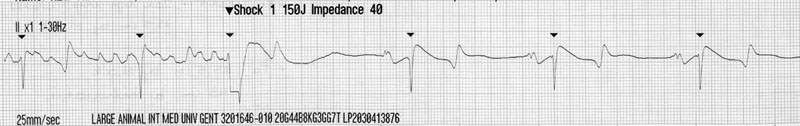
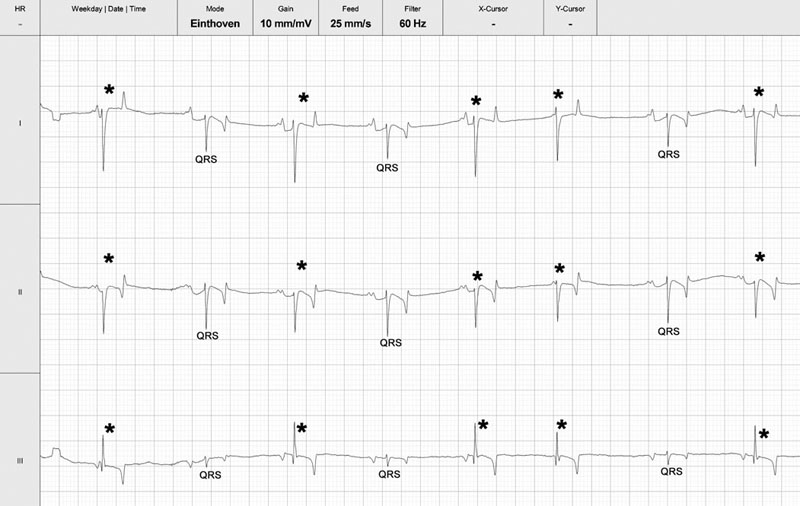
Cardiac murmurs and arrhythmias are detected commonly in performance horses, creating problems for veterinarians who must then determine their significance.1–4 This chapter will address the diseases and abnormalities affecting the heart and vessels of the athletic horse: The horse is equipped with enormous cardiac reserve (Chapter 31) and as a result, evaluation of the equine cardiovascular system at rest provides limited information relevant to function during intense exercise. Only when increased demand is placed on the heart during exercise will the effect of more subtle cardiac or vascular lesions become obvious. It is clearly important not to lose sight of the plethora of other reasons that cause horses to fail to perform to owner expectations.5 As has been emphasized elsewhere in this book, the probability of heart disease is low on the list of causes of poor performance, compared to orthopedic or respiratory disease. Clearly field, or treadmill, exercise tests provide the optimal environment to assess cardiac function in equine athletes, but it is often impractical or inappropriate to evaluate the heart at the limits of its reserve. As a result, the clinician must rely on clinical history to provide a subjective assessment of exercise tolerance and physical examination to assess the hemodynamic effect of any lesions present. In many cases, resting and exercising electrocardiography and echocardiography provide the additional information needed to determine a diagnosis and prognosis. Despite advances in technology, cardiac auscultation remains the most important technique for the diagnosis of cardiac disease in horses. It is important to develop a systematic, logical approach to cardiac auscultation for it to yield maximum information. The suggestion that equine clinicians find interpretation of auscultation findings difficult only serves to emphasize the importance of developing the necessary skills to perform this very important technique with confidence.6 • Always auscultate both sides of the chest. • Always palpate for an apex beat on both sides of the chest. (See Fig. 32.1.) • Always listen for at least 30 seconds to calculate the horse’s heart rate and establish the normality of its cardiac rhythm before worrying about the presence or absence of cardiac murmurs. • Always maintain a standard systematic approach for listening to the whole cardiac cycle at each valve area. Classically, landmarks for localization of cardiac valve areas have been described with reference to rib spaces and anatomical landmarks.7 The mental effort of remembering the landmarks, coupled with marked breed variation in thoracic conformation, makes this approach difficult to put into practice. It also encourages the clinician to remove the stethoscope from the horse’s chest, to examine each valve individually, potentially leaving wide areas of the cardiac auscultation area unexamined. An alternative approach is suggested. Palpation of the left apex beat (Fig. 32.1) provides an easy landmark for the mitral valve auscultation area. The apex beat should be palpated with the flat of the left hand. The stethoscope is then placed exactly over the apex beat. The point of maximal intensity (PMI) of sounds associated with the mitral valve is usually in this area. In this region the first heart sound, S1, caused by various vibrations at the onset of ventricular systole, should be loud. The third sound, S3, associated with the end of rapid early ventricular filling, might also be audible. It is important to appreciate that the normal diastolic sounds S3 and S4 are not always clearly audible in every horse. Once positioned at the apex beat, the examiner should initially concentrate only on cardiac rate and rhythm for at least 30 seconds, so that the heart rate and underlying rhythm are established before the examiner is distracted by the absence or presence of any cardiac murmurs. Then the examiner should concentrate specifically on both diastole and systole for a number of complete heart cycles, before the stethoscope is then moved slowly and deliberately in radiating directions around the mitral valve area while maintaining contact with the chest wall, until S1 is no longer clearly audible. The remaining valve areas on the left are then accessed by gradually moving the stethoscope in a cranial direction from the mitral valve area. Once more, the stethoscope remains in contact with the chest wall at all times. As the stethoscope is advanced slightly dorsal and cranial, the relative loudness of S1 and S2 is reversed, and S2 is accentuated relative to S1. As this occurs, the stethoscope is positioned at the point of maximal intensity of the semilunar valves. The first, more caudal and dorsal, valve encountered is the aortic valve. Once in the outflow and aortic and pulmonic valve areas, the clinician must again specifically listen for adventitious sounds in both systole and diastole, before performing the same radial scan of the area as for the mitral valve. S2 will be loudest over the pulmonary valve, which is situated more cranial and slightly ventral to the area in which S2 first becomes accentuated. Occasionally an audible splitting of S2 can be heard in the area of the pulmonic valve. Usually the sound is split due to earlier closure of the aortic valve compared to the pulmonic valve, but the situation can also be reversed in horses.8 The fourth heart sound, S4, composed of vibrations arising from active atrial contraction, is usually heard best in the area cranial and dorsal to the apex beat. It should also become standard to palpate for an apex beat on the right hemithorax. The vibrations are often palpable in narrow-chested athletic horses. If an apex beat is detected, it represents an excellent starting point for thoracic auscultation. The auscultation area is more cranial on the right, and forward placement of the forelimb is necessary in many horses (Fig. 32.2). The only valve sounds reliably heard in this area are associated with the tricuspid valve. After once more concentrating specifically on systole and diastole, a similar radiating survey, with the stethoscope in contact with the chest wall, should be performed around the point of maximal intensity of the heart sounds on the right side. Many clinicians find auscultation of the right side unrewarding, because the heart sounds are usually quieter here and there is more variation between horses. When difficulties arise, it is usually because the stethoscope is not positioned sufficiently cranial, the horse is excessively fat, or the horse’s leg is insufficiently protracted. • Dysrhythmias occur commonly in athletic horses9 and the majority, with the notable exception of atrial fibrillation (AF) and atrial flutter (AFL), usually do not affect performance. • In horses, use of limb leads for recording ECG traces is ill-advised. Wires and crocodile clips attached to their limbs are poorly tolerated and subject to a large amount of movement artifact. In addition, the pattern of depolarization of the equine ventricle precludes using multiple leads for assessment of cardiac size and mean electrical axis.10–12 • The base-apexlead system or modifications of it are used exclusively for rhythm diagnosis in equine medicine (Fig. 32.3). This lead system produces large complexes, which are easy to identify.13 Recording of multiple leads can be useful to identify buried P waves or slight alterations in QRS morphology (such as caused by fusion beats) (Fig. 32.4). • Diagnosis of arrhythmias has been greatly enhanced by improvements in technology that allow ECG recordings to be taken readily in resting and exercising horses (Figs 32.5 and 32.6). Digital ECGs, based on laptop and android technology are now readily affordable for equine veterinarians (Figs 32.4 and 32.5). Increased availability and practicability of this equipment means that disorders of cardiac rhythm are diagnosed more frequently in horses at rest and during exercise. Cardiac disease is a rare primary cause of poor performance in the equine athlete, but on the rare occasion that performance is affected by cardiac disease, dysrhythmia is the commonest underlying cause.5 Paradoxically, alterations in cardiac rhythm are common in athletic horses because of their normal high parasympathetic drive,14 so the equine clinician is faced with a wide variety of normal rhythms in resting horses. Bradydysrhythmias (slow rhythms) are normal findings in athletic horses, as are sinus and atrioventricular block.9,15 Despite this, on rare occasions, all of these normal rhythms can also result from cardiac disease, when the dysrhythmia causes serious decrements to performance and presents important risks to the rider and horse.16 Conversely, obvious arrhythmia such as atrial fibrillation might have no obvious effect on the performance of horses engaged in activities that are not aerobically challenging (dressage or show jumping). In contrast, if all of the cardiac reserve must be used, the effect of the same dysrhythmia on performance is devastating (racing, three-day eventing).17–19 Finally, cardiac rhythm disturbances often occur as a result of disease in other body systems or metabolic disturbances.20,21 In such cases, the arrhythmia is unlikely to be associated with primary heart disease. • Re-entrant rhythm disorders are usually characterized by an irregularly irregular rhythm (Figs 32.7–32.12). • Both abnormal rhythms can occasionally self-correct within hours to days of becoming established (Fig. 32.10). • AF is the commonest cardiovascular cause of poor performance in racehorses and those engaged in other aerobically challenging sports (eventing, hunting) but also jumping or dressage horses might present with loss of performance. • It can be an incidental finding in horses performing in less aerobically challenging disciplines. • AF can be associated with severe cardiac disease (Figs 32.13 and 32.14), or occur in the absence of any significant cardiac dysfunction. • Its presence leads to excessively high ventricular rates during exercise, increased prevalence of ventricular premature beats, ventricular tachycardia and occasionally R-on-T phenomenon (Fig. 32.11). These features will predispose affected horses to exercise-associated collapse and sudden death (Fig. 32.12). • Treatment depends on whether underlying cardiac disease is present (Fig. 32.14A and B). • Prognosis for successful treatment and return to previous athletic function in the absence of cardiac disease is good, but treatment failure and recurrence of the arrhythmia are also possible. Atrial fibrillation (AF) and atrial flutter (AFL) can occur in horses with no other evidence of cardiac disease, or these can be precipitated by atrial dilation secondary to underlying heart disease, most commonly long-standing mitral regurgitation17 (Figs 32.13 and 32.14). When AF occurs in isolation, it is often larger breed horses that are affected. Affected animals usually present with a history of poor performance. The more demanding the sports discipline, the more likely clinical signs are to occur. The lack of an atrial contribution to ventricular filling, which is especially important during exercise when the time available for early diastolic filling is reduced, is coupled with a very high ventricular response rate to the atrial arrhythmia. Both these factors combine to reduce the length of diastole and contribute to inadequate ventricular filling. This results in a deleterious effect on forward cardiac output and aerobic capacity in affected horses. The disproportionately high ventricular rate during AF is the result of an increased sympathetic tone that allows a high number of atrial impulses to be conducted to the ventricles. Instantaneous heart rates of over 300 bpm are often found (Fig. 32.11). At such high rates, ventricular filling is even more reduced, further decreasing cardiac output during exercise. In some horses, AF is associated with epistaxis.18,22 If AF develops suddenly, during fast work, there is an acute decrease in cardiac output, and affected horses can pull up suddenly, sometimes with ataxia and distress. Immediate thoracic auscultation reveals a rapid chaotic rhythm. Obvious performance decrements are not invariably the case, however, as affected horses can appear to work normally at lower intensity. The rhythm, once initiated, can be sustained, but short-lived paroxysmal AF also occurs during exercise (Fig. 32.10).23 Paroxysmal AF resolves in the minutes, hours or days following exercise and can be difficult to detect. As a result, AF is a possible cause for fading during racing or competition when horses are subsequently presented in normal sinus rhythm.24 AF can also develop in association with deranged electrolyte and fluid balance in endurance horses after endurance events. In such cases, the rhythm usually resolves spontaneously with rehydration and restitution of electrolyte balance. Clinical signs of atrial flutter are similar as for AF, although maximal heart rates during exercise equalize rapidly to the atrial flutter rate with withdrawal of parasympathetic tone; although the peak heart rates will be ultimately lower compared with that of horses affected by AF, the condition is usually still performance limiting (Fig. 32.8B). Auscultation findings in cases of AF will depend on the underlying cause. The irregularly irregular rhythm will be common to all cases of sustained AF, but in horses with AF secondary to atrial dilation from cardiac disease, there will also be an elevated heart rate, characteristic cardiac murmurs and physical signs of heart failure. Careful auscultation of these cases will reveal the underlying cause such as mitral regurgitation or ventricular septal defect. Horses with AF but no significant underlying heart disease, usually have a normal resting heart rate. Vagal tone at the atrioventricular node will cause waxing and waning of the cardiac rhythm that can appear deceptively similar to second-degree atrioventricular block.25 This feature can be confusing, but the two rhythms can always be differentiated with patient auscultation because an unexpected early beat will be always be detected (Fig. 32.9). The less common variant, atrial flutter, occasionally presents with a more regular and rapid rhythm when a constant ratio of flutter waves are blocked at the AV node. (Fig. 32.8B). The regular rapid atrial activity provoked by the flutter waves is occasionally audible if there are long diastolic intervals. Electrocardiography will reveal an irregularly irregular rhythm with a complete absence of P waves and normal QRS morphology. Cardiac rate will depend on the presence or absence of associated cardiac disease and atrioventricular conduction. Fibrillation waves are visible on the surface ECG and often change between coarse and fine fibrillation waves in the same horse (Fig. 32.7). When atrial flutter is present, P-like, often bifid, undulations at a rate of 170–250 per minute cycle continuously (Fig. 32.8A). Horses with atrial flutter have a regular or irregular ventricular rhythm depending on atrioventricular conduction and prevailing autonomic tone. At elevated heart rates, ventricular rhythm is regular because of a 1 : 1, 2 : 1, 3 : 1 atrioventricular conduction. RR intervals will be a constant multiple of the atrial flutter cycle length (Fig. 32.8B). In both AF and atrial flutter, echocardiography is important to determine the presence or absence of cardiac lesions and assess chamber size, when important cardiac disease cannot be ruled out from the physical examination and clinical history (Figs 32.13 and 33.14A and B). An exercising ECG should be performed if the owners decide against any treatment, even if the rhythm is an incidental finding and the horse’s performance is believed to be unaffected. This procedure will ascertain whether the horse is able to perform its duties comfortably within its reduced cardiac reserve and will confirm that withdrawal of parasympathetic tone during exercise does not result in accelerated conduction of wavelets, excessive tachycardia, or other malignant cardiac rhythms (Fig. 32.11) that could increase the horse’s risk of exercise-related injury, or predispose it to collapse and sudden death (Fig. 32.12). Paroxysmal and sustained AF can be associated with alterations in electrolyte status. Plasma or serum concentrations of cardiac troponin I (cTnI), markers of myocardial injury and inflammation, are rarely elevated in cases of AF, unless the condition is associated with toxic damage to the heart, or end-stage valvular of congenital heart disease. Mild elevations are often recorded in the first 24–48 hours after the rhythm becomes established but the significance of this elevation is unknown and could also be the result of mild exercise-associated elevations in cTnI.26 The therapeutic aims vary depending on the circumstances and cause of AF. When the horse is expected to perform and there is no evidence of underlying heart disease, therapy aims to convert AF to normal sinus rhythm and to prevent its recurrence. However, if AF is not associated with poor performance, provided underlying heart disease can be ruled out and the horse’s heart rate and rhythm appear to be acceptable during the horse’s normal activities, treatment might not be necessary. Nevertheless, recent clinical evidence that this rhythm can degenerate in some individuals during exercise means that horses affected with sustained AF could still be at a tangibly increased risk of collapse and sudden death during exercise than their peers. When AF coexists with heart failure, the abnormal rhythm should not be specifically treated. Treatment goals in this instance include control of ventricular response rate, reduction of volume overload by diuretics and angiotension-converting enzyme inhibitors, and cardiac afterload reduction by vasodilation.27,28 Successful cardioversion can be achieved by pharmacological treatment or by electrical cardioversion. The most commonly used pharmacological treatment consists of oral quinidine sulfate, a class 1A antiarrhythmic.29,30 Intravenous administration of quinidine gluconate has been described,31 but this preparation, though more convenient, is less effective when the arrhythmia is longstanding.30,32 In many countries, the intravenous preparation of quinidine gluconate is not available and production of quinidine sulfate has been discontinued. As a result quinidine must now be imported and as difficulty in obtaining quinidine sulfate increases, there has been a drive to investigate alternative treatments of equine AF. As a result of these efforts, the most promising new technique appears to be direct current transvenous electrical cardioversion (TVEC) under general anesthesia. This method is efficacious and it appears to have the least side effects and a high success rate for conversion of AF in many horses.33–35 Treatment of atrial flutter is similar to that of AF, but it can generally be expected to have a higher success rate because the arrhythmia is more organized and therefore amenable to conversion. Overdrive intra-atrial pacing has also been used successfully to treat refractory atrial flutter in one horse.36 Traditionally the administration of a test dose of quinidine sulfate (10 mg/kg per os) was given to check for idiosyncratic reactions before commencing treatment. However, this is not necessary and treatment is begun with 20 mg/kg quinidine sulfate administered via nasogastric tube (10 g per 500 kg horse). This dosage is repeated every two hours until sinus rhythm is restored, signs of intoxication develop, or a maximum total dose of 60–80 g (120 mg/kg) is achieved. Individual differences in quinidine resorption and elimination are considerable.37 Especially when mild signs of intoxication develop, the two-hour interval can be prolonged between two and six hours to avoid more severe intoxication. The longer quinidine can be kept at therapeutic level, the higher the statistical chance for conversion to sinus rhythm. Treatment must be performed in a quiet area with immediate IV access available. A continuous telemetric ECG should be available to monitor heart rate and rhythm throughout the treatment. Horses receiving quinidine should not be moved, as quinidine causes hypotension, through alpha receptor blockade.38 Signs of intoxication include urticaria, diarrhea, anorexia, weakness, ataxia and tachycardia.18,30 Nasal edema with stertorous breathing and depression are commonly observed after only a few doses. A relationship has been demonstrated between high plasma quinidine concentrations, ataxia and respiratory tract stridor, but not between plasma quinidine concentrations and tachycardia, diarrhea or colic.30 Conversion to normal sinus rhythm is less likely when there are signs of quinidine intoxication.30 Laminitis has also been reported after quinidine treatment, but this adverse effect appears to be rare. Sudden death can also occur and is most likely the result of a malignant ventricular rhythm (Fig. 32.15). In common with all class 1A antiarrhythmic drugs, quinidine has pro-arrhythmic properties.39 By lengthening the myocardial cell refractory period, quinidine increases the risk of severe ventricular rhythm disturbances.38 Because the drug increases AV nodal conduction, it has the potential to produce rapid supraventricular, as well as ventricular tachycardia.38 ECG should be monitored throughout the treatment and QRS and QT interval measured before each treatment. Although a 25% increase in QRS duration is reported as being clinically important,30 exact measurement is often difficult during AF due to superposition of f-waves. In addition, increased atrioventricular conduction during treatment can result in QRS broadening due to use-dependent sodium channel blockade and aberrant conduction. Therefore it is easy to confuse supraventricular tachycardia with ventricular tachycardia (Fig. 32.16A), although management is very different. Tachycardia is often encountered, most commonly due to supraventricular tachycardia (SVT). Low dose of alpha2-adrenergic agonist administration, provided significant signs of hypotension are absent, can depress atrioventricular nodal conduction sufficiently to immediately abolish supraventricular tachycardia and reduce QRS duration (Fig. 32.16B). Further emergency treatment includes intravenous administration of bicarbonate at 1 mEq/kg body weight to increase protein binding of quinidine and therefore decrease the concentration of unbound drug, which is the biologically active form. Supraventricular tachycardia can be treated by the intravenous administration of 0.002 mg/kg digoxin IV, or if unsuccessful, 0.03 mg/kg propranolol IV30 to slow AV nodal conduction. Digoxin, however, has a slow onset of action and concurrent use of quinidine and digoxin might increase toxicity of both quinidine and digoxin because of competitive protein binding. Propranolol should be administered with caution as it can enhance hypotension and negative inotropism. Life-threatening ventricular tachycardia can be treated with magnesium sulfate IV (4 mg/kg every two minutes up to 50 mg/kg) followed by lidocaine IV (0.25 to 0.5 mg/kg every five minutes up to 2–4 mg/kg). In many horses, cardioversion is a progressive event related to the cumulative quinidine dose. Intra-atrial electrograms show that the initial fibrillation ‘rate’ is usually between 300 and 450 depolarizations per minute. Due to quinidine, fibrillation becomes more organized and fibrillation rate decreases, thereby producing fibrillation waves that start to appear as flutter waves or P’ waves. Close to conversion, atrial rate can drop towards 170 to 100 per minute, while 2 : 1 conduction results in a ventricular rate between 50 and 85 bpm. Occasionally, the ECG can resemble sinus rhythm while in fact a fibrillation wave is still buried in each preceding QRS or T wave (Fig. 32.17). This means that AF is still present. Termination of treatment at this stage can result in gradual return of fine fibrillation waves as quinidine concentrations in plasma decline. This should not be regarded as early recurrence of AF. Horses that fail to revert to sinus rhythm after administration of 120 mg/kg quinidine, or that develop unacceptable adverse effects during cumulative dosing, sometimes respond to a second series of 20 mg/kg treatments after waiting 24-hours without further drug administration. Indeed some horses convert to normal sinus rhythm up to 24 hours after the final dose of quinidine has been administered without any additional drugs being administered. Such delayed conversion could be explained by slower quinidine elimination in some individuals, whereby the prolonged duration of a therapeutic quinidine level increases the statistical likelihood for cardioversion. The potential severity of quinidine’s side effects, have led to modifications of the traditional cumulative two-hourly regime and an alternative regime has been suggested.30 If a horse fails to respond to the initial two-hourly treatments, when signs of toxicity develop, or when the maximum dose of quinidine has been reached, quinidine administration is continued at six-hourly intervals. These workers also recommend that digoxin (0.01 mg/kg) be given orally every 12 hours until sinus rhythm is restored. From a theoretical point of view, digoxin tends to stabilize AF by increasing the number of wavelets circulating in the atria and decreasing their wavelength.40 However, digoxin has beneficial vagotonic effects on the atrioventricular node and is used therapeutically in humans and dogs to reduce the ventricular response to stable AF. It is therefore possible that a horse given digoxin might be better able to tolerate quinidine at its therapeutic levels without the development of tachycardia, thereby increasing the probability of conversion. The use of digoxin in the treatment of AF remains somewhat controversial. The class 1C antiarrhythmic flecainide is more efficacious than quinidine in returning human patients to sinus rhythm after sustained41 and paroxysmal AF42 and its use has been investigated in horses.43 Initial reports suggested that flecainide at 0.2 mg/kg/min IV over 10 minutes was effective in returning horses to normal sinus rhythm after AF was induced by rapid atrial pacing.44 However, use in horses with naturally occurring AF, but also in recent-onset experimental AF (G. van Loon, unpublished results), was associated with potentially dangerous wide-QRS tachycardia and risk for sudden death45 (Fig. 32.18). The class 3 agent amiodarone showed moderate efficacy (50%) in equine AF but its use was associated with adverse effects such as diarrhea and temporary hind limb weakness, especially when administered for longer than 48 hours.46,47 Because of its low bioavailability, amiodarone should be administered intravenously.48 Median half-lives of amiodarone and desethylamiodarone were 24.1 and 58.6 hours, respectively.48 In some countries, the cost of this product might be prohibitive. Other drugs such as propafenone (class 1C) and sotalol (class III), both with beta-adrenergic blocking activity, have been sporadically used in horses. No data on pharmacokinetics, adverse effects and pro-arrhythmic properties of these drugs in horses have been reported to date. Cibenzoline treatment in two horses with AF did not restore sinus rhythm and was associated with severe ventricular pro-arrhythmia.49 Direct current electrical cardioversion of tachyarrhythmias is a well-known and very effective technique for treatment of human tachyarrhythmias and can be applied via a transthoracic, transvenous or a combined approach. The adult horses’ size makes transthoracic cardioversion of AF very difficult. However, transvenous electrical cardioversion (TVEC) of AF has proven to be very effective with success rates of 90 to 98%.33,35 The technique also seems to be well suited for conversion of chronic AF cases or horses that failed to convert on quinidine.33,35 In a study of 72 treatments, treatment outcome was not predicted by AF duration as 71 horses converted.35 Although mild increases in cTnI are occasionally observed, increases have been judged to be clinically unimportant.50 As electrical cardioversion is painful, it always necessitates general anesthesia, which carries independent risks for morbidity and death.51 Despite the presence of AF, cardiovascular function was well maintained during anesthesia and was not affected by shock application. Cardiac index and stroke index increased and SAP decreased after cardioversion.52 The cardioversion procedure starts with insertion of two cardioversion catheters via the jugular vein in the standing horse. These catheters possess a large surface area electrode at the tip for shock delivery. Under ultrasound guidance, one catheter needs to be positioned in the right atrium while the second catheter is maneuvered 10–20 cm into the left pulmonary artery (Fig. 32.19). Both right and left parasternal images are useful for exact catheter localization. A pressure trace recording from the catheter gives additional insight into the position of the catheter top. On occasion, shock delivery can result in temporary asystole and, consequently. The use of an additional right ventricular temporary pacing catheter is also advised (Fig. 32.20A and B).53 When all catheters are in place, general anesthesia is induced. Catheter position is then confirmed by ultrasound and the catheters repositioned, as necessary. Radiography has also been used to check catheter and electrode location during anesthesia.54 The right ventricular pacing catheter is first connected to a temporary pacing unit. Both cardioversion catheters and a surface ECG are then connected to the defibrillator, which is operated in synchronous mode. This detects the R wave of the ECG and will automatically deliver the shock timed exactly on the R wave after the button of the defibrillator is pressed (Fig. 32.21). Correct detection of the R wave is vital as shock delivery on a T wave almost inevitably results in fatal ventricular fibrillation. When the defibrillator wrongly identifies the T wave as an R wave, position of the surface electrodes must be changed. Biphasic, rather than monophasic, shocks should be used because they are most effective. Incremental shocks are applied, starting at 125 to 360 J, with attempts being made to minimize total energy delivered. Most horses convert between 125 and 250 J, with an impedance of 30 to 50 ohms (Fig. 32.21).33,35 If cardioversion is not achieved, shock delivery after administration of an antiarrhythmic drug, such as amiodarone, occasionally promotes successful electrical cardioversion (G. van Loon, unpublished data). Also if there is a high frequency of atrial premature beats immediately after cardioversion, antiarrhythmic drugs can reduce the risk for immediate recurrence of AF (IRAF). After restoration of sinus rhythm, catheters are gently withdrawn and the horse is allowed to recover.
Diseases of the heart and vessels
Introduction
General approach to equine cardiology
Overall approach
Cardiac auscultation
Technique
Rules

Location of valve areas
Left hemithorax
Right hemithorax
Abnormalities of cardiac rhythm
General principles


Atrial fibrillation (AF) and atrial flutter (AFL)
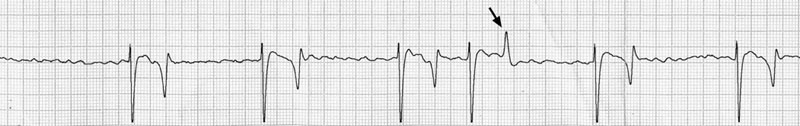
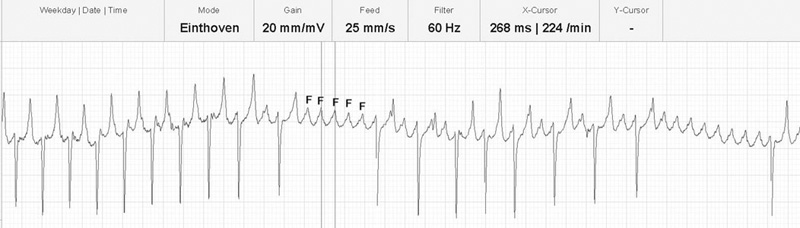
The ECG shows atrial flutter with a cycle length of 268 ms (rate 224 per minute).
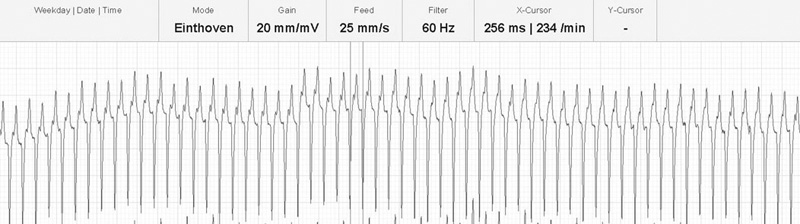
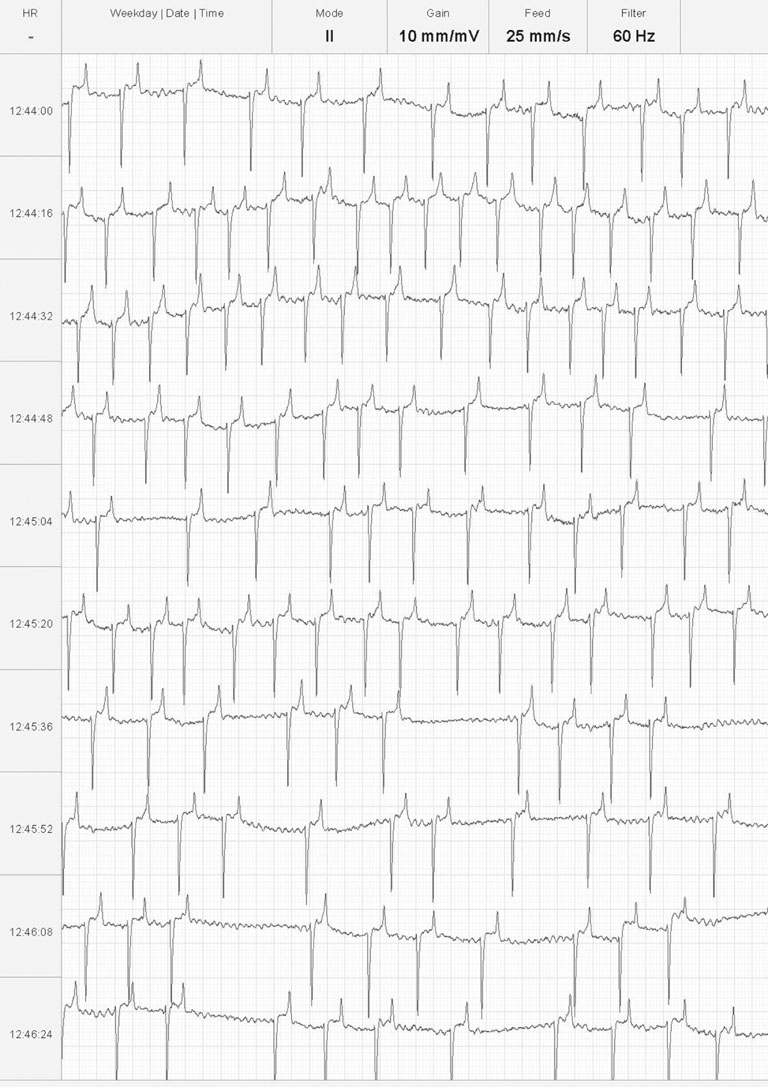
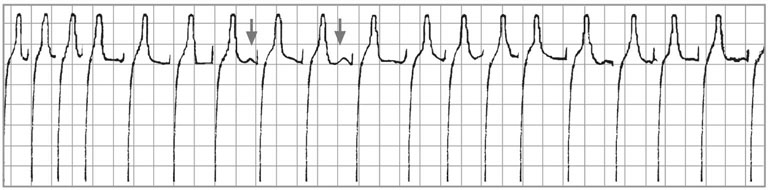
There are positive baseline undulations between the 7th and 8th and 9th and 10th beat that could be mistaken for ‘P’ waves. Closer inspection reveals them to be too close to the ‘QRS’ complex that follows and more characteristic ‘f’ waves are visible in the long diastolic interval before the final ‘QRS complex. This trace illustrates the high heart rate maintained by horses affected with paroxysmal atrial fibrillation.
The horse spontaneously converted to normal sinus rhythm within 5 hours of the abnormal rhythm becoming established.
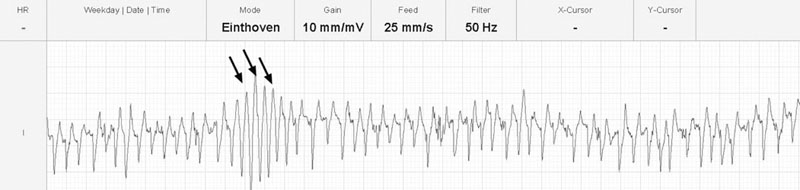
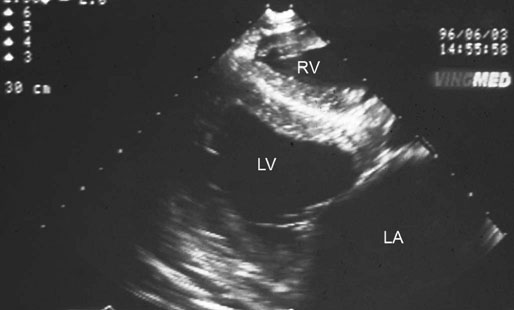
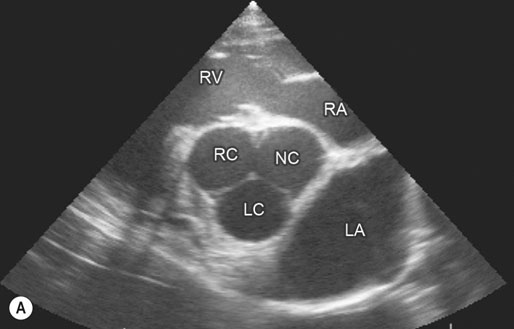
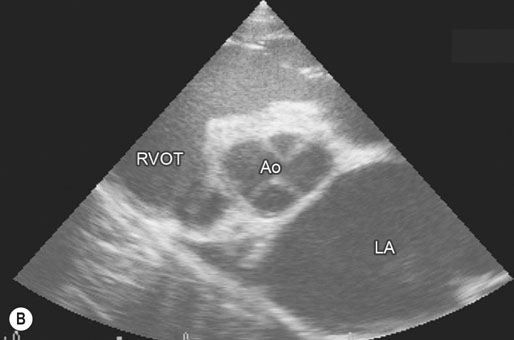
Image is derived from the right hemithorax with the transducer positioned to obtain a short-axis view of the atrium and aorta. Comparison of the relative width of the left atrium with respect to the aortic root provides useful reference for determination of left atrial enlargement, regardless of horse size. Normal horses have a left atrial to aortic root ratio of <1.2 : 1. Although there was a grade 2/6 murmur of mitral regurgitation, left atrial : aortic width ratio was within normal limits. The horse converted to normal sinus rhythm after administration of 45 g of quinidine per os. (LC = left coronary cusp of aortic valve; NC = non-coronary, RC = right coronary cusp, LA = left atrium, RV = right ventricle, RA = right atrium.)
(B) Two-year-old Thoroughbred flat racing filly with atrial fibrillation that presented with lethargy and poor exercise tolerance. Image is derived from the right hemithorax with the transducer positioned to obtain a short-axis view of the atrium and aorta. Visual inspection of the same image as Fig. 32.14A shows the greatly increased left atrial : aortic width ratio (2.9 : 1). The difference is accentuated as low output cardiac failure has reduced left ventricular stroke volume and blood pressure and concurrently aortic diameter has also reduced. The filly was treated using digoxin to control cardiac rate, diuretics to reduce congestion, an angiotensin-converting enzyme inhibitor (enalapril) to reduce cardiac afterload and a calcium sensitizing agent and phosphodiesterase inhibitor (pimobendan) to enhance myocardial contractility and further reduce afterload. Quinidine treatment is not appropriate when atrial fibrillation occurs secondary to left atrial enlargement. (Ao = aorta, RVOT = right ventricular outflow tract, LA = left atrium.)
Recognition
History
Physical examination
Special examination
Laboratory tests
Treatment and prognosis
Treatment of atrial fibrillation with quinidine sulfate
Regime
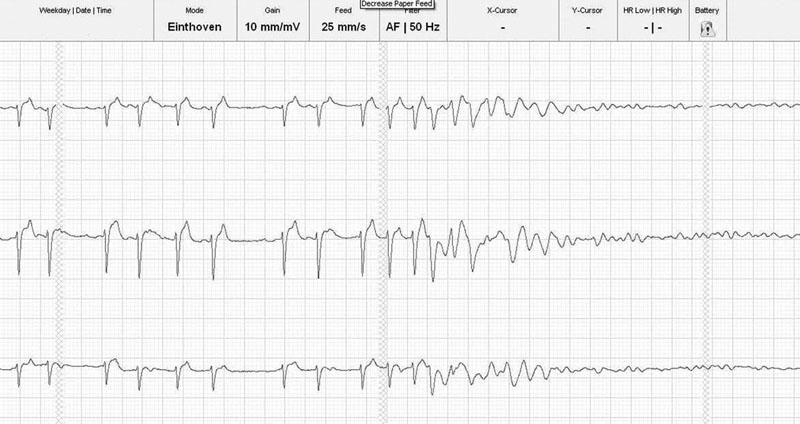
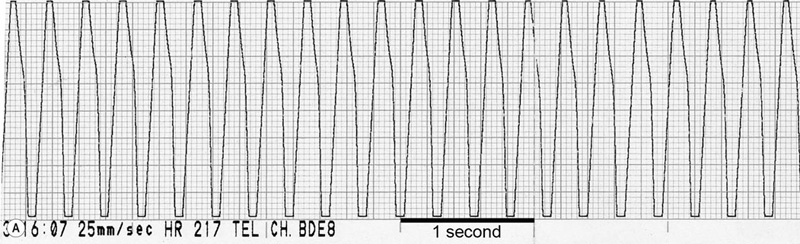
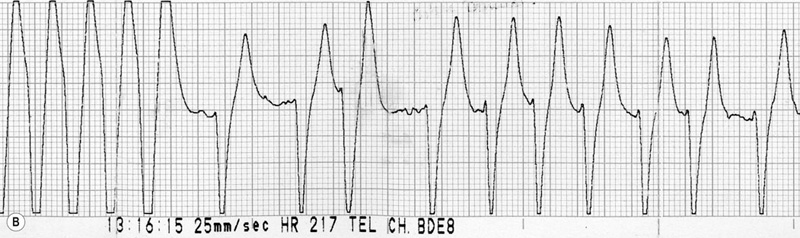
(B) ECG trace of the same horse as in Fig. 32.16A, 10 seconds after IV administration of 5 µg/kg detomidine. Note the sudden slowing of atrioventricular conduction and immediate shortening of the QRS complexes.
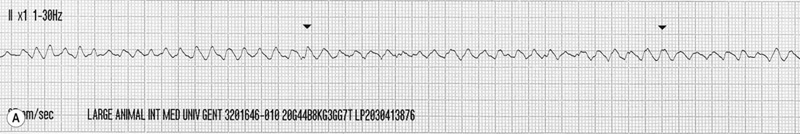
Other pharmacological treatments for atrial fibrillation
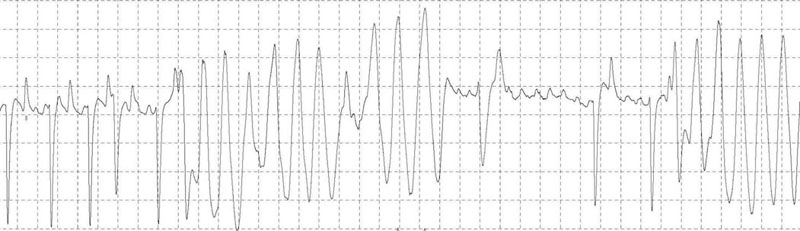
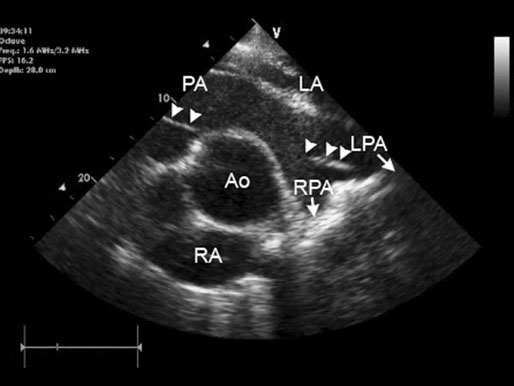
Transvenous electrical cardioversion of atrial fibrillation
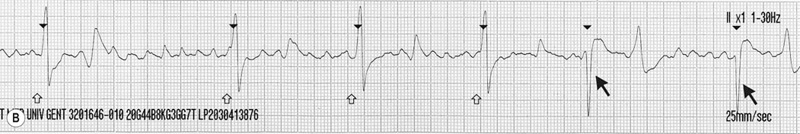
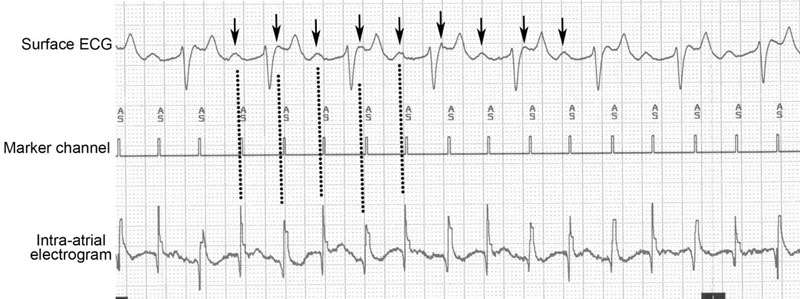
(B) Continuation of the ECG trace from Fig. 32.20A: right ventricular pacing (open arrows) is started and immediately restores ventricular function. Cardiac pacing is continued until the patient’s own ventricular depolarizations start to reappear (black arrows). Note the difference in QRS morphology between the paced and normal ventricular depolarizations. In this horse, subsequent 250 J shock delivery restored sinus rhythm.