Embryo Transfer
The potential for production of even higher number of embryos per year is possible through the use of multiple ovulation and embryo transfer (MOET). Protocols for superstimulation (superovulation) have been reported by several authors.1–4 Commercial alpaca embryo transfer in Australia reports an average of 2.5 to 3 embryos per uterine flush, with a potential for production of up to 21 embryos per individual per year.5 Pregnancy rates following transfer of embryos vary from 40% to 70%.5–7
Finally, the embryo transfer technique enables the preservation of endangered species such as vicunas, guanacos, and some breeds of alpacas and llamas through the use of interspecies embryo transfer.6,7 Cryopreservation of embryos could increase the application of this technology even more for genetics improvement and preservation of genetic diversity.
History
Studies on reproductive biotechnologies (artificial insemination and embryo transfer) in alpacas and llamas were initiated in the early 1960s at La Raya Research Station, IVITA, San Marcos University, Puno, Peru. Novoa and Sumar (1968) reported the first surgical collection of embryos from the oviducts of alpacas following a fertile Alpaca donors may be sedated with Butorphanol tartrate (0.05–0.1 mg/kg IM) or a combination Xylazine (0.1 mg/kg IM) and Butorphanol (0.05 to 0.1 mg/kg IM). Alpacas are preferably placed in a sternal recumbency on an elevated platform. Epidural analgesia is provided by Lidocaine 0.2 mg/kg with a maximum of 1 ml per 50 kg of Body Weight) bifurcation of the uterine horns (Figure 28-1). The embryos collected 4 days after mating were at different stages of development from two cells to a morula. The recovery rate was 80% in single ovulating females. In another experiment using the same technique, Sumar and Franco (1974) obtained 44 morulae from donors superstimulated with equine chorionic gonadotropin (eCG; 700 international units [IU]) and induced to ovulate with human chorionic gonadotropin (hCG; 1000 IU).9 All embryos were transferred surgically to recipients, which resulted in a 10% pregnancy rate.
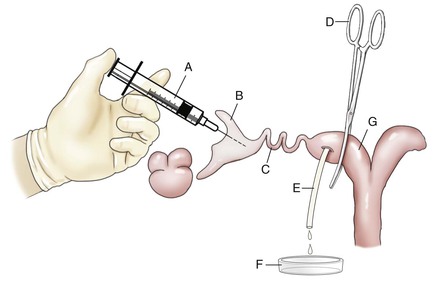
Figure 28-1 Illustration of the Surgical Embryo Collection Technique.
A, Syringe with blunt needle. B, Infundibulum. C, Oviduct. D, Curved forceps. E, Pipette inserted in the uterine horn. F, Glass dish. G, Uterine horn. (From Novoa, C., Sumar, J., 1968. Colección de huevos in vivo y ensayos de transferencia en alpacas. In: Tercer Boletín Extraordinario IVITA. Universidad Nacional Mayor de San Marcos, Lima, Perú, pp. 31–34.)
The first llama born via the nonsurgical collection and transfer technique was reported by Wiepz and Chapman (1985) in the United States.10 In this study, synchronization and ovulation of the recipient was induced by gonadotropin-releasing hormone (GnRH), and collection and transfer were done 7 days after GnRH treatment. One cria was born after transfer of two embryos to two synchronized females. Bourke et al. (1990) reported that superovulation can be achieved in llamas using pregnant mare’s serum gonadotropin (PMSG) and that embryos can be collected nonsurgically.11 From two llama donors, three embryos (all hatched blastocysts) were recovered by transcervical flushing 7 days after ovulation. Synchronization was achieved by simultaneous administration of 750 IU of hCG to both donor and recipients, immediately after mating of the donors. Two embryos were transferred to one of the recipients and one embryo to the second recipient. A single conceptus was visualized by ultrasonography in the recipient that had received two embryos.
Embryo transfer technology in alpacas and llamas has been relatively slow to develop until the last 10 years when renewed interest in the technology has led to more research on superovulation, embryo collection, and transfer.12,13 Commercial embryo transfer in alpacas and llamas is now offered in some countries, but it is still not fully accepted as a breeding technology in others.
Donor and Recipient Selection
Recipient Selection
The recipient pool may include animals with poor fiber production and quality and those eliminated from breeding because of hereditary disorders that are not life threatening (blue eyes, multicolor coats, etc.). Lactating females may be used, but not earlier than 30 days after parturition. A recent experiment in the highlands of Peru on a large number of donors and recipients clearly demonstrated the negative effect of lactation and body condition on pregnancy rates.14 Pregnancy rates following embryo transfer were significantly higher in nonlactating recipient alpacas (44%) than in lactating ones (18.2%). Lactation was associated with poor body condition in this study, most likely because of negative energy balance and weight loss that may have interfered with corpus luteum function.
Breeding Management and Timing of Embryo Collection in Non–superstimulated Donors
In practice, alpaca and llama embryos are collected nonsurgically from the uterine cavity. The embryo recovery rate depends greatly on the timing of embryo descent into the uterine cavity following ovulation and fertilization. Detailed studies on the chronology of embryo development and descent into the uterus in alpacas and llamas are scarce. At 3 days after mating, embryos recovered surgically from the uterine tube were at various stages ranging from two cells to a morula.8 In alpacas, Bravo et al. (1996) using females that were slaughtered 4, 7, and 10 days after copulation reported that embryos were still in the uterine tube at the morula stage at 4 days. A compact morula and blastocysts were recovered from the uterus, at day 7 and day 10, respectively, after breeding.15
Cárdenas et al. (1997) reported the chronology of embryo development following mating in alpacas (day 2 = 2–4 cells embryos; day 3 = 4–8 cells; day 4 = early morula; day 5 = compact morula, early blastocyst; day 10 = collapsed or elongated blastocyst; day 11–15, elongated blastocysts).16 In this study, no embryos were recovered from day 6 to day 9, but the reasons were not known. More recently, Cervantes et al. (2008) reported that the alpaca embryo enters the uterus on day 6 after mating at the hatched blastocyst stage and grows rapidly to almost double the size between day 6 and day 7 after mating.17 In llamas, Taylor et al. (2000) reported recovery rates of 55%, 79%, and 100% at days 7, 8, and 10 after mating, respectively.7 Other studies in alpacas and llamas showed that hatched blastocyst can be collected from the uterine cavity at 6 to 6.5 days after mating with or without GnRH administration.11
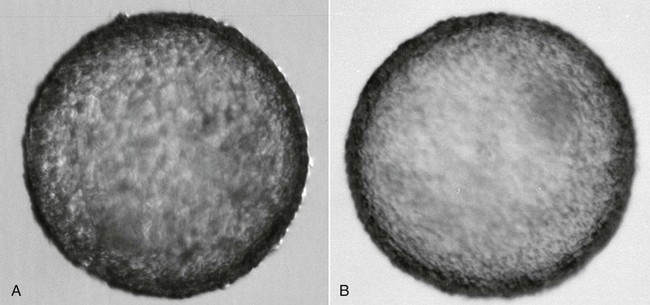
A general agreement exists in the literature that alpaca and llama embryos reach the uterine cavity at the hatched blastocyst stage (Figure 28-2). The mechanisms controlling the descent of the embryo into the uterine cavity in camelids are still not understood. It is possible that hatching from the zona pelucida needs to occur within the uterotubal junction to permit passage of the embryo. The great variation in the reported chronology of embryo development and timing of descent into the uterine cavity may be attributed to the variability in the timing of ovulation and other factors influencing the speed of development.18 On the basis of our experience, the ideal time for embryo collection is 7.5 days after mating. This timing ensures the highest rate of embryo recovery (80% to 100%). Camelid embryos seem to develop more rapidly than those of other species and undergo elongation most likely as part of maternal recognition of pregnancy.18,19
Embryo Collection Technique
Supplies needed for nonsurgical collection of embryos from alpacas and llamas are similar to those used in bovine embryo collection and consist of (1) a two-way Foley catheter (14 to 18 French [Fr]); (2) a stylette of 6.22 inch length with a clip to hold it within the catheter; (3) a sanitary plastic sleeve to prevent contamination of the catheter during introduction into the vagina; (4) a 6- or 12-mL syringe to inflate the Foley catheter balloon with the flushing medium; (5) an embryo filter placed on a graduated cylinder; (6) a “Y” tube for gravity irrigation of the uterus and return of the flushing medium (we suggest reducing the length of the tube, as commercial tubes are too large for uterine lavage in the alpaca or llama); and (7) searching and embryo washing dishes. All equipment should be sterile.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree