Drugs Used to Relieve Pain and Inflammation
Learning Objectives
After studying this chapter, you should be able to
1. Define terms related to the pharmacology of drugs used to relieve pain and inflammation.
2. Describe the anatomy and physiology associated with pain production and relief.
3. List and discuss the four steps involved in the production of pain sensation.
4. Understand the difference between physiologic and pathologic pain.
5. Understand the concepts of preemptive and multimodal pain therapy.
6. List physical signs associated with the expression of pain in animals.
8. List indications for the use of NSAIDs.
9. List potential adverse side effects of NSAIDs.
10. Describe the mechanism of action of the antihistamines.
11. Differentiate between the action of H1 and H2 histamine receptors.
12. List indications for muscle relaxants.
13. List the two major categories of corticosteroids and describe the effects of each.
15. List indications for the use of corticosteroids.
16. Describe potential adverse side effects of short-term and long-term corticosteroid use.
17. Describe the mechanism of action of local anesthetic agents.
Key Terms
Addison’s disease
Analgesia
Cushing’s disease
Histamine
Iatrogenic
Modulation
Multimodal analgesia
Nerve block
Neuropathic pain
Pathologic pain
Perception
Physiologic pain
Preemptive analgesia
Prostaglandin
Regional anesthesia
Somatic pain
Transdermal application
Transduction
Transmission
Visceral pain
Introduction
Pain has been defined by the International Association for the Study of Pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage.” It may occur alone or in combination with inflammation. Pain sensation arises in the terminal ends of sensory nerve fibers called nociceptors, which are located in every tissue of the body. Nociceptors may be activated through mechanical, thermal, and chemical stimulation to create a nerve impulse. Chemical stimulation may be derived from an exogenous source or from endogenous chemicals such as eicosanoids (prostaglandins), bradykinin, serotonin, and others released in response to tissue damage. These substances may create a “sensitizing soup,” which may create a lower threshold for nociceptors, amplifying the pain response (Muir, 2009)
Physiologic pain can be beneficial in that it can allow the animal to avoid damaging stimuli. Pathologic pain results from tissue or nerve damage and may be further classified according to its origin, severity, or duration. Visceral pain arises from hollow abdominal organs, peritoneum, heart, liver, and lungs while somatic pain arises from the musculoskeletal system. Somatic pain may be further described as superficial (arising from the skin) or deep (arising from periosteum, tendon, or joint tissues). Neuropathic pain can arise due to injury to the peripheral or central nervous system and is described in humans as “burning” or “shooting” (Gaynor, 2009). Pain can have varying degrees of severity (none, mild, moderate, or severe) and may be acute or chronic.
Severe or chronic pain may have an emotional content that activates sympathetic stimulation. It can be harmful because it can lead to stress and related problems such as gastrointestinal lesions, immunosuppression, delayed healing, hypertension, fatigue, abnormal behavior, and potential dysrhythmias. The ethical treatment of animals includes the “five freedoms”: freedom from pain, freedom from hunger and malnutrition, freedom from discomfort, freedom from disease and injury, and freedom to express normal behavior.
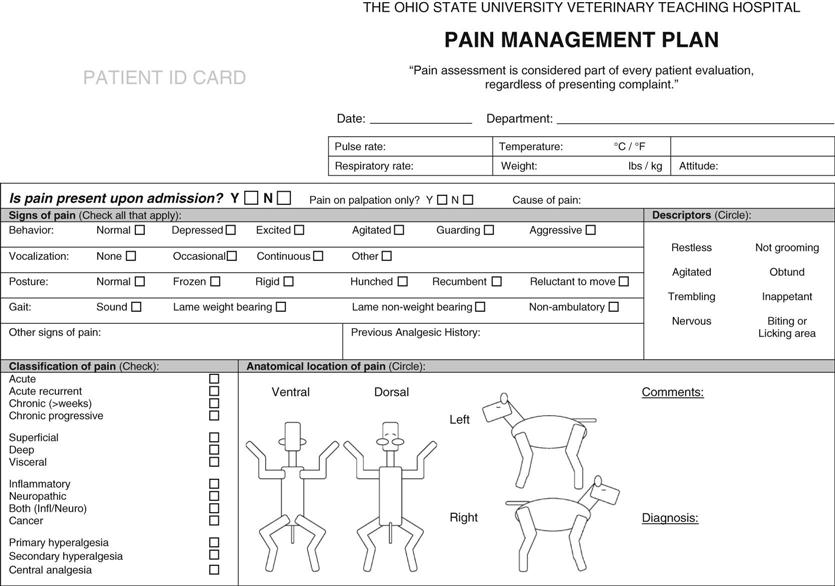
Assessment of pain in animals can be very difficult because of the dependence on nonverbal communication in veterinary medicine. Furthermore, animals differ from people in their pain response. It is important for wild animals to control the expression of pain to avoid predation or abandonment. Response to pain varies among individuals and may include increased heart rate, increased respiratory rate, mydriasis, salivation, vocalization, changes in facial expression, aggressive behavior, guarding of the painful site, restlessness, unresponsiveness, failure to groom, abnormal gait, and abnormal stance. A patient that is pain-free will be quiet and calm (Paddleford, 1999). Consult the Handbook of Veterinary Pain Management for pictures and descriptions of pain behaviors in animals. Pain scales and evaluation instruments (Figure 14-1) have been developed to make pain evaluation more objective.
Drugs used to control pain (analgesics) include nonsteroidal antiinflammatory drugs (NSAIDs), opioids, α-2 agonists, ketamine, and others. The body is able to produce its own opiate-like analgesic agents called endorphins and enkephalins. Efforts to synthesize these substances for commercial production have been unsuccessful.
At one time people believed that masking pain with analgesics could interfere with the diagnosis or treatment course of a disease. It is now known that animals in pain should be treated for humane reasons and so that the harmful side effects that accompany pain can be reduced. The treatment regimen may vary according to assessment of the severity and the origin of the pain. For best results, pain management intervention should be preemptive and include multimodal analgesia when possible.
Inflammation is a basic process that occurs in the body in response to tissue injury caused by physical, chemical, or biologic trauma. The objectives of this process are (1) to counteract the injury by removing or walling off the cause of the injury and (2) to repair or replace the damaged tissue. Clinical manifestations (cardinal signs) of inflammation include redness, heat, swelling, and pain. Although the process is designed to be protective, it can continue to become a source of further injury or damage (e.g., allergy, shock, or “proud flesh”).
Damage to cells from any source results in the release of several chemical mediators that may initiate or prolong the inflammatory response. These chemicals include prostaglandins, leukotrienes, histamine, cytokines, and other mediators. These substances cause helpful responses such as dilation and increased permeability of blood vessels that result in increased blood flow to the injured tissue. Enhanced blood flow brings plasma to dilute the offending agent, fibrin to immobilize it, and phagocytic cells to remove it. Redness, heat, swelling, and, to some extent, the pain of inflammation result from increased amounts of blood in the damaged tissue. The chemical mediators serve other beneficial functions such as attracting phagocytic cells to the area of concern (chemotaxis) and several potentially harmful functions such as initiation of bronchoconstriction (histamine), anaphylactic shock, pain (histamine), cell death, platelet aggregation, and intestinal spasm. The inflammatory process can be acute (anaphylaxis) or chronic (flea allergy and arthritis).
Drugs that are used to minimize the inflammatory process include NSAIDs, glucocorticosteroids, and several miscellaneous agents such as dimethyl sulfoxide (DMSO). Another process mediated by a chemical (or chemicals) released from damaged cells is fever. Fever is an increase in body temperature to above normal; it is an important clinical indicator of disease. The purpose of fever may include destruction of invading microorganisms by heat inactivation and facilitation of biochemical reactions in the body. (Most chemical reactions are accelerated by increased heat.)
Heat is generated by the metabolic activity of muscles and glands and is dissipated through radiation or conduction loss from the skin, sweat evaporation, and evaporation during panting. A “thermostat” in the hypothalamus regulates these mechanisms, which control body temperature.
A substance that can initiate a fever is called a pyrogen. An exogenous pyrogen is a foreign substance (e.g., bacteria, viruses) that when introduced into the body causes the release of an endogenous pyrogen (a chemical mediator such as prostaglandin) from white blood cells; this endogenous pyrogen causes resetting of the hypothalamic thermostat. The hypothalamus then activates processes to generate or conserve body heat: shivering to generate more heat, constriction of blood vessels in the skin to prevent radiation and conduction loss, and decreased sweating or panting to reduce evaporation loss. Damaged cells in some instances may release endogenous pyrogens in the absence of exogenous pyrogens. Drugs used to control fever are primarily NSAIDs.
Anatomy and Physiology
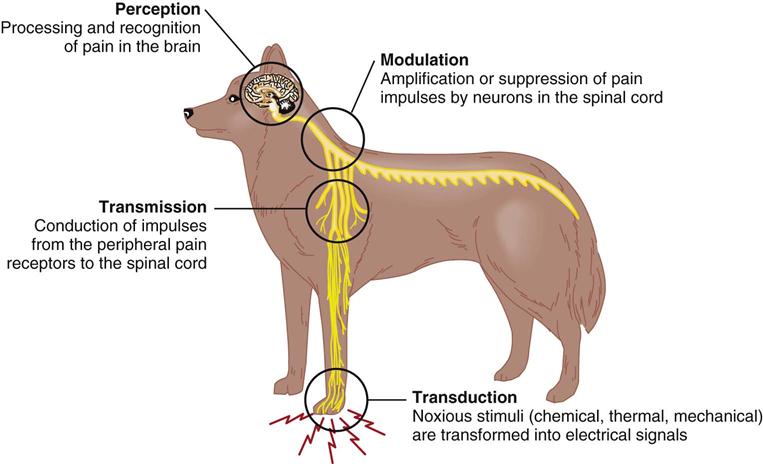
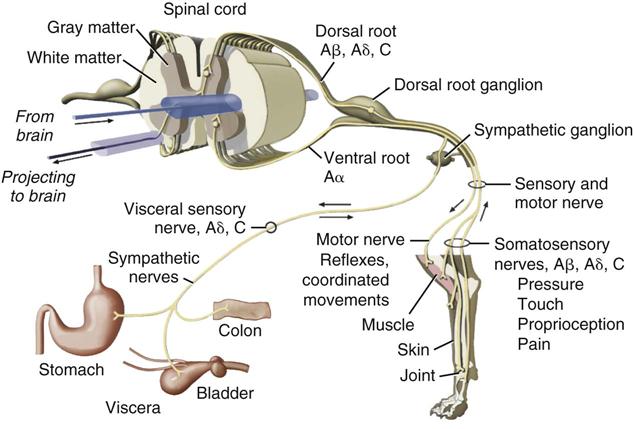
The production of pain sensation arises through a four step process: transduction, transmission, modulation, and perception (Figure 14-2). The first step called transduction occurs in nociceptors, which are terminal sensory nerve endings found in almost every tissue of the body. The nociceptor creates action potentials (electric impulses) in peripheral sensory nerves when activated by noxious stimuli. The second step is the transmission of the impulses to the central nervous system (CNS) by two fiber systems: type C unmyelinated fibers are responsible for dull, poorly localized pain (in humans), and type A delta fibers are responsible for sharp, localized pain (Ganong, 2003). Type A and type C fibers carry impulses to the dorsal horn of the spinal cord (Figure 14-3). This information then, in the third step, undergoes modulation (suppression or amplification) and is transmitted up the cord via the spinothalamic tract through the thalamus to the cerebral cortex, where processing occurs and recognition results in pain perception. If any part of this neuronal chain or the cortical interpretive area is nonfunctional, pain sensation does not occur. Multimodal therapy takes advantage of this concept to intervene at different sites within the pathways. Because an active cortex is required, the perception of pain can occur only in a conscious animal. It should be remembered that reflexive activity (e.g., withdrawal reflex) without pain recognition can occur as a result of nociceptor stimulation (see Chapter 4).
The perception of pain can be enhanced by phenomena called hyperalgesia and central sensitization (Boothe, 2012). Hyperalgesia occurs when the area of tissue injury becomes more sensitive and the threshold for subsequent stimuli decreases. This sensitivity can spread to surrounding uninjured tissue (secondary hyperalgesia). When the neurons of the spinothalamic tract of the spinal cord are stimulated repeatedly, they apparently become sensitized and discharge at a much lower threshold. This activity is called central sensitization, or “wind-up,” and is the rationale for the idea that pain control is enhanced if an analgesic is given before pain is generated.
When spinal cord lesions occur, superficial pain is inhibited before deep pain or lesion severity worsens. The absence of deep pain is often a poor prognostic sign.
As mentioned previously, pharmacologic intervention for pain control may target a single or multiple points of intervention in the pain process. Transduction can be inhibited by local anesthetics, opioids, NSAIDs, and other substances. Transmission of nerve impulses can be inhibited by local anesthetics and alpha-2 agonists. Modulation of pain impulses can occur in the spinal cord through the effects of local anesthetics, opioids, alpha-2 agonists, tricyclic antidepressants, NSAIDs, anticonvulsants, and other drugs. Pain perception in the cortex can be inhibited by the use of anesthetics, opioids, benzodiazepines, and alpha-2 agonists.
 Nonsteroidal Antiinflammatory Drugs
Nonsteroidal Antiinflammatory Drugs
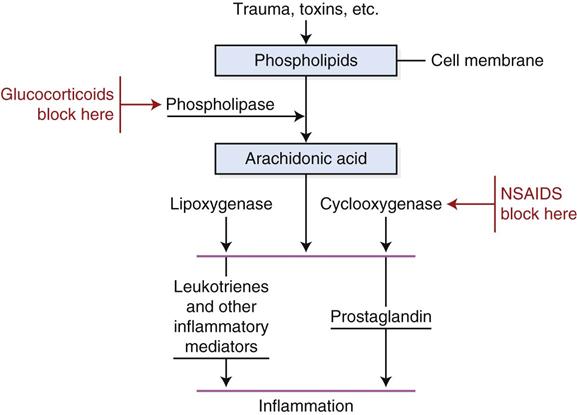
NSAIDs are very useful analgesic drugs that have been shown to work synergistically with opioids when used together. NSAIDs are thought to work by inhibiting an enzyme called cyclooxygenase (COX). Two forms (COX-1 and COX-2) of cyclooxygenase exist. COX-1 maintains physiologic functions such as modulation of renal blood flow and synthesis of gastric mucosa and is sometimes called the constitutive form (Paddleford, 1999). COX-2 is considered the induced (by tissue injury) form that promotes the formation of prostaglandin from cell membrane arachidonic acid (Figure 14-4). Generally, inhibition of COX-2 is responsible for effectiveness of NSAIDs whereas inhibition of COX-1 is associated with side effects. NSAIDs that selectively inhibit COX-2 are thought to produce fewer gastrointestinal side effects. Recent research has suggested that this scheme may be an oversimplification and that COX-2 may be constitutive in some tissues (Budsberg, 2009). COX-1 versus COX-2 selectivity may depend on the drug, the dose, and the species (Claude, 2013). Because the degree of COX-1 and COX-2 inhibition for many of the newer NSAIDs is not well-defined, preferential COX-2 inhibitor may be chosen over selective COX-2 inhibitor because some inhibition of COX-1 and COX-2 may occur (Boothe, 2012). COX-1 sparing has the same connotation as preferential COX-2 inhibitor.
Glucocorticoids exert their effects by blocking phospholipase, an enzyme necessary for the production of both prostaglandins and leukotrienes (intervention is provided earlier in the sequence of the formation of inflammatory mediators). Because the inflammatory reaction is blocked earlier by glucocorticoids, they are more effective antiinflammatory agents than are NSAIDs (Langston and Mercer, 1988). NSAIDs are often preferred, however, because they have fewer side effects and they promote analgesia and fever reduction. At this time, it is not known why glucocorticoids do not induce the analgesic and antipyretic effects of NSAIDs. It is also unknown why some NSAIDs provide relief of only mild pain (aspirin) and others provide relief of moderate to severe pain (flunixin). Some clinicians speculate that NSAIDs may act to varying degrees centrally to modulate spinal transmission of pain impulses (Paddleford, 1999).
The most common side effects of the NSAIDs are gastrointestinal ulceration and bleeding, which probably result from interference with the normal mucous coating of the stomach. Other side effects may include hepatotoxicity, nephrotoxicity, inhibition of cartilage metabolism, bone marrow suppression, and bleeding tendencies (from reduced platelet aggregation).
All pets should undergo a thorough physical examination and history, as well as appropriate laboratory tests, before NSAIDs are initiated. Clients should be advised to stop the use of these drugs and to contact their veterinarian if they observe side effects in their pets that are receiving NSAIDs. Pets receiving long-term treatment with NSAIDs should have periodic evaluations of liver and kidney function performed. Clients should be advised to watch their pets for anorexia, vomiting, changes in bowel movements, bloody or tarry stools, lethargy or other changes in behavior, seizures, jaundice, changes in urination (frequency, color, or smell), or changes in the condition of the skin.
Salicylates
Aspirin, a salicylate, is also known as acetylsalicylic acid. Its actions include the following:
These effects are thought to occur as a result of the ability of aspirin to inhibit an enzyme (COX) that is responsible for the synthesis of prostaglandin. Prostaglandin is a chemical mediator of the processes that lead to pain, fever, inflammation, and platelet aggregation. Its inhibition results in diminishing of each process.
Clinical Uses
Clinical uses of aspirin exist for most animal species and may include the following:
Dosage Forms
These include plain uncoated tablets, buffered uncoated tablets, enteric-coated forms, and boluses (large-animal applications). Many generic or brand names are available in many different strengths, including the following:
Adverse Side Effects
Adverse side effects of aspirin include gastric irritation, which can lead to ulceration and bleeding. Cats are highly susceptible to aspirin overdosage because of their inability to metabolize it rapidly; they should receive this drug only under the supervision of a veterinarian.
Pyrazolone Derivatives
Phenylbutazone
Phenylbutazone, a pyrazolone derivative, is an NSAID that is commonly used in veterinary medicine. Its actions include the following:
Clinical Uses
These include relief of inflammatory conditions of the musculoskeletal system of horses and dogs. Phenylbutazone is used extensively in horses for the treatment of lameness and for the relief of pain associated with colic. It sometimes is used in dogs and cattle for its antiinflammatory, analgesic, and antipyretic effects.
Dosage Forms
Dosage forms of phenylbutazone include parenteral injection, tablets, boluses, an oral paste, an oral gel, and powder.
Adverse Side Effects
These include gastrointestinal bleeding and bone marrow suppression.
Flunixin Meglumine
Flunixin is an NSAID that is labeled for use in horses and cattle. It has extralabel uses in other species. Its actions are related to its ability to inhibit COX and include the following:
Clinical Uses
Clinical uses of flunixin in horses include alleviation of pain associated with musculoskeletal disorders and colic. (Flunixin apparently has great ability to inhibit visceral pain.) Other uses in horses and other species include treatment of the following:
Dosage Forms
Dosage forms of flunixin include injectable, oral paste, and oral granule formulations.
Adverse Side Effects
These are limited in horses but may include swelling at the injection site and sweating. In dogs, vomiting, diarrhea, nephrotoxicity, and gastric ulceration may occur with long-term use.
Dimethyl Sulfoxide
DMSO is a clear liquid that was originally developed as a commercial solvent. It is noted for its antiinflammatory action and its ability to act as a carrier of other agents through the skin. Its antiinflammatory actions may be related to its ability to trap products associated with the inflammatory response. DMSO causes vasodilation when applied topically.
Clinical Uses
Clinical uses of DMSO are varied; however, the only labeled use for DMSO is for topical application to reduce acute swelling resulting from trauma in dogs and horses. DMSO has reportedly been used as the following:
• An adjunct to intestinal surgery (intravenously)
• A treatment for cerebral edema or spinal cord injury (intravenously)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree