Pamela A. Wilkins, Amelia R. Woolums, Consulting Editors ▪ Diagnostic Procedures for the Respiratory System Kara M. Lascola, Pamela A. Wilkins, Consulting Editors As with any disease process, acquisition of an accurate and appropriate history is the first step undertaken in evaluating the patient with a complaint thought to be related to the respiratory tract. Animals with respiratory disease may have widely varied histories and it is important to gather as much information as possible. Age and breed may play a role in the development of respiratory disease, such as congenital defects or neoplastic disease, or in inherited or acquired immunodeficiency syndromes seen in certain breeds. The environment in which the animal is maintained can contribute to the development or severity of respiratory disease, heaves in horses for example, and respiratory disease may become manifest following a change to a new environment. The work horses are expected to perform can lead to important diagnostic clues, and recent events, such as long-distance transport, can predispose horses to diseases such as pleuropneumonia. It is important to know if certain diseases are either endemic or epidemic where the horse is kept or has recently moved from; diseases such as strangles and Rhodococcus equi bronchopneumonia of foals come to mind. Any recent traumatic or potentially traumatic event should be noted. If possible, a thorough vaccination history should be obtained, as should an accurate history of any administered treatments or supplements and the patient’s response to those treatments. There are many presenting signs or chief complaints that should lead to more thorough evaluation of the respiratory system, some more directly associated with either the upper or lower respiratory tract. Findings or complaints associated with respiratory disease include nasal discharge, either bilateral or unilateral. Respiratory noise at rest or during exercise is commonly associated with abnormalities of the upper airway, as may be inequalities of airflow present at the nares. Normal animals may periodically cough or sneeze, but an increase in either activity may indicate involvement of the respiratory tract. Exercise intolerance or apparent decrease in the ability of the animal to exercise should prompt evaluation of the respiratory system. Other clinical signs that indicate thorough evaluation of the respiratory tract include, but are not limited to, abnormal breathing patterns (tachypnea, hyperpnea, dyspnea), cyanosis, hemoptysis, epistaxis, unusual swellings (facial, pharyngeal, cervical), lymphadenopathy, ataxia or reluctance to move, foul smell to the breath, weight loss, and ventral abdominal, sternal, or limb edema. The initial physical examination occurs at some distance from the patient and involves evaluation of the demeanor, posture, mental status, and way of movement of the patient. It is important to note if the patient has an abnormal stance, such as standing with the head and neck extended. If the patient is unwilling to move or stands with elbows abducted, this suggests pleural pain is present. Ideally, the respiratory rate can be determined by observation as can the respiratory pattern. While some respiratory diseases are not manifest at rest, important clues can be gained from observation of the patient at rest in many others. The normal resting respiratory rate of an adult horse is between 8 and 16 breaths per minute, for adult cattle 15 to 35 breaths/min; and sheep and goats, 12 to 20 breaths/min. There is some small abdominal component during the expiratory phase, which is, along with inspiration, an active process for horses. The normal rate for neonates is up to 60 breaths/min at birth and less than 30 breaths/min by 1 month of age; respiratory rate decreases toward the adult rate with age. High ambient temperature, fever, and excitement can all increase respiratory rate. Normal breathing, which is quiet and apparently effortless, is termed eupnea. The term dyspnea is a breathing pattern that is inferred by the observer to reflect difficulty in breathing; the animal will appear distressed, and the work of breathing is obviously increased, although the actual rate may be within normal limits. Other terms used to describe breathing patterns include tachypnea, characterized by rapid rate and shallow depth or low tidal volume; hyperpnea, with increased frequency and depth of breathing (an example would be during postexercise recovery); and apnea, in which there is no discernible breathing. Two additional terms include hypoventilation and hyperventilation, both of which require a change in arterial carbon dioxide partial pressure as a component of their definitions. Hyperventilation is a pattern that increases alveolar ventilation and causes arterial hypocapnia, whereas hypoventilation alters gas exchange in such a way to cause arterial hypercapnia, or retention of carbon dioxide. Closer examination can reveal some of the physical manifestations of presenting complaints listed previously. Beginning with the head, the clinician should determine that airflow is even from both nostrils, as differences can indicate either congenital or acquired abnormalities ranging from choanal atresia to the presence of upper airway masses. Abnormal respiratory sounds can sometimes be present at rest and may be heard at the nares. Abnormal breath odors may be prominent, particularly at the nares. The frontal and maxillary sinuses should be percussed; identification of abnormal resonance, usually dullness, may be made easier by performing this with the mouth held open. Palpation of the submandibular regions, larynx, and pharyngeal and cervical regions should be performed to identify any abnormal lymph node enlargement, masses, or areas of muscular atrophy. Both jugular veins should be checked for patency and the presence of any evidence of injection sites or infections that may contribute to abnormal upper airway function by interfering with normal recurrent laryngeal nerve or vagosympathetic trunk function. Coughing represents a nonspecific irritation of receptors in the airway and can be induced by many mechanisms. It can be, and usually is, a normal protective reflex that allows the animal to clear material from the airway. Cough can be associated with increased mucus production, production of other respiratory secretions, or decreased mucociliary clearance. In older horses cough is most commonly associated with heaves, whereas in younger horses cough is associated with infectious diseases and small airway inflammatory disease. Normal animals should not cough when the larynx or trachea is palpated. Nasal discharge can be unilateral or bilateral, scant or copious, clear, mucoid, mucopurulent, or even bloody. The nature and character of nasal discharge can provide some information about a possible source of the discharge, but this information should not be overinterpreted. Horses, for example, have a tendency to swallow excess airway secretions, and the volume of secretions may be underestimated. Whereas unilateral nasal discharge seems to suggest a source in front of the larynx, bilateral nasal discharge can be of either upper or lower airway origin. Skin depigmentation of the ventral nares or presence of mucoid material in feed or water containers is a clue to presence of nasal discharge. Hemoptysis is the coughing up of blood from the airways or lungs. It is important to determine conclusively that the blood has come from the respiratory system. Epistaxis is defined as blood seen at the nares and often originates in the nasal passages, sinuses, turbinates, nasopharynx, or equine guttural pouches, although the lung can be, and is, a source on occasion, as in exercise-induced pulmonary hemorrhage (EIPH) or following lung biopsy. Bilateral epistaxis generally indicates bleeding caudal to the choanae. Because animals tend to swallow excessive respiratory secretions, bleeding can be occult and may not be seen unless the animal drops its head toward the ground. Significant blood loss can occur in this manner, unseen by owners. Examination of the oral mucous membranes may reveal cyanosis, bluish discoloration of the oral, nasal, or vulvar mucous membranes. Cyanosis does not become apparent until 5 mg/100 mL of deoxygenated hemoglobin, about one third of the total normal hemoglobin, is present; this reflects a profound decrease in oxygen saturation of hemoglobin and is suggestive of severe hypoxemia. As it is the total quantity of deoxygenated hemoglobin that lends the mucous membranes the bluish color, very anemic patients may lack sufficient deoxygenated hemoglobin to appear blue, which makes appreciation of cyanosis impossible in these patients. One caveat is that all newborns are cyanotic for the first few breaths and only become pink when they have established neonatal, as opposed to fetal, cardiorespiratory circulation and have opened their lungs to allow for gas exchange. It is important that auscultation of the thorax take place in as quiet an environment as possible. Additionally, auscultation of the lung fields should be performed under two breathing conditions: eupnea and hyperpnea, with hyperpnea induced by the use of a rebreathing bag. Some common misconceptions regarding the use of a rebreathing bag exist. Simply occluding the animal’s nostrils is inadequate to fully examine the patient, as is using a rectal sleeve as a rebreathing bag. The purpose is to cause the animal to rebreathe its own expired carbon dioxide, not to necessarily deprive it of oxygen. Rebreathing expired carbon dioxide results in increased Paco2, which stimulates deeper and more frequent breathing efforts, making recognition of abnormal lung sounds simpler. The bag used should be large enough to accommodate 2 to 3 times the normal tidal volume of the animal and should be held in such a manner as to prevent the bag from occluding the patient’s nostrils. Once the bag is removed, the animal will usually take several very deep breaths and the examiner should take advantage of these very large breaths to reexamine areas where suspicious sounds were heard during rebreathing. Animals with significant lung pathology will not tolerate the bag well, may cough when the bag is removed, and may require more time to return to baseline respiratory patterns when the bag is removed. Normal breath sounds are those produced by turbulence within the tracheobronchial tree and may vary considerably depending on location within the lung, breathing pattern, and condition of the animal.1 Only airways from the larynx to segmental bronchi contribute to sound generation; bronchial and vesicular sounds represent larger airway flow events. Vesicular sounds—the quietest sounds, heard over the middle and diaphragmatic lung regions—correlate best with regional ventilation and mainly represent segmental bronchial sounds; they do not represent air flow in terminal conducting airways and alveoli, which is silent due to the nature of its flow. Bronchial sounds are louder and heard best over the trachea and base of the lung. Common abnormalities found during auscultation include ventral areas of dullness if pleural effusion is significant, dorsal areas of dullness or hyperresonance with pneumothorax, and dorsal harsh lung sounds. The degree of variation in normal lung sounds is large, and auscultatory findings do not always correlate well with the degree of lung abnormality. That said, abnormal lung sounds are always potentially clinically important. Adventitious lung sounds are divided into short discontinuous sounds called crackles and longer continuous sounds called wheezes, replacing the older terms rales and rhonchi, respectively. Crackles are most commonly generated by sudden pressure equalization when collapsed airway segments open. Although an air/fluid interface is required, crackles do not necessarily imply excessive secretions or pulmonary edema. They are often end-inspiratory and associated with reinflation of atalectatic lung. Crackles may be normal when auscultated in the previously down lung of a laterally recumbent neonate. Disease processes that generate crackles include pneumonia, interstitial fibrosis, chronic obstructive lung disease, congestive heart failure, and atelectasis.2 Wheezes commonly represent oscillation of airway walls before complete closing (expiratory) or opening (inspiratory). Intrathoracic airways are usually involved in expiratory wheezes and include the lower trachea, main, lobar, and segmental bronchi. Disappearance of a wheeze after coughing indicates secretory rather than tissue component origin. Disease processes responsible for wheezes include airway stenosis or external compression; airway lumen compromise by foreign body, purulent material, cyst, or neoplasm, and airway wall thickening as in chronic bronchitis; and bronchoconstriction. Expiratory wheezes are a hallmark of obstructive lung diseases such as heaves. Crackles and wheezes may be variably present. A final category of adventitious sounds is the “rubbing” or “creaking” sounds generated by sliding or stretching of inflamed pleural surfaces, commonly termed pleural friction rubs. Percussion of the thorax is performed by methodical tapping over the intercostal spaces of the thorax using a variety of instruments, including plexors, pleximeters, spoons, fingers, neurologic hammers, and hands. It is an inexpensive and useful component of the physical examination and should be performed in all patients where the respiratory system is suspect. Percussion of the thorax can reveal hyporesonance (dullness) ventrally when pleural effusion is present, reveal hyperresonance dorsally in pneumothorax, and cause some patients to exhibit pleurodynia during the examination. Other conditions that can alter resonance of the thorax include, but are not limited to, diaphragmatic hernia with intrathoracic intestine, pericardial effusion, pulmonary and pleural abscessation, and consolidated lung. The point at which a change occurs from resonant to dull can be marked with adhesive tape. Thus the outline of aerated lung immediately beneath the chest wall is delineated. It is usually impossible to fully delineate the lung field cranially because of body fat and triceps musculature. There is a distinct region of cardiac dullness for all species on the left side. Percussion allows delineation of pleural effusion and intrathoracic masses or consolidated lung up to 7 cm beneath the pleural surface, but cannot distinguish between them. The procedure should be performed whenever pleural effusion is suspected on the basis of auscultatory findings and in all ruminants as part of the physical examination to uncover occult pneumonia.3 The upper airway can be directly examined with the aid of an endoscope, the only limitations being the size of the patient, the patency of the airway, and the size of the available equipment. Standard flexible fiberoptic endoscopes, available to most practitioners and present now in virtually all referral hospitals, allow direct examination of the nasal passages, ethmoid turbinates, nasal maxillary opening of the sinuses, pharynx, guttural pouch openings, larynx, and cranial trachea (Fig. 31-1). Smaller (8 to 10 mm in diameter) endoscopes can be readily introduced into the equine guttural pouches, with the aid of a biopsy instrument, and longer endoscopes (more than 150 cm long with diameters greater than 10 mm) are commonly employed to examine main stem bronchi and their initial branches in large animals.4 Smaller species, such as camelids, may require the use of a smaller diameter (3 to 5 mm) endoscope or bronchoscope. Small brushes, used for collecting exfoliated cells for cytologic study, and a variety of biopsy instruments can be used for sampling the airway. Airway endoscopy in the horse has evolved to include dynamic endoscopy (e.g., treadmill, overland endoscopy) during exercise to evaluate the dynamic function of the upper respiratory tract.3,4a Objective measurements can be made by use of videoendoscopy with slow motion or freeze-frame features.3 Treadmill endoscopy is available at specialty practices and typically is performed at high speed (12 to 14 m/s) using incremental standardized testing protocols. Overland endoscopy has recently become more readily available to certain specialty practices and is performed during ridden exercise in the field with endoscopy equipment that is attached to the horse.4a Advantages of overland endoscopy include the ability to evaluate the horse under saddle and in natural conditions. Standardizing exercise tests in the field may, however, be more challenging. Sedation/tranquilization will aid many standing endoscopic examinations, but examinations aimed at evaluating pharyngeal and/or laryngeal function are best performed without any form of chemical restraint that might alter function. Most horses will allow standing examination of the upper airway with only physical restraint, such as judicious use of a nose twitch. Introduction of the endoscope into the trachea may elicit coughing, particularly in horses but less so in cattle. Small ruminants, such as sheep and goats, may require local tracheal anesthetic administration in the form of 2% lidocaine administered through small tubing passed through the biopsy channel of the endoscope. If lidocaine is used, it must be diluted and must not reach a toxic dose in small ruminants. Diluted topical 2% lidocaine can similarly be used in horses and cattle, if need be, for evaluation of the distal trachea, main stem bronchi, and larger bronchial tree branches. Horses are more sensitive to tracheal and bronchial stimulation and are more likely to require topical anesthesia than are cattle. Guttural pouch diseases and upper airway abnormalities, such as pharyngeal lymphoid hyperplasia, laryngeal hemiplegia, epiglottic entrapment by arytenoepiglottic folds, dorsal displacement of the soft palate, pharyngeal cysts, retropharyngeal masses, and epiglottic deformities, are best diagnosed by endoscopic examination. Direct tracheobronchoscopic examination is best for the diagnosis of EIPH if it is performed within a few hours of exercise. Bronchoscopy with a longer endoscope may be useful for evaluating additional lower airway abnormalities, such as recurrent airway obstruction in horses. The degree and nature of airway secretions accumulating in the trachea can be easily assessed with an endoscope, and accumulated secretions may be sampled by aspirating the secretions through small tubing introduced into the trachea through the biopsy channel. Because the endoscope has passed through the nonsterile upper airway, these samples are best suited for cytologic, not microbiologic, evaluation but may be fully compatible with evaluation using molecular diagnostic techniques.5–8 Endoscopy has also been used to help remove foreign bodies from the airway, generally aided by the biopsy instrument. Radiographs are indicated when the clinician suspects a congenital anomaly involving any thoracic structure; infectious disease of the pleura, pulmonary parenchyma, tracheobronchial tree, or mediastinum; pneumothorax or pneumomediastinum; thoracic neoplasia of any origin; or trauma. Radiographs are frequently performed along with thoracic ultrasonographic evaluation. If significant accumulation of pleural fluid is suspected based on physical examination findings, the ultrasonographic portion of the examination should be performed first and radiographs obtained following drainage of excess fluid, as fluid may obscure potentially important parenchymal disease. The equipment needed to perform radiographic evaluation of the upper airway is available in most private practices, while most large referral and university practices will have the equipment needed to perform thoracic radiography in larger patients such as adult horses and cattle. Conventional and digital radiography are available to most practitioners. Digital radiography has replaced conventional radiography in many practices and referral clinics because of recent improvements in affordability and the range of systems offered. Advantages to these systems include portability and speed of image processing along with a greater range of radiographic exposures and tools for image manipulation.9 Because of its configuration, the thorax in adult horses and cattle is filmed as standing lateral, generally requiring a series of three to four separate but overlapping images; thus the benefit of the ventrodorsal view in which the two lungs may be compared is lost. Obtaining standing radiographs bilaterally may aid in localizing lesions to one hemothorax or the other. Neonates and small ruminants can be more readily handled and retained in recumbent positions, allowing for multiple recumbent views. Other imaging modalities, such as computed tomography (CT) and magnetic resonance imaging (MRI), are available at select referral practices and may aid in the characterization of certain upper airway disorders in large animal species. In horses, CT has been used in the diagnosis of sinonasal disease, particularly ethmoid hematomas,10 and MRI has been used for the premortem diagnosis of laryngeal dysplasia associated with branchial arch abnormalities.11 The application of CT and MRI toward routine use in the diagnosis of airway disease in large animal species is limited because of availability of equipment, cost of procedure, the need for general anesthesia in most instances, and size restrictions. Imaging of pulmonary disease is limited to immature animals or smaller species such as camelids.12–14 Skull and cervical radiographs offer diagnostic information for evaluation of the upper respiratory tract. For large animal species, standing lateral skull films are easily obtained, and, with practice and adequate sedation, ventrodorsal and oblique projections can be obtained in most patients. Certain difficult patients may require general anesthesia in order to obtain radiographs of diagnostic quality. Skull radiographs image the sinuses, pharynx, and larynx, allowing for assessment of anatomic dimensions of the pharyngeal and laryngeal structures. Sinuses affected by neoplasia or inflammation may show abnormal tissue density, a horizontal fluid line on a standing lateral film, bone lysis around the affected sinus, or alveolar periostitis. Thorough evaluation of the sinuses and nasal passages requires lateral, dorsoventral, and oblique views. Foreign bodies can be assessed in many cases. The equine guttural pouches are evident on lateral skull projection, and abnormal fluid accumulation, distortion by enlarged retropharyngeal lymph nodes, and emphysema can be radiographically apparent. Radiographic assessment of the thorax of large animals remains preferable to ultrasonographic examination for detection of diffuse parenchymal diseases, such as interstitial pneumonia, pulmonary edema, equine multinodular pulmonary fibrosis (EMPF), fungal pneumonia, acute lung injury (ALI), acute respiratory distress syndrome (ARDS), chronic disorders, and deep parenchymal or mediastinal abscesses. Unfortunately, many radiographic changes in equine respiratory disorders tend to be nonspecific or, in certain diseases, such as EIPH, inflammatory airway disease, or heaves, minimal to nonexistent. Four types of radiographic patterns are described for the thorax: alveolar (airspace), interstitial, bronchiolar, and vascular. Opaque areas coalesce and fully obliterate vessels and bronchi in the alveolar pattern, air bronchograms may be prominent, and this pattern is common in pulmonary edema, pulmonary hemorrhage, EMPF, ALI, ARDS, lung consolidation, and neoplasia. Interstitial patterns are the most common pattern noted in equine thoracic radiographs and are characterized by a blurring of the edges of pulmonary vessels, a diffuse increase in lung density, and variable reticular, linear, and nodular opacities. The reticular pattern is most commonly associated with more diffuse infectious lung diseases, pulmonary edema, interstitial pneumonia, and pulmonary fibrosis, whereas the irregular linear pattern is seen most commonly with resolving bronchopneumonia. A nodular pattern is seen with abscesses, granulomata, and neoplasms. It is rare to see a pure bronchial pattern in a horse; it is usually seen in association with an interstitial pattern. An exception is paired linear opacities or numerous small circular opacities (donuts) representing thickening of large or medium airways in equine bronchitis/bronchiolitis. The vascular pattern is seen in horses radiographed immediately after exercise or in animals with left to right cardiac shunts. Finally, extraparenchymal problems such as pleural effusions or free gas may be seen on thoracic radiographs of large animals. Thoracic radiology may be used for evaluation of potential rib fracture, but it is far less sensitive than thoracic ultrasonography in this regard. Thoracic ultrasonography, a companion to thoracic radiography, is useful for diagnostic, therapeutic, and prognostic evaluation of the extraparenchymal thorax, the pleural space, and the peripheral (superficial) parenchyma of the lung. Unlike thoracic radiography, for which specialized equipment is needed to image the adult large animal, thoracic ultrasonography is an imaging technique readily available to most practitioners. In many instances it is superior to thoracic radiography as an imaging method; examples include evaluation of pleural effusions, assessment of thoracic trauma, evaluation of neoplasms or granulomata, detection of mediastinal masses/abscesses, and guidance of transthoracic lung biopsy.15,16 Ultrasonography is considered greatly superior to thoracic radiography in the detection of rib fractures.17 This imaging technique should be considered for complete evaluation of any large animal with suspected or diagnosed pulmonary disease. Ultrasonography is generally performed with the patient standing, although in neonates lateral recumbency may be preferred or even necessary, and sound waves are generated by piezoelectric crystals, transmitted to the area of interest through a skin coupling gel with subsequently reflected echoes detected by the same crystal. Echo signals from all tissue interfaces are displayed on a screen, which can be photographed for a permanent record or stored digitally. Air trapped beneath the haired skin can interfere with the process, as can excessive skin dirt, so preparation of the acoustic window usually involves hair removal and cleansing in order to get the best image possible. Although ultrasound waves will not penetrate the aerated portion of the lung, limiting the examination to extraparenchymal surfaces in normal horses, ultrasonography is superior to thoracic radiography for the evaluation of these areas of the chest. Small amounts of pleural fluid that would be missed on auscultation, percussion, or thoracic radiographs can be detected, and the amount and character of pleural effusion in each hemithorax can be evaluated separately.15 Clear fluid is anechoic, but inflammatory cells, gas, and fibrin are echogenic, causing opacities that can be seen floating in pleural fluid and altering the general echogenicity of the fluid. Because of this, ultrasound is the method of choice for diagnosis and monitoring of pleural space disease. Ultrasonography should be used to guide catheter placement for drainage of accumulated fluid in the pleural space. The pleural surfaces are imaged well by ultrasound with thickened or roughened areas easily detected. Lack of normal independent movement of the visceral and parietal pleural surfaces during the respiratory cycle, suggestive of adhesion formation, can be readily monitored.15,16 Consolidated lung is a better acoustic medium than aerated parenchyma and can be well visualized. If there is pleuropneumonia with consolidation or atelectasis caused by compression of the ventral lung by pleural effusion, it will be evident. Pulmonary abscesses or masses extending to the lung surface can be imaged and ultrasound can be used for guidance during transthoracic biopsy.15,16 Thoracic radiography remains superior to ultrasound in the diagnosis of pulmonary parenchymal disease and pneumothorax, but combined the two techniques will improve patient management diagnostically and therapeutically. Nuclear medicine imaging is a very specialized technique available at a few university and private specialty referral practices. Gamma-emitting radioisotopes such as krypton-81m or technetium-99m can be used with an external detector (gamma camera) to assess regional pulmonary ventilation and perfusion in the horse. The procedure is safe and painless. Anesthesia is not needed, and the only requirement is that the patient stand quietly in front of the gamma camera. After the study, the patient must be kept in an isolated area to allow decay and excretion of the radiopharmaceutical (normally no more than 48 hours), and, of course, all pertinent radiation regulations must be strictly adhered to. The radioisotope is bound to albumin aggregates of 10 to 15 micrometers in diameter. When injected into a peripheral vein, that is, the perfusion scan, the aggregates become trapped in the pulmonary arterial vasculature. Given even and thorough mixing in the right ventricle, the resulting image illustrates the perfusion distribution of the pulmonary arterial system. The ventilation scan is generated when the horse inhales aerosolized radioisotope particles through a closed circuit system.18 The particles are small enough in diameter to be deposited in the alveoli and small conducting airways with the gamma camera recording the sites of deposition. Together, the ventilation and perfusion scans allow for evaluation of the ventilation/perfusion ( Arterial blood gas determinations are the most sensitive indicator of respiratory function readily available to the clinician. The most easily accessed arteries for sampling are the metatarsal, temporal, facial, and brachial arteries (Fig. 31-2). In cattle the coccygeal artery on the ventral aspect of the tailhead is easily accessible. Heparin is the only acceptable anticoagulant for blood gas samples, and all gas bubbles must be removed and the syringe capped to prevent equilibration of the sample with room air. Use a short (1-inch) small-gauge (25-gauge generally) needle and a 1- to 3-mL syringe for most samples. The syringe and needle can be purchased preheparinized especially for arterial blood gas samples, or regular syringes and needles may be heparinized by aspirating a small volume of heparin into the syringe via the needle and then forcefully expelling the air and heparin from the syringe three times. This minimizes the effect heparin might have on any reported values from the blood gas analyzer. Pulsation of blood from the needle, spontaneous filling of the syringe, and bright color of the blood all confirm a successful arterial puncture. If successful arterial puncture is questionable, then a comparison sample may be drawn from the jugular vein. Once the sample has been drawn, the vessel should be manually compressed for 2 to 5 minutes to prevent hematoma formation. If the sample will not be analyzed within 10 minutes, it should be placed on ice to slow metabolism of blood cells. The patient’s body temperature at the time of sampling should also be recorded, as results are frequently reported at both 37° C and at the actual temperature of the patient, called temperature-corrected values, for pH, Po2, and Pco2 as these values are known to be temperature variable. Clinically either set of values may be used for evaluating efficacy of treatment, but comparisons should only be made between samples reported similarly as either temperature corrected or not. Portable arterial/venous blood gas analyzers have become relatively inexpensive. Because of their ease of use and rapid return of results, they are now making arterial blood gas analysis more practical for use in the field and the technique is no longer reserved for large institution or referral practices.22,23 Awareness of potential device-, species-, or age-related differences in reference ranges, as well as awareness of any limitations in accuracy, is critical for proper interpretation of results. It is veritably impossible to manage severe respiratory disease without knowledge of arterial blood gas parameters. Pulse oximetry is also being more commonly employed in some institutions and referral centers. These monitors measure oxygen saturation of hemoglobin, useful for diagnosing severe hypoxemia or recognizing desaturation events, but provide no measure of actual arterial oxygen and carbon dioxide partial pressures. The most common abnormalities recognized with arterial blood gas analysis in an animal that is breathing room air are hypoxemia with normocapnia or hypocapnia and hypoxemia with hypercapnia. There are five primary means by which hypoxemia develops in any animal. For our purposes, hypoxemia is defined as decreased oxygen tension of the arterial blood (decreased Pao2) and hypoxia is defined as decreased oxygen concentration at the level of the tissue, with or without hypoxemia. Hypoxia results from hypoxemia, decreased perfusion of the tissue bed in question, or decreased oxygen carrying capacity of the blood due to anemia or hemoglobin alteration. Hypoxemia develops from (1) low partial pressure of oxygen in the inspired air, such as seen in high altitude or in an error mixing ventilator gas, resulting in lower than anticipated partial pressure of oxygen (altered Fio2); (2) hypoventilation; (3) Hypoxemia is usually treated with intranasal humidified oxygen insufflation at 4 to 10 L/min for neonates and 10 to 15 L/min in adults. Hypercapnia is not easy to treat. It is important to try to distinguish between acute and chronic hypercapnia. Acute hypercapnia is usually accompanied by a relatively dramatic decrease in blood pH of 0.008 pH units for each 1-mm Hg increase in Paco2. This respiratory acidosis can promote circulatory collapse, particularly in the concurrently hypoxemic and/or hypovolemic patient. The effects of more chronic CO2 retention are less obvious as the time course allows for adaptation. The pH change is less, about 0.003 pH units per 1-mm Hg increase in Paco2, as it is balanced by enhanced renal absorption of bicarbonate by the proximal renal tubule. Most patients with acute respiratory distress are in the acute stages of respiratory failure, but chronic adaptation will begin to occur within 6 to 12 hours and will be maximal in 3 to 5 days. An increase in bicarbonate will be noted, particularly if the acidosis is primarily respiratory in origin, and pH may be within the normal range. Alveolar gas exchange is readily estimated by determining the alveolar-arterial (A-a) gradient for oxygen, computed by subtracting the Pao2 measured by the arterial blood gas from the calculated alveolar oxygen partial pressure (PAo2). The PAo2 is effectively estimated using the partial pressure of inspired oxygen (Pio2) as follows:24 The Pio2 equals the total barometric pressure (760 mm Hg) minus the partial pressure of water vapor (47 mm Hg) multiplied by the fraction of room air that is oxygen (0.21) and thus equals 150 mm Hg for room air. For patients on supplemental inspired oxygen, the practitioner must remember to recalculate the Pio2 with the new oxygen fraction (Fio2) (or, if at altitude, the Po2 in the inspired gas), which is only possible in patients receiving inspiratory gas through a closed system. The Paco2 is obtained from the arterial blood gas measurement. The A-a gradient is normally only 4 to 10 mm Hg; an increase beyond this indicates impaired gas exchange within the lungs, most often the result of A second useful measure is the Pao2/Fio2 ratio, a component of most definitions of ALI and ARDS.25 The Pao2/Fio2 ratio equals the Pao2 obtained from the arterial blood gas divided by the Fio2, the oxygen fraction in the inspired gases. The normal Pao2/Fio2 ratio is greater than 300 mm Hg, a ratio less than 300 mm Hg is consistent with a potential diagnosis of ALI, and a ratio less than 200 mm Hg suggests ARDS, a more severe form of ALI. A consensus statement recently defined the conditions of equine neonatal acute respiratory distress syndrome and equine acute lung injury (EqNARDS and EqNALI).26 Although similar to the definitions for ALI/ARDS in humans and adult veterinary species, the definition of EqNARDS and EqNALI account for the relative hypoxemia of normal neonatal foals compared with adults during the first week of life.26 For this reason, over the first week of life the Pao2/Fio2 increases from less than 115 mm Hg to less than 190 mm Hg in EqNARDS and from less than 175 mm Hg to less than 280 mm Hg in EqNALI.26 The ranges of normal arterial blood gas values for various species are listed in Table 31-1. TABLE 31-1 Normal Arterial pH and Pco2 Values for Various Species (Nonneonate) The major functions of the lungs are to transport gas from the periphery to the site of gas exchange (i.e., the “bellows” function) and to provide gas exchange with the blood, facilitating gas transport to the tissues. The first of these is assessed by means of pulmonary function tests, and the second by arterial blood gas evaluation, discussed above. Historically pulmonary function tests have primarily been used in horses and in most cases as a research tool in veterinary teaching hospitals. Pulmonary function testing has also recently been described in awake adult llamas27 and alpacas.28 Portable technologies, such as flowmetric plethysmography, allow the use of noninvasive techniques in the field, and the awareness of their clinical utility to practitioners has grown.29,30 Measurements of pressure, flow, and volume allow computation of a variety of ventilatory variables. Their ability to demonstrate airway obstruction and airway hyperresponsiveness31 makes them clinically valuable in the diagnosis and characterization of inflammatory airway disease (IAD) and recurrent airway obstruction (RAO) in horses.31 They may also provide an objective means with which to monitor response to therapy.30–35 These techniques are described in greater detail in a later portion of this section. Various spaces in the respiratory system can be aspirated or lavaged for diagnostic or therapeutic purposes. The most commonly performed procedure is the tracheobronchial aspirate. By aspirating from the airways caudal to the larynx, a sample without pharyngeal contamination is obtained. In both the horse and the ruminant the procedure is performed with the animal standing. Sedation or restraint may be needed. A small area over the trachea in the middle third of the neck is clipped and routinely sterilely prepared. The skin is anesthetized using a local block of 2% lidocaine, generally less than 3 mL given as a “bleb” subcutaneously, and a small stab incision is made. A trocar or angiocatheter needle is introduced on the midline between muscle bundles, with the beveled edge facing ventrally to decrease the opportunity for inadvertent cutting of the tubing when it is introduced or manipulated, and the ventral tracheal wall is punctured between two cartilaginous rings. The distal end of the trocar or needle is then advanced distally in the trachea, with the bevel facing ventrally, taking care not to lacerate the dorsal tracheal mucosa. Sterile polyethylene tubing or the catheter from the angiocatheter is introduced through the trocar or needle for about 30 cm. A needle or sharp trocar should be withdrawn to prevent severing the tubing or catheter, but a cannula with rounded edges may be left in place. Approximately 20 to 30 mL of nonbacteriostatic sterile saline solution is introduced quickly. Intermittent aspiration is performed as the tubing is gradually withdrawn. The tubing can be advanced again if a guarding cannula has been left in place to prevent introduction of skin contamination. Additional saline solution aliquots can also be introduced. Once an adequate sample has been obtained, the tubing is completely withdrawn, followed by the withdrawal of any guarding catheter or trocar. Injectable antimicrobial solution or suspension can be infiltrated at the skin incision site if a septic sample is suspected, and in horses and small ruminants a sterile dressing can be applied for 24 hours if desired. Possible complications include subcutaneous emphysema (usually peritracheal but may extend into the mediastinum), local cellulitis, or cutting of the catheter at the needle and loss into the airway. The latter is usually resolved because the catheter is rapidly coughed up, but good technique should prevent this complication. If necessary, the severed catheter can be retrieved endoscopically. The sample should be cultured for aerobic bacteria. Anaerobic colonization is possible, and appropriate cultures should be made if these organisms are suspected (evidence of pleural effusion, consolidation, abscessation, fetid breath, history of aspiration). For patients with prior antimicrobial therapy, it is advised to discontinue antibiotics for 72 to 96 hours before culture, although a recent study has shown reliable recovery of bacteria using bronchoalveolar lavage fluid from foals receiving therapy.6 Airway aspiration can also be performed during routine endoscopy of the trachea using an aspiration catheter advanced through the endoscope biopsy channel, but there is potential for pharyngeal contamination. Results comparing culture from a protected aspiration catheter passed through an endoscope compared favorably with traditional percutaneous tracheobronchial aspirate.5 A direct smear and Gram stain can be used as an initial guide for antimicrobial therapy pending culture results. Cytologic evaluation can be extremely valuable in differentiating among infectious, allergic, parasitic, and neoplastic processes. Transtracheal aspirates from clinically normal horses contain columnar ciliated epithelial cells, a few neutrophils, and multiple mononuclear cells. Increased percentages of neutrophils and the presence of mast cells, eosinophils, giant cells, and hemosiderophages have been found in aspirates from normally performing Thoroughbred racehorses, indicating some airway inflammation in “normal” equine atheletes.8 Mucus, large spores, and fungal hyphae may be found in the absence of airway disease and must not be overinterpreted. In cases of pneumonia, neutrophils may constitute 40% to 90% of the cellular sample. Bacterial pneumonia causes a more degenerate appearance of neutrophils, and intracellular bacteria may be found. Equine lungworm is characterized by the presence of large numbers of eosinophils and occasionally a larva. In ruminants the most important information gathered in patients with bronchopneumonia is usually the result of culture and antimicrobial sensitivity testing. Bronchoalveolar lavage (BAL) involves obtaining a sample from the terminal airways and alveolar region and is described in horses, cattle, and camelids.28,36,37 BAL is performed using a long endoscope (2 to 3 m) or BAL (e.g., double-lumen or cuffed Bivona [Smiths Medical, Norwell, Mass.]) or MILA (MILA International, Elanger, Ky.) tubes introduced through the nares. Endoscopic BAL allows for more exact placement of the end of the endoscope, so a clear understanding of the anatomic location of the distal airway lavage is available. It also allows for the characterization of lower airway secretions and mucosal inflammation. Use of the BAL tube is a blind technique, but most frequently the dorsal lung of one hemithorax is sampled. BAL is contraindicated in horses that are expected to exercise within 24 hours of the procedure and in any animal demonstrating signs of respiratory distress, marked tachypnea, paroxysmal cough, or significant hypoxemia. Proper sedation is critical for performing BAL. In horses, the addition of butorphanol may reduce cough that is typically associated with this procedure. Instillation of dilute 2% lidocaine, particularly when passing the tube into the trachea or past the level of the carina, may also decrease coughing associated with this procedure. Passage of the tube into the trachea can be facilitated by stretching the head horizontally. The endoscope or tube should be passed until it is wedged in the most distal bronchus. If using a cuffed BAL tube, the cuff should be inflated to seal the airway once wedged. In adult horses, it is recommended to infuse a total of 300 to 500 mL of warm (37°) sterile physiologic saline in aliquots of 60 to 250 mL. Smaller lavage volumes may be necessary in smaller animals. Fluid may be instilled via prefilled syringes or via a solution administration set using a pressure bag with fluid bag or pressure bulb with fluid bottle. The BAL sample is collected via continuous aspiration with syringes or a suction pump using low suction pressure. Aliquots should be pooled for analysis. The presence of surfactant (foam) and turbidity on gross inspection is suggestive of a properly obtained sample. BAL has the advantage of sampling the airways nearest the parenchymal region, but only a limited area of the lung is sampled instead of the pooled secretions from a tracheobronchial aspirate. Thus bronchoalveolar lavage may be superior to tracheobronchial aspirate in evaluation of horses with diffuse chronic lung diseases of the peripheral airways, and molecular analysis of fluid is performed for identification of certain viral or bacterial species. Cytologic findings of BAL and tracheobronchial aspirates correlate poorly, and a tracheobronchial aspirate should be performed prior to a BAL if an infectious process is suspected. Bronchoalveolar lavage cytology is valuable in evaluation of fungal infections, inflammatory airway disease, and the assessment of therapeutic response. Normal cell distribution of BAL fluid in healthy horses reveals predominantly macrophages (50% to 70%) and lymphocytes (30% to 50%) with lower percentages of neutrophils (<5%), mast cells (<3%), and eosinophils (<0.1%). Macrophages are the predominant cell type (89%) in BAL fluid of healthy calves,37 whereas alpacas may have a greater abundance of neutrophils (10%).28 Aspiration from the pleural space is a simple, easily performed, inexpensive procedure that can be both diagnostic and therapeutic. In the horse with septic or neoplastic effusions, sedation is often unnecessary, because the procedure causes only minimal additional discomfort. Following ultrasonographic evaluation of the thorax, a point is chosen where drainage or fluid sampling would seem most appropriate, frequently found in the sixth or seventh intercostal space 10 cm dorsal to the olecranon and above the lateral thoracic vein. The area should be clipped, if it was not clipped for the thoracic ultrasound examination, and surgically prepared. Multiple sites may be needed in horses with loculated pockets of fluid in the pleural cavity and these sites should also be chosen using ultrasonography. The skin and intercostal tissue down to the pleura are anesthetized with lidocaine, and a stab incision is made. A sterile 2- to 3-inch teat cannula or bitch catheter is introduced immediately cranial to the rib border to avoid the intercostal nerve and vessel along the caudal aspect of the ribs. The cannula should be attached to sterile intravenous extension tubing and a three-way stopcock. While the cannula is advanced bluntly through the parietal pleura, a sudden loss of the force required to advance is felt. Aspiration should be attempted at this time. The orientation of the cannula can be varied to reach as much fluid as possible. Normally only a few milliliters of straw-colored fluid are obtained. In cases of pleural effusion, as much as 30 L may be removed from each side of the chest (Fig. 31-3). If fluid is excessive, the tubing can be extended over a bucket for gravity drainage, or a vacuum pump with a fluid trap can be attached. When the procedure is complete, a purse-string suture is placed around the stab incision and the cannula is withdrawn while the suture is tightened. In cases where the effusion is large and expected to continue forming for several days, the initial drainage can be performed by placing a chest tube instead of a puncturing the pleural space with a teat cannula. If a chest tube is to be left in place, it should be secured with a Chinese finger trap suture and the end covered by a Heimlich valve to prevent aspiration of air into the thorax through the tube. If the thorax is being drained rapidly, the patient should be watched carefully for signs of distress, as draining of large volumes can alter cardiovascular parameters significantly. Increasing opacity, presence of fibrin clumps, and malodor of pleural fluid all suggest relative progression from transudate to septic exudate containing inflammatory cells and debris. A putrid odor suggests the presence of anaerobic bacteria. Samples should be cultured for aerobic and anaerobic organisms. A white blood cell (WBC) count of 10,000/mL or less is considered normal; fewer than 60% are normally neutrophils, the remainder being lymphocytes and macrophages. The proportion and total number of neutrophils increase with pleuritis. Erythrocytes are normally not present in the absence of a traumatic tap. The protein concentration is normally less than 3.5 g/dL, and pH should be approximately 7.4. Additional metabolic values that give early indication of sepsis can be obtained on pleural fluid samples collected after filtration through a blood administration set to remove fibrin and debris potentially detrimental to analytic equipment. Pleural fluid pH, Pco2, and concentration of glucose, lactate, and bicarbonate can be directly compared with similar analysis of venous blood from the patient. A septic pleural exudate is acidic, with decreased glucose and bicarbonate but increased lactate and Pco2 compared with venous blood concentrations or tensions, apparently reflecting metabolic activity of phagocytic cells and bacteria and development of an anaerobic environment.38 Of these values, low pleural fluid glucose concentration (<40 mg/dL) has the best correlation with sepsis.39 Neoplastic cells may be found in cases of lymphosarcoma, adenocarcinoma, or other neoplasms. Equine gastric squamous cell carcinoma occasionally presents with neoplastic pleural effusion. If neoplastic effusion is suspected but diagnostic cells do not exfoliate into the pleural fluid, pleuroscopy under sedation and local anesthesia can be used directly to visualize and obtain biopsy samples of intrathoracic lesions. The technique of pleuroscopy is beyond the scope of this chapter. Mediastinal fenestrations may be occluded by fibrin and cell debris; therefore each side of the thorax should be evaluated separately. In the horse a transtracheal aspirate for culture should also be performed because of the common association of pleuritis with bacterial pneumonia and pulmonary abscessation. Although identical organisms are generally isolated from both samples, this is sometimes not the case.
Diseases of the Respiratory System

General Evaluation of the Patient with Respiratory Disease
History
Presenting Signs or Chief Complaints
Physical Examination
Additional Diagnostic Evaluation of the Respiratory Tract
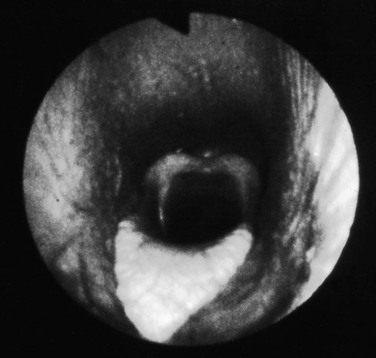
Endoscopy
Radiography
Ultrasonography
Nuclear Medicine Imaging
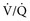 ) ratio, important in evaluation of certain respiratory problems such as EIPH (high
) ratio, important in evaluation of certain respiratory problems such as EIPH (high 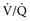 areas), pulmonary thromboembolism (high
areas), pulmonary thromboembolism (high 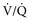 areas), and heaves (low
areas), and heaves (low 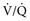 areas).19 Additional uses are in the evaluation of deposition in the lung of aerosolized radiolabeled medications such as albuterol19 and the evaluation of mucociliary clearance or tracheal mucus transport. In the latter, the time a bolus of radioisotope requires to cover a given tracheal distance is recorded in millimeters per minute and compared with normal ranges.21
areas).19 Additional uses are in the evaluation of deposition in the lung of aerosolized radiolabeled medications such as albuterol19 and the evaluation of mucociliary clearance or tracheal mucus transport. In the latter, the time a bolus of radioisotope requires to cover a given tracheal distance is recorded in millimeters per minute and compared with normal ranges.21
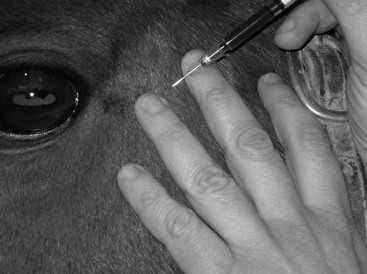
Arterial Blood Gas Analysis
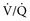 mismatch; (4) diffusion limitation; or (5) intrapulmonary or intracardiac right to left shunting of blood. Mild to moderate hypoxemia is not an uncommon finding in neonates, but it must be evaluated in terms of the current age of the foal and its position. The difficulty in obtaining the sample must also be considered, as severe struggling can variably affect the arterial blood gas results. If the lung is significantly involved in the underlying pathology, such as with severe pneumonia, ALI, or ARDS, increased Paco2 may very well be present, representing respiratory failure.
mismatch; (4) diffusion limitation; or (5) intrapulmonary or intracardiac right to left shunting of blood. Mild to moderate hypoxemia is not an uncommon finding in neonates, but it must be evaluated in terms of the current age of the foal and its position. The difficulty in obtaining the sample must also be considered, as severe struggling can variably affect the arterial blood gas results. If the lung is significantly involved in the underlying pathology, such as with severe pneumonia, ALI, or ARDS, increased Paco2 may very well be present, representing respiratory failure.
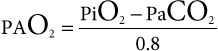
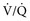 mismatching. Only an estimate of the A-a gradient can be calculated in patients receiving intranasal insufflation of oxygen, as neither the Fio2 nor the Po2 is known.
mismatching. Only an estimate of the A-a gradient can be calculated in patients receiving intranasal insufflation of oxygen, as neither the Fio2 nor the Po2 is known.
Species
Blood pH
Pco2 (mm Hg)
Bovine
7.32-7.45
35-53
Ovine
7.32-7.54
37-46
Equine
7.32-7.44
38-46
Caprine
7.42-7.46
33-38
Respiratory Function Testing
Collection and Evaluation of Respiratory Secretions
Tracheal Aspirates and Bronchoalveolar Lavage.
Thoracocentesis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Diseases of the Respiratory System
Chapter 31