Chapter 6 Disorders of Calcium
Hypercalcemia and Hypocalcemia
Calcium is required in the body for many vital intracellular and extracellular functions, as well as for skeletal support. Ionized calcium (iCa or Ca2+) is required for enzymatic reactions, membrane transport and stability, blood coagulation, nerve conduction, neuromuscular transmission, muscle contraction, vascular smooth muscle tone, hormone secretion, bone formation and resorption, control of hepatic glycogen metabolism, and cell growth and division.491 Intracellular calcium ions are one of the primary regulators of the cellular response to many agonists and serve as “an almost universal ionic messenger,” conveying signals received at the cell surface to the inside of the cell.463 In addition to serving as an intracellular messenger, the iCa concentration in the extracellular fluid (ECF) regulates cell function in many organs, including the parathyroid gland, kidneys, and thyroid C cells by binding to a newly identified cell membrane-bound calcium-sensing receptor.80 Normal homeostatic control mechanisms usually maintain the serum calcium concentration within a narrow range and guarantee an adequate supply of calcium for intracellular function. These mechanisms must be disrupted for hypercalcemia or hypocalcemia to develop. Abnormal serum calcium concentrations are of diagnostic value and contribute to the development of lesions and clinical signs. Technological advances in the measurement of serum iCa concentration, parathyroid hormone (PTH), parathyroid hormone-related protein (PTHrP), and vitamin D metabolites have provided tools that allow greater diagnostic accuracy in the investigation of calcium disorders.
Normal physiology
Overview of calcium homeostasis
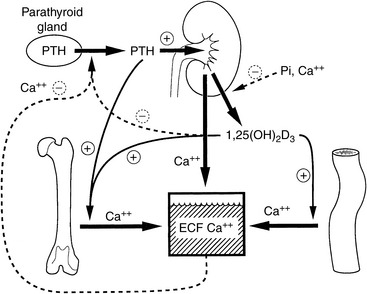
Regulation of serum calcium concentration is complex and requires the integrated actions of PTH, vitamin D metabolites, and calcitonin (Fig. 6-1). PTH and calcitriol (1,25-dihydroxyvitamin D3) are the main regulators of calcium homeostasis and have major regulatory effects on each other.478 PTH is largely responsible for the minute-to-minute control of serum iCa concentration, whereas calcitriol maintains day-to-day control. In the fetus, the parathyroid glands and placenta produce PTHrP, which binds to PTH receptors and regulates calcium balance.582 After birth, the parathyroid glands modify their pattern of hormone secretion and produce predominantly PTH. Other hormones, including adrenal corticosteroids, estrogens, thyroxine, growth hormone, glucagon, and prolactin, have less influence on calcium homeostasis but may play a role during growth, lactation, or certain disease states.
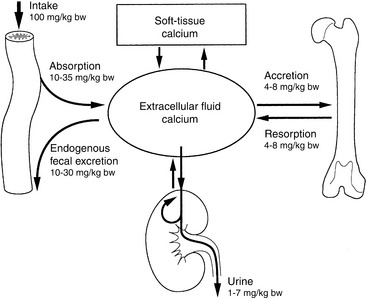
The intestine, kidneys, and bone are the major target organs affected by calcium regulatory hormones. These interactions allow conservation of calcium in the ECF by renal tubular reabsorption, increased intestinal transport of calcium from the diet, and internal redistribution of calcium from bone (Fig. 6-2). The intestine and kidneys are the major regulators of calcium balance in health.176 Normally, dietary calcium intake equals the amount of calcium lost in urine and feces. The enteric absorption of calcium depends on the physiologic status of the intestines (e.g., acidity, presence of other dietary components, integrity of the villi or presence of small intestinal disease, and degree of enterocyte stimulation by calcitriol). Non–protein-bound calcium is filtered by the glomerulus and undergoes extensive renal reabsorption. This process results in reclamation of more than 98% of the filtered calcium in health.146,482
The skeleton provides a major supply of calcium and phosphorus when intestinal absorption and renal reabsorption inadequately maintain normal serum calcium concentrations. Bone calcium mobilization is important in the acute regulation of blood calcium.433 Calcium and phosphorus can be mobilized from calcium phosphate in the bone ECF compartment, but these stores are rapidly depleted. The osteoblast is critical in limiting the distribution of calcium and phosphate between bone and ECF, and exchangeable bone water is separated from ECF water by the combined membranes of osteoblasts lining bone surfaces. For greater or prolonged release of calcium from bone, osteoclastic bone resorption must be activated. Osteoclasts secrete acid and proteases that result in dissolution of the mineralized matrix of bone and mobilize calcium and phosphorus.
Extracellular iCa concentration is the actively regulated fraction of total calcium (tCa).81,115 When blood iCa concentration decreases, PTH secretion is stimulated. PTH exerts direct effects on bone and the kidneys and indirect effects on the intestine through calcitriol. PTH increases synthesis of calcitriol by activating renal mitochondrial 1α-hydroxylation of 25-hydroxycholecalciferol. Calcitriol increases calcium absorption from the intestine and acts with PTH to stimulate osteoclastic bone resorption.104 Calcitriol is necessary for differentiation of osteoclasts from precursor mononuclear cells. PTH increases osteoclast number and stimulates osteoclast function to increase bone resorption and the release of calcium from bone to blood. Calcitriol also induces renal transport mechanisms activated by PTH that increase tubular reabsorption of calcium from the glomerular filtrate, thus preventing calcium loss in urine.404
Calcium distribution within the body
Approximately 99% of body calcium resides in the skeleton and is stored as hydroxyapatite, Ca10(PO4)6(OH)2. Most skeletal calcium is poorly exchangeable, and less than 1% is considered readily available. The small amount of rapidly exchangeable bone calcium arises from the ECF in bone that is present between osteoblasts and osteocytes and the bone matrix. Almost all of the nonskeletal calcium resides in the extracellular space, although small and biologically important quantities are found intracellularly.491
Extracellular Calcium
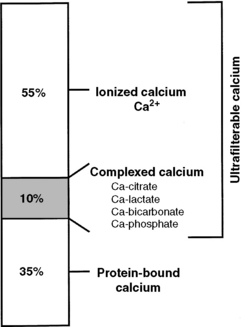
Calcium in plasma or serum exists in three fractions: ionized (iCa), complexed (bound to phosphate, bicarbonate, sulfate, citrate, and lactate), and protein bound (Fig. 6-3). In clinically normal dogs, protein-bound, complexed, and iCa account for approximately 34%, 10%, and 56% of serum tCa concentration, respectively.516 Ionized calcium is the most important biologically active fraction in serum, although an active biologic role for complexed calcium has been suggested.571 No biologic role for protein-bound calcium has been identified other than as a storage pool or buffering system for iCa.
Intracellular Calcium
Intracellular iCa is an important secondary messenger in the response to biochemical signals (such as hormones) transduced through the cell membrane.462,491 Therefore, intracellular iCa concentrations are maintained at a very low level (approximately 100 nM), 10,000-fold less than the serum concentration. This permits rapid diffusion into the cytoplasm from the ECF or endoplasmic reticulum. Intracellular calcium is rapidly buffered by cytosolic proteins and is transported into organelles or to the outside of the cell after an increase in intracellular iCa. If intracellular iCa is not maintained at a low concentration, it leads to toxicity and eventual cell death.
Most intracellular calcium is sequestered in organelles or bound to cellular membranes or proteins.276 Sequestration of iCa in mitochondria blunts an increase in cytosolic iCa, whereas endoplasmic reticulum serves as a reservoir to increase cytosolic iCa when necessary. Binding of calcium to specific cytosolic or membrane proteins is an efficient method for regulation of intracellular iCa concentration. Protein binding provides intracellular iCa buffering and also may act as a messenger system when protein configuration and activity are altered. Calbindin, calmodulin, and troponin C are important intracellular calcium-binding proteins.57
Cell Membrane Calcium Ion Sensing Receptor
In 1993, a novel iCa-sensing receptor was cloned and sequenced.78 The iCa receptor plays an integral role in iCa balance by regulating parathyroid chief cells, C cells, and renal epithelial cells.77,251 In parathyroid chief cells and C cells, the iCa receptor directly regulates intracellular iCa concentration, which controls PTH and calcitonin secretion. Ionized magnesium (iMg) is also an agonist of the iCa receptor. Stimulation of the iCa receptor caused by increased extracellular iCa concentration in the kidneys decreases NaCl, iCa, and iMg reabsorption in the proximal convoluted tubule and decreases water reabsorption in collecting ducts. This results in greater excretion of iCa and iMg in a more dilute urine.
Genetic diseases have been described related to both inactivating and activating mutations of the calcium receptor gene.23 Inactivating mutations lead to severe neonatal hypercalcemia when homozygous and to familial hypocalciuric hypercalcemia when heterozygous.562 Activating mutations of the calcium receptor produce hypoparathyroidism and hypocalcemia.564 Autoantibodies produced against the calcium receptor may either disable it, producing hyperparathyroidism with hypercalcemia,427,472 or activate it, producing hypoparathyroidism.219,293 Drugs that bind the Ca2+-sensing receptor may be useful in treating disorders of the parathyroid gland.
Parathyroid hormone
Structure
PTH is an 84-amino acid single-chain polypeptide that is synthesized and secreted by chief cells of the parathyroid glands.478 The amino acid sequences of PTH are known for the dog, cow, pig, rat, chicken, and human,313,488 and most mammals appear to have very similar amino-terminal portions of the molecule.404 Whereas the conserved amino end of PTH is vital for binding to cell membrane receptors, the role of the carboxyl terminus is to serve as a guide for PTH through the cellular secretory pathway.329
Synthesis and secretion
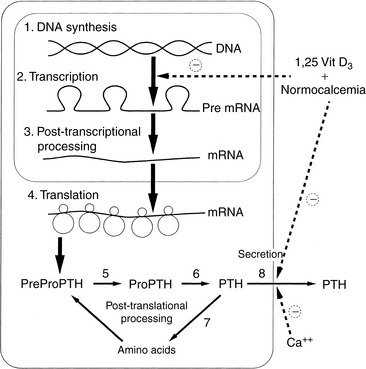
Synthesis, secretion, and degradation of PTH by chief cells are closely related. Little PTH is stored within the parathyroid glands,231 and synthesis of new specific messenger RNA (mRNA) and translation to PTH are required to maintain secretion.535 After secretion, PTH has a short half-life (3 to 5 minutes) in serum; thus, a steady rate of secretion is necessary to maintain serum PTH concentrations. Circulating PTH has many forms, not all of which have bioactivity,71,413 leading to potential confusion in assay interpretations.508,560,625
The amount of PTH available for secretion is a function of the balance of synthesis and degradation within chief cells (Fig. 6-4). Calcitriol, via the vitamin D receptor (VDR), and extracellular iCa concentration, via effects on the plasmalemmal calcium receptor,108,109,470 control these parathyroid cell processes. Because calcitriol regulates expression of the calcium receptor gene,99 calcitriol can be considered to exert overall control over PTH synthesis and secretion by the parathyroid cells. In general, the parathyroid gland has evolved most of its regulatory strategies to protect against hypocalcemia, with sensitive control of PTH synthesis and secretion being the dominant sites for regulation.82,536 However, high serum iCa concentrations increase the rate of degradation of PTH within the gland to protect against hypercalcemia.313
Except for minor diurnal variation, PTH secretion is relatively constant but may have a mild pulsatile pattern in response to minor fluctuations in the concentration of serum iCa.81 A relatively low rate of PTH secretion is needed normally to maintain serum iCa concentration. The basal secretory rate of PTH is approximately 25% of the maximal rate, and PTH is constantly secreted during normocalcemia. Complete inhibition of PTH secretion is not achieved even in the presence of severe hypercalcemia.313
Hypocalcemia is the principal stimulus for PTH secretion, but epinephrine, isoproterenol, dopamine, secretin, prostaglandin E2, and stimulation of nerve endings within the parathyroid gland may have minor effects.231 High concentrations of serum and intracellular iCa inhibit PTH secretion via increased arachidonic acid62,101 and possibly subsequent eicosanoid production.101 The control at PTH mRNA synthesis is also critically important.535
Calcitriol also plays an important role in the regulation of PTH synthesis and secretion.538 Calcitriol inhibits PTH mRNA synthesis537 and stimulates synthesis of the calcium receptor.99 These relationships explain the requirement for adequate blood concentrations of calcitriol to maintain the ability of the parathyroid gland to respond to changes in extracellular calcium concentrations.349,405 Increased intracellular iCa may also cooperate with calcitriol to reduce PTH synthesis in chief cells by inhibiting the expression of calreticulin (a blocker of VDR action).526,600 Animals with uremia and reduced serum calcitriol concentrations have poorly regulated chief cell function that results in renal secondary hyperparathyroidism,217,401 but a significant part of the hyperparathyroid response in uremic patients is the result of a glandular hyperplasia caused by the changes of both calcitriol and serum phosphorus.9 Serum phosphorus concentrations are generally considered to regulate PTH secretion principally by indirect means. Renal calcitriol synthesis is reduced early in uremia by modest hyperphosphatemia, and the plasma iCa concentration may decrease because of reduced effects of calcitriol on the intestine, bone, and kidneys. Markedly increased serum phosphorus concentrations (as seen in advanced renal failure) can lower the serum iCa concentration (mass law effect), resulting in an increase in PTH secretion because of the lowered calcium, but these effects do not occur early in renal failure when serum phosphorus is only moderately increased.401
Serum magnesium concentration has little role in the control of PTH secretion under normal conditions, but PTH secretion can be inhibited by very high concentrations of serum iMg.478 Paradoxically, hypomagnesemia or magnesium depletion also results in an inability to secrete PTH, but the cellular mechanism of this effect is unclear. This effect may be partially caused by reduced sensitivity of cell membrane receptors to iCa in the presence of low serum iMg concentrations.231,382
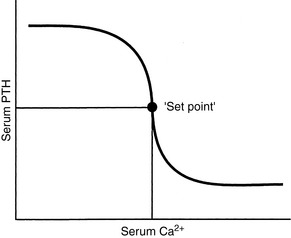
Set-Point for PTH Secretion
The set-point for PTH secretion is defined as the ECF iCa concentration that occurs at the serum PTH concentration that is midway between maximal and minimal values of PTH obtained experimentally.81 Normal serum iCa concentration is maintained slightly higher than the set-point; thus, PTH release normally is less than half-maximal (Fig. 6-5).
The rate of PTH secretion is inversely proportional to the concentration of extracellular calcium, but this proportional secretion of PTH occurs only over a narrow range corresponding to a serum tCa concentration of 7.5 to 11.0 mg/dL.231 An inverse sigmoidal curve with a steep slope results when the relationship between serum iCa concentration and PTH secretion is plotted over a larger range of calcium concentrations (see Fig. 6-5).81 This ensures large changes in PTH secretion for relatively small changes in iCa concentration in the physiologic range and precise control of serum iCa concentration. An approximate 10% decrease in serum iCa concentration elicits a nearly maximal PTH secretory response. The rate of decrease of serum iCa concentration is also important, and rapid decreases in serum iCa result in larger increases in PTH secretion. A 2% to 3% decrease in iCa concentration, if rapid in onset, may result in a 400% increase in PTH secretion.81
The cell membrane calcium receptor is responsible for establishing the relationship of the set-point for PTH secretion and extracellular iCa concentration.598 The calcium receptor regulates PTH secretion indirectly by controlling the intracellular iCa concentration by means of (1) release of iCa from intracellular stores, and (2) cell membrane calcium channels. Calcium channels span the parathyroid chief cell membrane and are important in allowing extracellular iCa access to the interior of the cell.186 The calcium channels are controlled by intracellular iCa concentration82 and membrane regulatory G proteins, which interact with the cell membrane calcium receptor.24
Calcitriol plays an important role in controlling the parathyroid gland set-point by regulating (1) synthesis of the cell membrane calcium receptor,76,99 (2) synthesis of cell membrane G proteins, and (3) function of cell membrane calcium channels.404 Therefore, adequate calcitriol is necessary to maintain the set-point for PTH secretion. The regulation of calcium receptor expression by calcitriol explains the observed “calcium set point” aberrations in control of PTH secretion in those with uremia.356 These patients have deficits in calcitriol production,116,617 as well as resistance in uremic parathyroids to calcitriol160,435; thus, they are less able of inducing synthesis of adequate numbers of calcium receptors.
Although regulations at each parathyroid cell may fail, thus producing abnormally increased PTH,214,460 changes may also be seen in the maximal secretory capacity dependent mostly on parathyroid cell numbers.506 It is likely that increased PTH secretion in patients with renal secondary hyperparathyroidism is primarily caused by parathyroid gland hyperplasia.157 One important role of calcitriol therapy in these patients is to prevent or reverse the parathyroid cellular hyperplasia.100,156,403
Inhibition of PTH Synthesis and Secretion
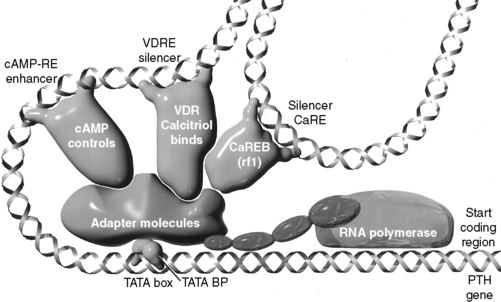
This topic has become important with the understanding of the toxicity of PTH in animals and humans with chronic renal failure (CRF) and accompanying secondary hyperparathyroidism.11,357,401,438 Recently, increased awareness of PTH toxicity stems from established relations to cardiovascular disease135 and mortality.546 PTH secretion is inhibited by increased serum iCa concentration,535,537 and the initial effect to decrease PTH secretion is rapid (occurring within 2 to 3 minutes), mediated by the calcium receptor with a cascade of resulting intracellular events67,137,251 and involving mediation by arachidonate.8 Slower effects are caused by inhibition of synthesis of PTH mRNA and its translation to hormone (Fig. 6-6).535
The osteocyte-derived phosphatonin43 fibroblast growth factor-23 (FGF-23) is both protectively phosphaturic229 and inhibitory of PTH secretion.203 FGF-23 is induced by calcitriol304 and in a feedback loop, FGF-23 inhibits calcitriol synthesis.229 The calcimimetic cinacalcet (Sensipar), previously used as an alternative to calcitriol’s PTH suppression13 during kidney disease,543,630 has recently been shown to be contraindicated131 due primarily to hyperphosphatemic consequences.284 High phosphorus is increasingly recognized193,291,359 as the major driver of cardiovascular calcification,280,367 which is the major cause of mortality in human patients with chronic renal disease.387 Calcitriol, in part due to its induction of FGF-23304 with its phosphaturic effects,229 can protect against vascular calcification,358,603,627 which is likely instrumental in the now widely recognized improved survival associated with use of calcitriol621 and other active vitamin D metabolites.620 Cinacalcet, although it suppresses PTH,630 fails to affect FGF-23, so with PTH suppressed, there is no protection against hyperphosphatemia caused by failure of renal excretion. Oral calcitriol improves survival in human renal failure patients before dialysis,584 a group that corresponds to the dogs and cats with chronic renal failure for which veterinary use of calcitriol has been recommended.401 A large body of work now demonstrates in humans the life protection conferred by calcitriol,617,643 and in placebo-controlled studies in the dog.456
Calcitriol is an important inhibitor of PTH synthesis, and it completes a negative feedback loop from the kidneys because PTH stimulates renal calcitriol synthesis. Short and long negative feedback loops complement each other to control normal secretion of PTH.313 The long negative feedback loop is completed when an increased serum iCa concentration results from PTH stimulation of renal calcitriol production and subsequent enhanced gastrointestinal absorption of calcium. This effect takes hours to develop because calcium-binding proteins associated with calcium absorption must be induced in enterocytes.72,601 The short negative feedback loop is mediated by the binding of calcitriol to VDRs in parathyroid cells, with inhibition of transcription of the PTH gene.535 The calcitriol receptor (VDR) is expressed in parathyroid chief cells at concentrations equal to those in intestinal epithelial cells that regulate calcium absorption in the gastrointestinal tract. The VDR was found to be depleted in the parathyroid glands of dogs and humans with uremia because of a lack of renal production of calcitriol.75 After the VDR binds calcitriol, the VDR-calcitriol complex acts in the nucleus of the parathyroid chief cells by binding to specific regions of the PTH gene called vitamin D response elements (VDREs) and inhibiting transcription of the PTH gene (see Fig. 6-6).313,401 For calcitriol to suppress synthesis of PTH, a normal concentration of iCa must be present because it would be inappropriate to suppress PTH synthesis in a hypocalcemic patient.
Clearance and metabolism of parathyroid hormone
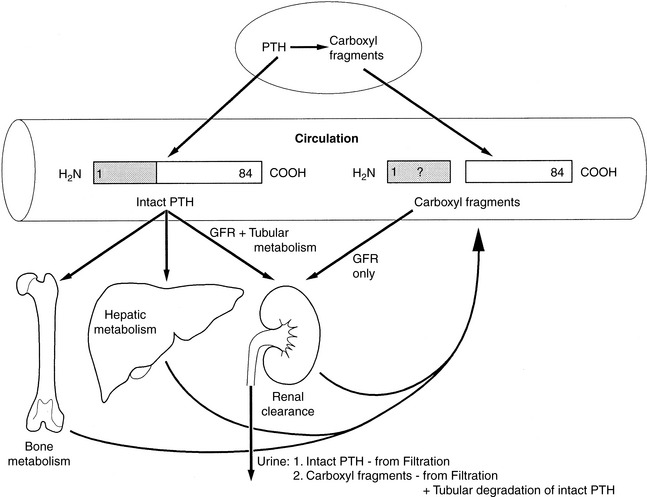
The intact PTH molecule (84 amino acids) circulates in the bloodstream with a half-life of 3 to 5 minutes and is removed by fixed macrophages.313,478 A significant amount of cleavage is close to the amino terminus of the PTH molecule. Regardless of where the endopeptidase cleavage occurs, the amino-terminal portion of PTH is completely degraded within the phagocytes.
The kidneys and bone also participate in destruction of intact PTH. Fragments of PTH are filtered by the glomeruli. This mechanism of excretion is most important for the excretion of the carboxyl-terminal PTH fragments because carboxyl-terminal PTH (released from either the parathyroid gland or Kupffer cells) is cleared only by glomerular filtration (Fig. 6-7). The carboxyl-terminal fragments of PTH are not important for calcium metabolism. The circulating half-life of carboxyl-terminal PTH is much longer than that of intact PTH, and serum concentrations of carboxyl-terminal PTH can be very high during primary or secondary hyperparathyroidism and can be nonspecifically increased during renal failure.
Actions of parathyroid hormone
An important action of PTH on bone is to mobilize calcium from skeletal reserves into ECF.102 The increase in blood calcium concentration results from an interaction of PTH with receptors on osteoblasts that stimulate increased calcium release from bone and direct an increase in osteoclastic bone resorption.393
The response of bone to PTH is biphasic. The immediate effects are the result of increasing the activity of existing bone cells. This rapid effect of PTH depends on the continuous presence of hormone and results in an increased flow of calcium from deep in bone to bone surfaces through the action of an osteocyte-osteoblast “pump” in order to make fine adjustments in the blood calcium concentration.433 The later effects of PTH on bone are potentially of greater magnitude and are not dependent on the continuous presence of hormone. Osteoclasts are primarily responsible for the long-term action of PTH on increasing bone resorption and overall bone remodeling.102,393
PTH also has the potential to serve as an anabolic agent in bone and stimulate osteoblastic bone formation.201,552 Intermittent administration of exogenous 1-34 PTH has been reported to increase bone mass in humans and animals.554
The ability of PTH to enhance the renal reabsorption of calcium is of considerable importance. This effect of PTH on tubular reabsorption of calcium is caused by, in part, a direct action on the distal convoluted tubule.631 PTH may also increase calcium reabsorption in the ascending thick limb of Henle’s loop indirectly by increasing the net positive charge in the nephron lumen and creating a stimulus for diffusion out of the lumen. PTH also regulates the conversion of 25-hydroxycholecalciferol to calcitriol and other metabolites of vitamin D.
Parathyroid Hormone C-Terminal 7-84 as PTH Antagonist
It was originally thought that PTH 35-84 and other fragments cleaved between residues 24 and 43 dominated the carboxyl-terminal fragments of PTH secreted by chief cells. The C-terminal fragments can be measured using C-terminal-specific immunoassays. The function of PTH 35-84 and its receptor is unknown, but it may regulate bone cell function. The larger C-terminal fragment, PTH 7-84,279 may be significantly increased in renal secondary hyperparathyroidism386 and can antagonize the effects of PTH 1-84 in vivo.321 The antagonistic action of PTH 7-84 is likely attributable to binding to an alternate PTH receptor and not to the PTH1 receptor that is used by PTH 1-34 and PTH 1-84.148,414
Parathyroid Hormone Receptor
The receptor for N-terminal PTH (amino acids 1 to 34), the region important in calcium regulation, has been cloned and sequenced in humans, dogs, and other species.1,416,542 It is a seven-transmembrane domain receptor that is expressed in renal epithelial cells, osteoblasts, and some other cells. The N-terminal regions of PTH and PTHrP bind this receptor with equal affinity. The PTH receptor is also located on many cell types, such as dermal fibroblasts, that are not associated with the action of PTH. It is assumed that the receptor functions as the binding protein for PTHrP in these tissues. The currently used terminology for this receptor is the PTH1 receptor, but it is often described as the PTH/PTHrP receptor. The PTH2 receptor is present in the brain and binds to both PTH and tuberoinfundibular peptide but not to PTHrP.249
Parathyroid hormone-related protein: a polyhormone
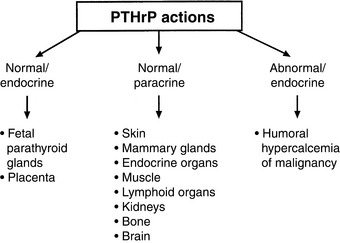
PTHrP is not strictly a calcium-regulating hormone, but it was identified in 1982 as an important PTH-like factor that plays a central role in the pathogenesis of humoral hypercalcemia of malignancy (HHM).480 PTHrP is produced widely in the body and has numerous actions in the developing fetus and adult animal independent of its role in cancer-associated hypercalcemia.451 This is in contrast to PTH, which is produced by the parathyroid glands and functions principally in regulation of calcium balance. PTHrP has multiple actions that are specific to the N-terminal, midregion, and C-terminal regions of the protein, making PTHrP a true polyhormone.
Some of the actions of PTHrP involve normal regulation of calcium metabolism.491 For example, PTHrP functions as a calcium-regulating hormone in the fetus and is produced by the fetal placenta.343 In the adult, PTHrP circulates in the blood in low concentrations (<1 pM) but is produced by many different tissues and functions principally as an autocrine, paracrine, or intracrine cellular regulator. PTHrP is produced by the lactating mammary gland and is secreted into milk. Mammary gland production of PTHrP likely facilitates mobilization of calcium from maternal bones and may play a role in the transport of calcium into milk during lactation.628,629 PTHrP acts as an abnormal systemic calcium-regulating hormone and mimics the actions of PTH in patients with HHM. PTHrP not only plays a major role in most forms of HHM but also has been demonstrated in many normal tissues, including epithelial cells of the skin and other organs; endocrine glands; smooth, skeletal, and cardiac muscle; lactating mammary glands; placenta; fetal parathyroid glands; bone; brain; and lymphocytes.451,478 Therefore, PTHrP functions as (1) a hormone in an endocrine manner in the fetus and lactating dams,582 (2) a paracrine factor in many fetal and adult tissues, and (3) an abnormal hormone in an endocrine manner in adults with HHM (Fig. 6-8). PTHrP is necessary for normal endochondral bone formation in the fetus and neonate. Knockout of the PTHrP gene results in short-limb dwarfism and death at birth as a result of a failure of cartilage proliferation at the growth plates and premature ossification.287
PTHrP is a 139- to 173-amino acid peptide originally isolated from human and animal tumors associated with HHM.480 PTHrP shares 70% sequence homology with PTH in its first 13 amino acids. The N-terminal region of PTHrP (amino acids 1 to 34) binds and stimulates PTH receptors in bone and kidney cells with affinity equal to that of PTH, so that PTHrP functions similarly to PTH in patients with HHM.124,423 The midregion of PTHrP is responsible for stimulating iCa uptake by the fetal placenta,343and the C-terminal region can inhibit osteoclastic bone resorption.181
The complementary DNA (cDNA) for canine and feline PTHrP has been cloned and sequenced.492,555 The sequence of canine PTHrP cDNA and gene indicated that the dog PTHrP gene is more closely related to the human PTHrP gene than are the PTHrP genes in rats, mice, and chickens.226 The deduced amino acid sequence of the N-terminal region (amino acids 1 to 36) is identical in five mammalian species (dog, cat, human, rat, and mouse), and there is a high degree of homology of the midregion of PTHrP in these species.350,492,551,555,633 The high degree of interspecies homology indicates the importance of the N terminus and midregion in the function of PTHrP.
There is less homology of the C-terminal region of canine PTHrP with that from other species. The function of the C-terminal region is unknown. PTHrP (107 to 111) and PTHrP (107 to 139) may inhibit osteoclastic bone resorption.182,548 Increased urine concentrations of C-terminal PTHrP have been demonstrated in humans and mice with cancer-associated hypercalcemia275,288 and in patients with renal failure.89 Increased C-terminal PTH is also seen in the sera of patients with renal failure and indicates that the kidneys are an important site of excretion of C-terminal PTHrP. C-terminal PTHrP may have a longer serum half-life than N-terminal or midregion PTHrP.
Parathyroid hormone-related protein in the fetus
Fetuses maintain higher concentrations of serum iCa than their dams. Fetal parathyroid glands produce low levels of PTH,105 and PTHrP functions to maintain iCa balance in the fetus.342,343 PTHrP is secreted by fetal parathyroid chief cells, and PTHrP is produced by the placenta, which is necessary for iCa uptake by the fetus.628 The midregion of PTHrP is the most active portion that stimulates iCa and iMg transport by the placenta. The placenta expresses the iCa-sensing receptor, which may contribute to the regulation of placental calcium transport.309 PTHrP is also produced by the uterus, where it is important in permitting relaxation of the smooth muscle of the muscularis as the fetus grows.565
Vitamin D
Vitamin D (calciferol) is classified as a secosteroid hormone.261 In tetrapods, the role of vitamin D via the calcitriol-activated VDR has evolved into one dominated by calcium regulatory mechanisms, but the roles in primitive species, including regulation of detoxification enzymes, have commonly been retained in more evolved life forms.596,613 These pleiotropic actions of vitamin D330 include, among others, important roles as antiproliferative and prodifferentiative mediators25 working in part via control of DNA replication164 and roles as immunomodulators,238 including effects on glomerulonephritis431 and encephalitis.205 A role of calcitriol to regulate expression of the insulin receptor has been described,345 as has a role in muscle.140 Of particular interest in uremic patients is the calcitriol increase of erythroid proliferation via burst-forming units.20 These pleiotropic effects of calcitriol can be related to important clinical applications in patients with renal or other metabolic disease.252 They may explain the clinical improvements noticed in dog and cat uremic patients treated preventively with low doses of calcitriol401 that were accomplished when calcitriol was used before any PTH elevation had occurred.
Vitamin D metabolism
The cholecalciferol (parent vitamin D3 of animal origin) metabolites 25-hydroxyvitamin D3 (calcidiol), 1,25-dihydroxyvitamin D3 (calcitriol), and 24,25-dihydroxyvitamin D3 are the most important of at least 30 metabolites. In domestic mammals, the same three metabolites derived from vitamin D2 (ergocalciferol of plant origin) are equally bioactive; thus, generic use of the terms 1,25-dihydroxyvitamin D and calcitriol is assumed to include metabolites of vitamin D3 or D2 derived from animal or plant origin, respectively. The 25-hydroxyvitamin D that is produced in liver is the major circulating form of vitamin D209 and serves as a pool for further activation by 1α-hydroxylation or catabolism by 24-hydroxylation.243,421 Only 25-hydroxylation and 1α-hydroxylation are important in the function of vitamin D.139
Synthesis
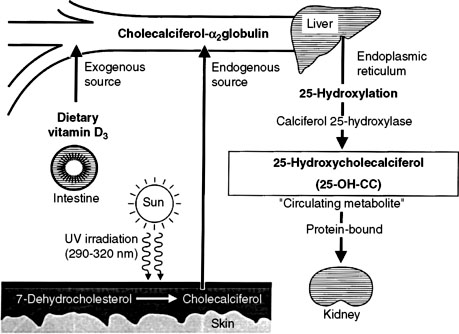
In humans, the requirement for vitamin D can be met by consumption of vitamin D2 or D3 or by synthesis of vitamin D3 (cholecalciferol) in the skin. Cholecalciferol is synthesized in the skin from 7-dehydrocholesterol after exposure to ultraviolet light. 7-Dehydrocholesterol forms previtamin D3 in the presence of ultraviolet B light at 288 nm, followed by further thermal conversion from pre vitamin D3 to vitamin D3.253 Dogs and cats inefficiently photosynthesize vitamin D in their skin and consequently are dependent on vitamin D in their diet.264 Vitamin D ingested in the diet is absorbed intact from the intestine.
Vitamin D-binding protein transports vitamin D to the liver and other target sites (Fig. 6-9).129 Hydroxylation of vitamin D occurs in the liver to produce 25-hydroxyvitamin D (calcidiol). The 25-hydroxylase activity is not influenced by calcium or phosphorus.209 Calcidiol does not have any known action in normal animals,139 but during vitamin D intoxication, high levels of calcidiol are produced by the liver and can induce hypercalcemia.
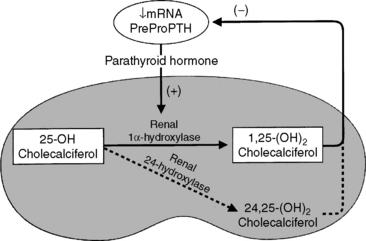
The most important step in bioactivation of vitamin D occurs as 25-hydroxyvitamin D is further hydroxylated to calcitriol in the proximal tubules of the kidneys.243 This reaction is tightly regulated by ionic and hormonal control mechanisms that modulate the activity of the hydroxylase enzyme systems (Fig. 6-10). The two principal enzyme systems involved are 25-hydroxyvitamin D-1α-hydroxylase (resulting in active calcitriol formation) and 25-hydroxyvitamin D-24R-hydroxylase (the first step of catabolism to inactive vitamin D metabolites). The activities of these enzymes are reciprocally regulated.421
The 1α-hydroxylase enzyme activity is localized within mitochondria of the convoluted tubules and portions of the straight proximal tubules of the kidneys. Little extrarenal 1α-hydroxylation of 25-hydroxyvitamin D occurs in other tissues except in human and rat placenta and skin and in some lymphoproliferative disorders.5,159 The 24-hydroxylation can also metabolize calcitriol, generating 1,24,25-trihydroxyvitamin D as the first step in the major catabolic pathway of calcitriol to biologically inactive calcitroic acid.261 Inactive vitamin D catabolites are excreted through the bile into feces, which is the only important excretory route; less than 4% is excreted into urine.139
Stimulation of Calcitriol Synthesis
Serum PTH, calcitriol, phosphorus, and calcium concentrations are the principal regulators for renal calcitriol synthesis.243 Chronic changes in serum calcium concentration regulate the synthesis of calcitriol, and these calcium changes can override signals from serum phosphorus and PTH concentrations.267 Deficiencies of phosphorus, calcium, and calcitriol lead to increased calcitriol formation.402 Low calcium or calcitriol concentrations lead to increased serum PTH concentrations. In the kidneys, PTH mediates dephosphorylation of renal ferredoxin (renoredoxin) and results in increased synthesis of calcitriol.212,533 Renoredoxin is the regulatory constituent of the 1α-hydroxylase enzyme system and is inhibited by phosphorylation in the presence of high concentrations of phosphorus or calcium in the renal tubule.243 PTH not only activates the renal 1α-hydroxylase but also induces synthesis of the enzyme from the renal gene encoding it.158,159
Several drugs and hormones have effects on vitamin D metabolism, some of which are stimulatory.65 Hypocalcemia and calcitonin directly stimulate 1α-hydroxylation independent of PTH.70 Estrogens increase calcitriol synthesis after up-regulation of PTH receptors in the kidneys,70 and testosterone may also increase calcitriol synthesis.640 Reduced dietary calcium intake can lead to stimulation of renal 1α-hydroxylase in the absence of detectable hypocalcemia.640
Inhibition of Calcitriol Synthesis
Calcitriol synthesis is inhibited by calcitriol, hypercalcemia, FGF-23, and phosphate loading.70,203,243 Calcium directly and indirectly inhibits calcitriol synthesis.175 The indirect action is caused by inhibition of PTH synthesis and secretion, thus removing the stimulus provided by PTH. The inhibitory effects of chronic hypercalcemia can override the stimulatory effects of increased PTH concentrations in calcitriol production, as may occur in primary hyperparathyroidism.267 The inhibitory effects of high concentrations of phosphorus on calcitriol synthesis are important and affect the activity of existing enzyme molecules.401,402
Actions of Calcitriol
Calcitriol is the only natural form of vitamin D with significant biologic activity.139,467 It is approximately 1000 times as effective as parent vitamin D and 500 times as effective as its precursor calcidiol (25-hydroxyvitamin D) in binding to the natural calcitriol receptor (VDR) in target cells.404 Calcitriol increases serum calcium and phosphorus concentrations, and its major target organ for these effects is the intestine.72 However, there is also an important contribution from bone,549 and calcitriol stimulates the kidneys to reabsorb both calcium and phosphorus from the glomerular filtrate. Calcitriol has multiple indirect effects on calcium balance, including up-regulation of calcitriol receptors in patients with uremia, regulation of PTH synthesis and secretion by the parathyroid gland,635 and prevention or reversal of parathyroid gland hyperplasia in the uremic patient.202,401
The calcitriol receptor
The VDR for calcitriol is present in many tissues in addition to bone, kidneys, intestine, and parathyroid gland.237 The importance of calcitriol in tissue is proportional to the abundance of the VDR in the cells, and this is highly regulated.311 Intestinal epithelial cells and parathyroid gland chief cells have equal and high concentrations of VDR. VDR genetic polymorphisms are thought to generate variation of efficiency of the VDR.84,110 Calcitriol initially dissociates from its serum binding protein, diffuses across the cell membrane, and binds with its receptor.
Effects of Calcitriol on the Intestine
Calcitriol enhances the transport of calcium and phosphate from the intestinal lumen to plasma across the enterocyte.73,601 Energy in the form of adenosine triphosphate (ATP) is required to transport calcium from the enterocytes into the blood and to absorb phosphate from the intestinal lumen. Calcitriol induces synthesis of the plasma membrane calcium pump (ATPase) that removes calcium from the enterocytes432 and the Na+-phosphate cotransport protein that transports phosphorus into the enterocyte. In addition, calcitriol increases the brush border permeability to calcium and induces the synthesis of calbindin-D 9k.125,567 Calbindins serve as buffers to protect enterocytes from toxic concentrations of calcium ion while ferrying calcium across the cell.601 Calcitriol also directly stimulates rapid calcium transport (transcaltachia) across the enterocyte.418 Normal dogs have a progressive decrease in the number of calcitriol receptors and calbindin concentrations that regulate the efficiency of calcium absorption in enterocytes from the duodenum to the ileum.307 Longer transit times in certain portions of the intestinal tract (e.g., ileum) can still lead to significant calcium absorption despite low transport efficiency.601
Effects of Calcitriol on Bone
Calcitriol is necessary for bone formation and mineralization because it ensures an adequate source of calcium and phosphorus from the intestinal tract. Deficiencies in vitamin D lead to impaired bone growth, such as rickets in growing animals and osteomalacia in adults.478 Calcitriol is necessary for normal bone development and growth because it regulates the production of multiple bone proteins produced by osteoblasts, including alkaline phosphatase (ALP), collagen type I, osteocalcin, and osteopontin.19,544 Calcitriol is also necessary for normal bone resorption because it promotes differentiation of monocytic hematopoietic precursors in the bone marrow into osteoclasts.549 This relationship between calcitriol and osteoclasts explains the dependence of PTH on calcitriol for optimal bone resorption.403
Effects of Calcitriol on the Kidneys
An important effect of calcitriol in the kidneys is direct inhibition of 25-hydroxyvitamin D-1α-hydroxylase in the renal tubule, preventing overproduction of calcitriol.467 In addition, calcitriol facilitates calcium and phosphorus reabsorption from the glomerular filtrate.318 Calcitriol is necessary to work with PTH to reabsorb urinary calcium into blood. Glomerular podocytes contain the VDR for calcitriol and respond to low doses of calcitriol with decreased injury and loss of podocytes.316 In glomerulonephritis, low doses of calcitriol decreased mesangial proliferative nephritis, which involved calcitriol abrogation of inflammatory mediators interleukin (IL)-1α, tumor necrosis factor-α (TNF-α), and IL-6 in the mesangium.430 Although calcitriol has generally been thought to protect the kidneys during CRF by preventing the damage from excess PTH,403,635 it is becoming clear that calcitriol has direct beneficial effects on the diseased kidney as well.
Effects of Calcitriol on the Parathyroid Gland
Calcitriol inhibits the production of PTH in the parathyroid gland by direct and indirect means.531,537 Binding of calcitriol to its receptor in parathyroid chief cells directly inhibits PTH synthesis. Second, calcitriol stimulates intestinal calcium absorption, which indirectly reduces PTH secretion by increasing serum iCa concentration. Calcitriol suppression of PTH synthesis is dose dependent and occurs before serum iCa concentration is increased by the delayed effects of calcitriol on intestinal calcium transport.540 Calcitriol may be considered the primary controlling factor for transcription of the PTH gene and subsequent synthesis of PTH because suppression of PTH synthesis cannot occur in the absence of calcitriol even in the presence of hypercalcemia (see Fig. 6-6).402,537 PTH secretion decreases 12 to 24 hours after exposure to calcitriol. Whereas PTH stimulates renal calcitriol synthesis, calcitriol is a negative regulator of PTH. Long-standing calcitriol deficiency results in chief cell hypertrophy and hyperplasia, demonstrating that calcitriol is important in limiting cellular proliferation in the parathyroid gland.537 Calcitriol treatment of uremia in dogs and humans has resulted in regression of parathyroid gland hyperplasia.202,404 Calcitriol can be used to prevent development of hyperparathyroidism in dogs and cats with early stages of CRF.401 This has proved to be highly successful and is consistent with developing thinking in the human medical profession.642
Calcitriol in cancer therapy?
Many studies focus on the benefits of calcitriol therapy in cancer.222,254 Part of the great interest stems from the antiproliferative role of calcitriol,25 with specific effects on DNA replication genes164 and with a potentially important effect on proliferation of blood vessel endothelial cells.42 Studies are focused on human prostate cancer312 and also on breast and colon cancers.254 Although a discussion is beyond the scope of this chapter, its dynamic character indicates it will be important for many years to come.
Calcitonin
Calcitonin is a 32-amino acid polypeptide hormone that is synthesized by C cells in the thyroid gland.389,478 An important role of calcitonin is to limit the degree of postprandial hypercalcemia. This effect, in concert with PTH, acts to maintain serum iCa concentration within a narrow range. Calcitonin is secreted in response to hypercalcemia and also to a calcium-rich meal. Calcitonin secretion increases during hypercalcemia, but the effects of calcitonin on normal calcium homeostasis are considered to be minor. The major target site for calcitonin is bone, where it inhibits osteoclastic bone resorption. The effects of calcitonin in bone are transitory, which has limited the usefulness of calcitonin as a treatment for hypercalcemia. At high doses, calcitonin may promote urinary calcium excretion.81
Normal homeostatic response to hypocalcemia
Hypocalcemia elicits corrective responses that are mediated by PTH and calcitriol.478 Acute effects occur in seconds to minutes; subacute effects occur over several hours; and chronic effects occur over days to weeks. A marked increase in PTH secretion occurs in response to mild hypocalcemia, and this response occurs in seconds. Acute secretion of preformed PTH can maintain PTH concentrations for 1 to 1.5 hours during hypocalcemia. Hypocalcemia decreases the proportion of PTH that is degraded in the parathyroid chief cells, making more PTH available for secretion. This effect is relatively rapid (approximately 40 minutes). During increased PTH secretion, renal calcium reabsorption and phosphorus excretion are increased within minutes, whereas bone mobilization of calcium and phosphate occurs within 1 to 2 hours.
After several hours of hypocalcemia, increased PTH secretion stimulates the synthesis and secretion of calcitriol. Increased intestinal transport of calcium and phosphorus into blood follows, providing an external source of calcium in addition to the internal mobilization from bone. Hypocalcemia increases transcription of the PTH gene and synthesis of PTH mRNA, enhancing the ability of the chief cells to produce PTH. This effect also occurs within hours of hypocalcemia. Over days or weeks of hypocalcemia, further increases in PTH secretion are achieved largely by hypertrophy and hyperplasia of chief cells in the parathyroid gland.497 In addition, the proportion of chief cells actively synthesizing PTH is increased.
Normal homeostatic response to hypercalcemia
Most of the effects that occur during hypercalcemia are the opposite of those described earlier for hypocalcemia.478 Hypercalcemia results in decreased PTH secretion, increased intracellular degradation of PTH in chief cells, and decreased PTH synthesis. Increased calcitonin secretion is stimulated in an attempt to minimize the magnitude of hypercalcemia. In addition, hyperplasia of C cells in the thyroid gland results if the hypercalcemic stimulus is sustained, but this mechanism is ineffective for controlling hypercalcemia because of the transitory effect of calcitonin on osteoclastic bone resorption.420,484 Calcitriol synthesis is decreased both through direct inhibition by iCa and as a result of decreased stimulation because of decreased PTH concentration.
Diagnostics
Table 6-1 lists the normal values for serum tCa,115 iCa,114 PTH,404,577 PTHrP,489 and vitamin D metabolites that are useful in the diagnostic workup of patients with calcium disorders.478
Table 6-1 Normal Serum Concentrations
| Dog | Cat | |
|---|---|---|
| Total Calcium | ||
| mg/dL | 9.0-11.5 | 8.0-10.5 |
| mmol/L | 2.2-3.8 | 2.0-2.6 |
| Ionized Calcium | ||
| mg/dL | 5.0-6.0 | 4.5-5.5 |
| mmol/L | 1.2-1.5 | 1.1-1.4 |
| Parathyroid Hormone (PTH) | ||
| Intact (pmol/L) | 2-13* | 0-4* |
| N-terminal (pg/mL) | 5-55 | 8-28 |
| Parathyroid Hormone Related protein (PTHrP) (pmol/L) (intact or N-terminal) | <1.0* | <1.0* |
| 25-Hydroxyvitamin D | 60-215* | 65-170* |
| (calcidiol) (nmol/L) | ||
| 1,25-Dihyroxyvitamin D | ||
| (calcitriol) (pg/mL) | ||
| Adults | 20-50 | 20-40 |
| 10-12-week old | 60-120 | 20-80 |
* Data from Endocrine Diagnostic Section, Diagnostic Center for Population and Animal Health, Lansing, Michigan.
Total calcium
Despite the fact that only the iCa fraction is physiologically active, the calcium status of animals is usually initially based on evaluation of the serum tCa concentration. Measurement of tCa concentration is more readily available than iCa measurement, but it does not always accurately reflect the iCa concentration of the patient. The serum tCa concentration has been assumed to be directly proportional to iCa, but in many clinical conditions, this may lead to erroneous interpretation of laboratory data. In humans with disorders of calcium balance, measurement of serum tCa concentrations failed to predict serum iCa concentrations in 31% of all patients566 and in 26% of patients with renal disease.88 In 1633 canine samples, diagnostic disagreement between serum iCa and tCa was 27%, and in dogs with CRF, this disagreement was 36%.519 In cats, serum iCa concentrations were only moderately correlated with serum tCa concentrations,142 and a 40% diagnostic disagreement between serum iCa and tCa measurement was noted in 434 cats.518 In dogs, tCa measurement overestimated normocalcemia and underestimated hypocalcemia,519 and in cats, hypercalcemia and normocalcemia were underestimated, and hypocalcemia was overestimated when using serum tCa concentration to predict iCa status.518
Analytical Methods
Fasting serum or heparinized plasma samples should be submitted for analysis. Oxalate, citrate, and ethylenediaminetetraacetic acid (EDTA) anticoagulants should not be used because calcium is bound to these chemicals and becomes unavailable for analysis.623
Serum tCa concentrations vary with the method used. Isotope dilution with subsequent mass spectrometry constitutes the definitive method for calcium measurement but is not readily available.200 For clinical determination of serum tCa concentration, simple colorimetric reactions and spectrophotometry are usually employed using automated or manual methods. Ortho-cresolphthalein complexone is a metal dye that is commonly used to form a color complex with calcium. This method is accurate and reproducible.200 Hemolysis can result in formation of an interfering hemoglobin-chromogen complex that falsely increases measured calcium concentration. High concentrations of bilirubin falsely decrease, and acetaminophen and hydralazine falsely increase serum tCa concentration. Lipemia can result in spuriously high calcium concentrations,380 with values exceeding 20 mg/dL in some instances of severe lipemia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree