] did not appear to have been the primary stimulus for aldosterone release (McKeever et al., 1991a). The primarily supported mechanism for the release of aldosterone appears to be a proportional increase in the plasma renin–angiotensin–aldosterone cascade, where an increase in renin results in the generation of angiotensin I and angiotensin II, with angiotensin II stimulating the production and release of aldosterone (Costill et al., 1976; Kosunen and Pakarinen, 1976; McKeever et al., 2000; Zambraski, 1990). The relationship between plasma aldosterone concentration and exercise intensity has been reported in horses running on a treadmill. Aldosterone increased from concentrations around 20 to 50 pg/mL at rest to almost 200 pg/mL at a speed of 10 m/s (Kokkonen et al., 2002; McKeever et al., 1992b). A linear relationship was found between exercise intensity and aldosterone concentration. However, unlike renin, aldosterone concentration did not reach a plateau at maximal heart rate (HRMAX). Another study found that during submaximal exercise, increases in plasma aldosterone concentration paralleled changes in renin but that the magnitude of the increase in renin (66%) was less than the relative increase (709%) in aldosterone concentration (McKeever et al., 1991a). The authors concluded that factors other than renin also affected the release of aldosterone in the horse (McKeever et al., 1991a). Of all the parameters reported, a significant increase in plasma [K+] may have served as the strongest stimulus for the release of aldosterone (McKeever and Hinchcliff, 1995). An increase in plasma [K+] as small as 0.3 milliequivalent per liter (mEq/L) can be sufficient to stimulate the secretion of aldosterone, independent of the renin–angiotensin cascade, through the conversion of cholesterol to pregnenolone or at a later step in the biosynthetic pathway (McKeever and Hinchcliff, 1995). This is consistent with the acute homeostatic requirements of the horse, since a major perturbation in electrolyte homeostasis observed during endurance exertion was an increase in plasma [K+] and not a drop in plasma [Na+] (McKeever et al., 1992b). As with humans, aldosterone has a minimal role in the acute response to exercise in horses. However, aldosterone concentration remains elevated for hours after exercise and may affect the long–term reabsorption of sodium and water (Convertino, 1991; Hyyppa et al., 1996; McKeever, et al., 2002b) by the kidneys and by the intestinal tract (Bridges and Rummel, 1984; Kokkonen et al., 2002) The major glucocorticoid secreted by the adrenal glands is cortisol. However, some cortisone, corticosterone, and deoxycorticosterone are also produced (Dickson, 1970; Willmore and Costill, 1994). Cortisol, cortisone, and corticosterone can be found in a ratio of 16:8:0.5 in the plasma of horses; therefore, most exercise studies have focused on cortisol (Dickson, 1970). Cortisol undergoes diurnal variation, with peak levels found in the early morning between 06:00 and 10:00 and lowest levels found in the late evening and overnight periods (Dickson, 1970; Willmore and Costill, 1994). Although glucocorticoids are typically, and correctly, referred to as “stress” hormones, they are also released under a multitude of normal situations not necessarily characterized as stressful. Thus, it is the appropriateness of their release and the magnitude of their release that would indicate whether a given physiologic or psychological disturbance (or stressor) can be classified as a mere perturbation or a true threat to homeostasis. Release of cortisol allows an individual to tolerate and adapt to challenges to homeostasis that occur in everyday life. To this end, the functional effects of cortisol fall into two major categories: (1) substrate mobilization and (2) immune modulation (Dickson, 1970; Willmore and Costill, 1994). During acute exercise, itself a distinct challenge to homeostasis and a unique stressor, cortisol stimulates substrate mobilization by enhancing gluconeogenesis, mobilizing free fatty acids through lipolysis, and increasing the availability of amino acids through protein degradation (Willmore and Costill, 1994). At the same time, cortisol release decreases glucose utilization by nonessential tissues to spare it for use by the central nervous system (Willmore and Costill, 1994). One could speculate that such an action could delay the onset of central fatigue that occurs during endurance exertion when blood glucose concentrations drop (Farris et al., 1998; McKeever, et al., 2002b). The increase in protein catabolism results in amino acid availability as a source of energy when glucose levels begin to drop. They are available after exercise to repair tissues and for the synthesis of enzymes involved in many cellular pathways. Cortisol’s modulation of immune function results from its actions as an antiinflammatory agent and suppressor of immune reactions (Dickson, 1970; Willmore and Costill, 1994). Many studies have demonstrated that cortisol is increased in the horse during a wide variety of exercise activities, from racing, to polo, to endurance rides (Caloni et al., 1999; Crandell et al., 1999; Horohov et al., 1999; Hyyppa, 2001; Snow and MacKenzie, 1977; Snow and Rose, 1981). The release of cortisol in the horse appears to be affected both by intensity and duration of exercise (Snow and MacKenzie, 1977; Snow and Rose, 1981). However, excessive increases in cortisol concentrations following exertion can be a marker of too much exercise. Prolonged cortisol recovery times as well as either inappropriately high or low plasma concentrations of cortisol may be a marker of overtraining in the horse. As mentioned above, postexertion effects of cortisol may elicit a permissive effect that is beneficial for training adaptation by preventing the immune system from eliciting an inflammatory and immune reaction to acute exercise (Horohov et al., 1999; Toutain, et al., 1995). Several studies on horses have followed cortisol levels for extended periods following exercise. Those experiments demonstrated that exercise caused a sixfold increase in adrenal cortisol secretion and a two- to three-fold increase in plasma cortisol concentration. Urinary cortisol concentrations also increased threefold with a return to baseline levels by 10 hours after exertion (Toutain, et al., 1995). The authors also noted a substantial increase in liver clearance of cortisol. Prior studies have suggested that peak postexercise cortisol concentrations are reached earlier in trained horses and that trained horses have a faster cortisol recovery time (Snow and MacKenzie, 1977; Snow and Rose, 1981). On average, peak cortisol levels were observed at about 30 minutes after exertion. Much of this enhanced recovery would be dependent on optimal training volume and rest to produce training adaptations. Still, improved training status may improve the potential to rapidly switch from a catabolic state to an anabolic state. The pancreas is a V–shaped organ that lies along the duodenum. Structurally, most of the pancreas is composed of acini, which function as exocrine cells secreting digestive enzymes and bicarbonate into the small intestine via the pancreatic duct. The endocrine function of the pancreas is mediated by the cells of the islets of Langerhans. These specialized cells are arranged as branching cords that are surrounded by a large network or plexus of capillaries. The cells of the islets are classified into three types: (1) the α-cells which produce glucagon, (2) the β-cells which produce insulin, and (3) the δ-cells which produce somatostatin (Dickson, 1970; Willmore and Costill, 1994). Of these hormones, the most important during exercise are insulin and glucagon because of their actions in the control of glucose metabolism. Insulin functions as part of the feedback system controlling blood glucose concentration (Dickson, 1970; Willmore and Costill, 1994). Insulin is primarily a glucose “storage” hormone because it facilitates glucose uptake by the cells, promotes glycogenesis, and inhibits gluconeogenesis (Dickson, 1970; Giraudet et al., 1994; Ralston, 1992; Willmore and Costill, 1994). As such, it is arguably the most potent anabolic hormone. At rest, insulin is the “key” that opens the cellular door to allow uptake of glucose. However, during exercise, the working muscles take up glucose without insulin. Thus, insulin is very important during the recovery from exercise when glycogen repletion is most active (Dickson, 1970; Davie et al., 1994; Davie et al., 1999; De La Corte et al., 1999; Willmore and Costill, 1994). The insulin response to acute exercise has been well documented with the horse which, like humans and other species, suppresses insulin during exercise (Duren et al., 1999; Geor et al., 2000; Snow and Rose, 1981). This suppression appears to have a threshold of 50% of
Endocrine and immune responses to exercise and training
Endocrine system and hormones
Major endocrine glands and hormones
Anterior pituitary hormones
Growth hormone
Thyrotropin
![]() O2max) in humans. This breakpoint is common to several hormonal systems, including the observed increases in catecholamines, adrenocorticotropic hormone (ACTH), cortisol, plasma renin activity (PRA), etc. (Freund et al., 1991; Jimenez et al., 1993; Nagata et al., 1999). Interestingly, exercise duration beyond 40 minutes appears to also cause an increase in TSH. This observed increase during longer steady-state exertion is similar to the response of other metabolic hormones and may be related to substrate mobilization and attempts to prevent the onset of central fatigue mechanisms. Data on the effects of acute exercise and training on TSH in equine athletes are lacking.
O2max) in humans. This breakpoint is common to several hormonal systems, including the observed increases in catecholamines, adrenocorticotropic hormone (ACTH), cortisol, plasma renin activity (PRA), etc. (Freund et al., 1991; Jimenez et al., 1993; Nagata et al., 1999). Interestingly, exercise duration beyond 40 minutes appears to also cause an increase in TSH. This observed increase during longer steady-state exertion is similar to the response of other metabolic hormones and may be related to substrate mobilization and attempts to prevent the onset of central fatigue mechanisms. Data on the effects of acute exercise and training on TSH in equine athletes are lacking.
Adrenocorticotropic hormone
![]() O2max, the ACTH response was rapid, and concentrations increased in a linear fashion until the end of the exercise test (Nagata et al., 1999). Postexercise concentrations fell rapidly, returning to baseline within 60 to 120 minutes (Nagata et al., 1999). These responses are similar to those reported for humans and other species (Nagata et al., 1999; Willmore and Costill, 1994). With chronic exposure to a stressor, as would happen during overtraining, regulation of the HPAA is challenging, as exhaustion may result from the increased allostatic load.
O2max, the ACTH response was rapid, and concentrations increased in a linear fashion until the end of the exercise test (Nagata et al., 1999). Postexercise concentrations fell rapidly, returning to baseline within 60 to 120 minutes (Nagata et al., 1999). These responses are similar to those reported for humans and other species (Nagata et al., 1999; Willmore and Costill, 1994). With chronic exposure to a stressor, as would happen during overtraining, regulation of the HPAA is challenging, as exhaustion may result from the increased allostatic load.
Endorphins, enkephalins, and dynorphins
![]() O2max. Interestingly, this is the same point where one sees a curvilinear increase in the catecholamines, plasma renin activity, plasma lactate concentration, and several other variables. This suggests a close interplay between these factors in the transition from low-intensity and primarily aerobic exercise to higher-intensity exercise with an increasing anaerobic component (Convertino et al., 1983; Freund et al., 1991; Jimenez et al., 1993; Nagata et al., 1999). Duration of exercise also appears to affect the magnitude of the endorphin response with greater plasma concentrations as horses approach fatigue (McLaren et al., 1989; Mehl et al., 2000). Training appears to alter the endorphin response to acute exertion with greater plasma concentrations observed in the postexertion peak occurring between 5 and 10 minutes after exertion (Malinowski et al., 2003).
O2max. Interestingly, this is the same point where one sees a curvilinear increase in the catecholamines, plasma renin activity, plasma lactate concentration, and several other variables. This suggests a close interplay between these factors in the transition from low-intensity and primarily aerobic exercise to higher-intensity exercise with an increasing anaerobic component (Convertino et al., 1983; Freund et al., 1991; Jimenez et al., 1993; Nagata et al., 1999). Duration of exercise also appears to affect the magnitude of the endorphin response with greater plasma concentrations as horses approach fatigue (McLaren et al., 1989; Mehl et al., 2000). Training appears to alter the endorphin response to acute exertion with greater plasma concentrations observed in the postexertion peak occurring between 5 and 10 minutes after exertion (Malinowski et al., 2003).
Posterior pituitary hormones
Arginine vasopressin
Thyroid
Triiodothyronine and thyroxine
Calcitonin
Parathyroid gland
Adrenals
Hormones produced by the adrenal medulla (catecholamines)
Primary hormones produced by the adrenal cortex
Aldosterone
Cortisol
Pancreas
Insulin
![]() O2max, which coincides with the increase in catecholamines seen during exercise (McKeever, 2002). Mechanistic studies have demonstrated the link between exercise-induced increases in sympathetic drive, particularly due to epinephrine’s inhibitory effects on insulin, and changes in insulin and glucagon secretion in the horse (Geor et al., 2000). Functionally, this allows the animal to increase gluconeogenesis to maintain blood glucose concentrations during exercise (Geor et al., 2000; Willmore and Costill, 1994). Suppression of insulin and maintenance of blood glucose concentration prevents the onset of central mechanisms of fatigue (Farris et al., 1998; McLaren et al., 1989). Much of the recent work on the insulin response to exertion has centered on the composition and timing of pre-exercise feeding (Crandell et al., 1999; Frape, 1988; Hyyppa et al., 1999; Pagan and Harris, 1999; Poso and Hyyppa, 1999). High-carbohydrate feeds are beneficial for optimal muscle glycogen synthesis to fuel exercise. However, the resultant increase in blood glucose seen after a horse eats a high-carbohydrate ration usually evokes an increase in insulin secretion. The goal of recent research has been to prevent this feed-induced spike in insulin that would tend to decrease blood glucose right before or during exercise (Williams et al., 2001a). This phenomenon has often been referred to as “rebound hypoglycemia” (Jentjens and Jeukendrup, 2002). Recent research in humans has suggested that rebound hypoglycemia may be influenced by the timing of carbohydrate intake and the intensity of the exercise but that any hypoglycemic effects may have little, if any, impact on performance (Moseley et al., 2003).
O2max, which coincides with the increase in catecholamines seen during exercise (McKeever, 2002). Mechanistic studies have demonstrated the link between exercise-induced increases in sympathetic drive, particularly due to epinephrine’s inhibitory effects on insulin, and changes in insulin and glucagon secretion in the horse (Geor et al., 2000). Functionally, this allows the animal to increase gluconeogenesis to maintain blood glucose concentrations during exercise (Geor et al., 2000; Willmore and Costill, 1994). Suppression of insulin and maintenance of blood glucose concentration prevents the onset of central mechanisms of fatigue (Farris et al., 1998; McLaren et al., 1989). Much of the recent work on the insulin response to exertion has centered on the composition and timing of pre-exercise feeding (Crandell et al., 1999; Frape, 1988; Hyyppa et al., 1999; Pagan and Harris, 1999; Poso and Hyyppa, 1999). High-carbohydrate feeds are beneficial for optimal muscle glycogen synthesis to fuel exercise. However, the resultant increase in blood glucose seen after a horse eats a high-carbohydrate ration usually evokes an increase in insulin secretion. The goal of recent research has been to prevent this feed-induced spike in insulin that would tend to decrease blood glucose right before or during exercise (Williams et al., 2001a). This phenomenon has often been referred to as “rebound hypoglycemia” (Jentjens and Jeukendrup, 2002). Recent research in humans has suggested that rebound hypoglycemia may be influenced by the timing of carbohydrate intake and the intensity of the exercise but that any hypoglycemic effects may have little, if any, impact on performance (Moseley et al., 2003).![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Endocrine and immune responses to exercise and training
Only gold members can continue reading. Log In or Register to continue