Embryo Preservation and in Vitro Production of Embryos
Reproductive biotechnology techniques, which include artificial insemination with cooled or frozen-thawed semen, embryo transfer (ET), and in vitro embryo production (in vitro fertilization [IVF], intracytoplasmic sperm injection [ICSI], and cloning) are widely used in domestic species such as bovines and equines. The industry that surrounds these species promotes the research and development of these biotechnologies, but research in this area in South American camelids (SACs) has been minimal. Complex reproductive characteristics of these species contribute to this lack of research. The induced nature of ovulation, the short lifespan of the corpus luteum (CL), the difference in luteolytic activity between the two uterine horns, the short period for maternal recognition of pregnancy (MRP), the highly viscous semen, and the low sperm concentration in camelids constitute serious challenges to researchers. Nevertheless, some assisted reproductive techniques (e.g., synchronization of ovarian follicular development, ovarian superstimulation, and ET) have gained a greater increase in knowledge, whereas others show less advances (artificial insemination, IVF, and ICSI), and certain basic reproductive physiology such as MRP signaling remains unclear.1,2
In Vitro Embryo Production
In vitro production of embryos demands a large quantity of oocytes that can be fertilized. The methods used to obtain cumulus oocyte complexes (COCs) are aspiration or dissection of follicles from slaughterhouse ovaries; aspiration of follicles surgically exposed during a laparotomy (llamas; alpacas); and ultrasound-guided transvaginal aspiration of follicles.3–11
Postmortem Oocyte Collection
The use of slaughterhouse ovaries has the advantage of providing a large quantity of oocytes, but the main disadvantage is that they need to be in vitro matured (IVM). Upon review of the literature, it is evident that maturation procedures for llama oocytes are not optimal. The first report on IVM llama oocytes was published in 1992 by Del Campo et al.12 Complete maturation requires both nuclear maturation and cytoplasmic maturation. Most researchers have reported 39% to 80% nuclear maturation of oocytes between 24 and 36 hours on the basis of oocytes reaching the metaphase II stage (MII) of development.4,12 No reports on the evaluation of cytoplasmic maturation of llama oocytes from slaughterhouse ovaries are available. The most commonly used marker for cytoplasmic maturation is the evaluation of the movement of the cortical granules toward the periphery of the oocyte. This evaluation may be carried out by using confocal laser microscopy and different fluorochromes. A consensus seems to exist that the llama oocyte from slaughterhouse ovaries needs approximately 36 hours in culture to mature to the MII stage.4,12 In addition, because oocytes are recovered from slaughterhouse, it is not known if follicles considered as preovulatory were in the growing or the regressing phase, which would affect the quality of the oocyte.
In Vivo Collection of Oocytes
Ovarian Stimulation
Follicular wave inhibition is an important step in increasing the response to superovulatory treatment. Various protocols based on progestagen or progestagen–estradiol treatments have been suggested to inhibit follicular development prior to initiation of superstimulation.13–19
Gonadotropin (follicle-stimulating hormone [FSH] and equine chorionic gonadotropin [eCG] either individually or combined) are administered in the absence of any follicle greater than 5 mm in diameter.6,14,18,20
Oocyte Recovery
The technique with the highest percentage of recovery in llamas is the aspiration of follicles via laparotomy. The COCs may be obtained from over 80% of the follicles that are aspirated from alpacas and llamas.6,21 A relationship exists between the size of the follicle and the stage of maturation of the COC that is recovered.6 Chaves et al. recovered a total of 46 oocytes from the 83 follicles aspirated in vicunas (recovery rate: 55.4%).22 However, this method requires a team of specialists in surgery and anesthesia and an operating theater. This surgical technique is also too invasive and cannot be repeated frequently. An alternative for in vivo oocyte recovery is ovum pickup using the ultrasound-guided transvaginal method, which is quick and nontraumatic. Only a few reports on the use of this technique in llamas are available. The COC recovery rate varies (52%, 74%, and 77%) in superstimulated females.9–11 Both follicle aspiration techniques (laparotomy and ultrasound-guided transvaginal aspiration) have the disadvantage of causing possible bleeding after aspiration and subsequent formation of ovariobursal adhesions.
In Vivo Oocyte Maturation
As mentioned earlier, the rate of in vitro oocyte maturation in llamas remains variable. As camelids are induced ovulators, in vivo oocyte maturation within the follicles could be promoted by the induction of a surge of luteinizing hormone (LH). This observation is reinforced by the fact that in bovines, in vivo maturation is more efficient for reaching the blastocyst stage compared with in vitro maturation. Meiosis startup may be induced by exogenous administration of gonadotropin-releasing hormone (GnRH) analogs such as buserelin or human chorionic gonadotropin (hCG). Superstimulation with eCG is associated with a larger proportion of expanded COCs as well as COCs in metaphase II, compared with the treatment with FSH.4 Trasorras et al. (2009) obtained 94% (98 of 104) COCs in the expanded stage from follicles 7 millimeters (mm) or greater in diameter in females superstimulated with eCG.6 No compact COCs (0 of 104) were recovered. Buserelin administration has been shown to be beneficial for recovering a larger quantity of expanded COCs that can be used directly in assisted reproductive techniques without previous maturation.6 Chaves et al. studied oocyte nuclear and cytoplasmic maturation in superstimulated vicuna females with 750 international units (IU) of eCG.22 Oocytes were fixed immediately after surgical aspiration. Evaluation was carried out by confocal laser microscopy, using propidium iodide (PI) to stain nuclear material and fluorescein marked peanut agglutinin (FICT-PNA) to stain the cortical granules. These authors found that 45% of the oocytes showed nuclear maturation but that only 9% showed cytoplasmic maturation also. These results demonstrated the beneficial effect of the superstimulation treatment despite the absence of induction of ovulation.
Embryo in Vitro Production Techniques
Few reports have been published on IVF in camelids. The first IVF in llamas was carried out by Del Campo et al.23 In this study, the authors used slaughterhouse oocytes and epididymal spermatozoa in co-culture with oviduct cells. Out of the 234 supposed zygotes cultured, only 4.7% (11 of 234) developed to the hatched blastocyst stage. Gomez et al. reported the first production of llama–alpaca cross-bred embryos after heterologous IVF using slaughterhouse alpaca oocytes and llama epididymal spermatozoa in co-culture with bovine oviduct cells.21 They used a small number of oocytes for fertilization and subsequent in vitro culture (n = 5); after 6 days, all reached the morula stage, but none continued to develop.
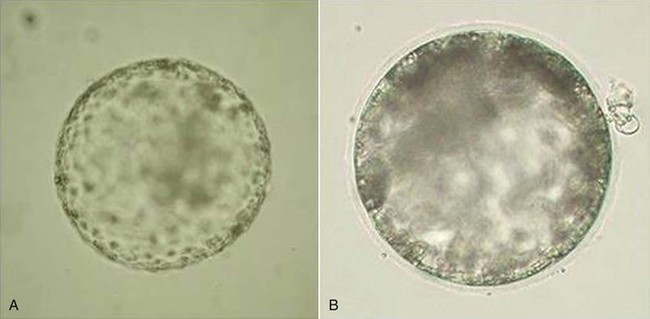
Our group has also worked with in vitro production of camelid embryos using two assisted reproduction techniques: IVF and ICSI.24–26 The work by Conde et al. represents the preliminary development of IVF and ICSI using raw llama semen collected through electroejaculation. For the first time, they obtained embryos that were produced in vitro and developed to the expanded blastocyst stage (Figure 29-1). Nevertheless, the results obtained to date with regard to early embryo development are very poor because the quality of the blastocysts produced in vitro is lower than that of blastocysts produced in vivo. In other species, regardless of culture media, embryos produced in vitro have lower number of blastomeres compared to embryos produced in vivo.27 Although we have obtained pregnancies via ET using in vivo produced embryos, the best-quality in vitro–produced camelid embryos still have not resulted in pregnancy after transcervical transfer of the embryos to the uterus of previously synchronized females.28 However, in the camel (Camelus dromedarius), this objective has been achieved after the transfer of in vitro–produced embryos that were cultured in a semidefined medium.29 Application of this type of assisted reproductive technique in genetically superior llamas would bring about a significant increase in the number of embryos produced in vitro and would improve the reproductive efficiency of these species, both domestic and wild, in which the birth of a live in vitro produced offspring has not been achieved. This biotechnology is also fundamental to maintaining females and males with some specific acquired reproductive disorders and thus preventing fertilization or preserving pregnancy.
Nuclear Transfer
The principle of nuclear transfer or cloning is relatively simple. The karyoplasts of a cell from the donor animal are transferred into the cytoplasm of an oocyte from which genetic material has been removed. The oocyte is enucleated by removing the metaphase plate and polar body by using a small-diameter pipette through micromanipulation. The donor cell is then combined with the enucleated oocyte (cytoplast) by electrofusion, and the recombined oocyte is activated to stimulate embryonic development. This technique requires a large quantity of oocytes. Only one report of nuclear transfer in llamas has been published.30 These authors used adult male llama fibroblast cell lines obtained from a skin biopsy and IVM oocytes recovered from superstimulated females after ovariectomy. Of a total of 80 reconstructed couplets, 62.5% were successfully fused, followed by cleavage rates of 32% to 40%. A total of 11 embryos (stage 8 cells to morula) were transferred to synchronized recipient llamas, but no pregnancies were detected 14 days later. Nevertheless, the application of this technologic advance was successful in the dromedary camel. Wani et al. obtained the first clone of this species born alive from a reconstructed embryo by using cumulus cells.31
In Vitro Culture of Embryos
Systems used for in vitro embryo culture include co-culture of different types of cells or the use of defined or semidefined synthetic culture media. The following have been used in different species: oviduct epithelial cells, granulosa cells, synthetic oviductal fluid (SOF), Charles Rosenkrans (CR) media 1, CR 2 media, potassium simplex optimization medium (KSOM), and Dulbecco’s modified eagle’s medium (DMEM-F12).2,23,29,32 The use of co-culture with somatic cells is also an acceptable technique for producing embryos.33 Nevertheless, this undefined medium makes it difficult or almost impossible to examine the nutritional requirements of the embryos and contributes to variability in the composition of the culture system.34 In addition, the cells present in the culture medium may compete with the embryos for the nutrients, or the metabolic waste may have a deleterious effect on embryo development. The use of defined culture media allows evaluation of the nutritional conditions necessary for the development of embryos to stages that are optimal for their transfer. The designing of these media is based on embryo physiologic and metabolic dynamics and on the reduction of intracellular stress. Furthermore, data obtained from the oviduct microenvironment are taken into account. In SACs, no data have been published on the rate of production and the composition of the fluid produced by the oviduct. Early embryo development in vivo in llamas seems to be rapid compared with other species such that morulae have been recovered in vivo from llama oviducts 3 days after ovulation.35 For this rapid growth to be possible, the contribution of an adequate microenvironment with nutrients, ions, hormones, proteins, amino acids, and growth factors is necessary and probably present in the oviducts of these species.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree