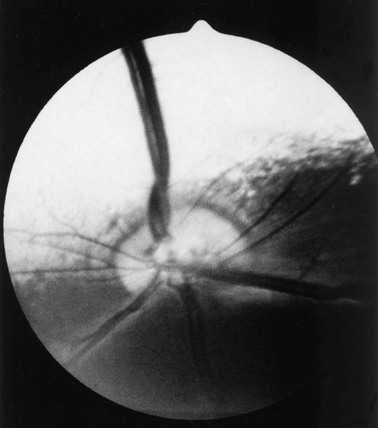
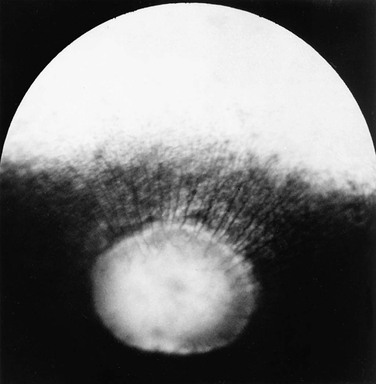
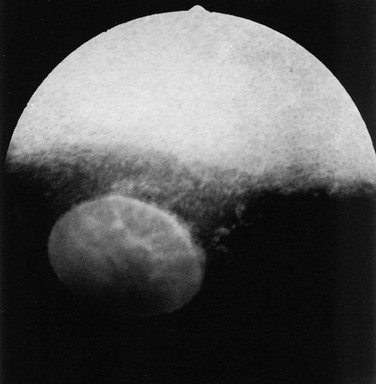
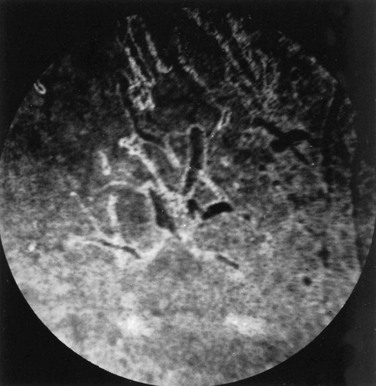
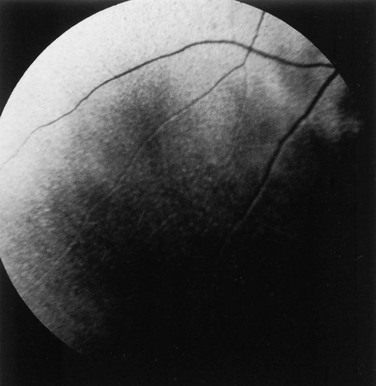
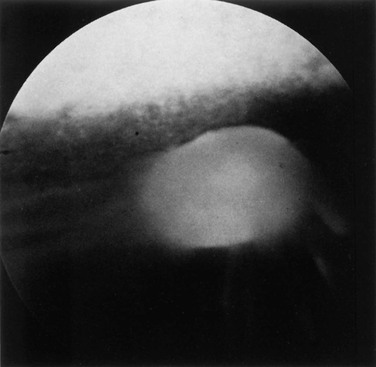
Ellen Belknap, Anne J. Gemensky Metzler, Consulting Editors Renee T. Carter* The clinician’s approach to the ophthalmology patient should be similar to the approach taken to patients evaluated for other systemic disorders. Prior to conducting a complete ophthalmic examination, basic information as to the patient’s signalment (species, breed, age, coat color, gender), use, and medical history should be obtained. A complete ophthalmic examination is then conducted in a stepwise methodical pattern to ensure all aspects of the eye are evaluated and important diagnostic steps are not overlooked. Reasons for presentation for ophthalmic examination include: • Abnormal appearance of one or both eyes (i.e., asymmetry or color change) • Presence of ocular discharge • Change in the performance/behavior of the patient • Follow-up of a previously diagnosed ophthalmic condition A series of questions regarding the clinical signs observed, the duration and clinical course of the condition, change in activity/behavior, previous eye problems, and whether other animals have been affected should be ascertained. The clinician should also obtain information on medications the owner has used to treat the eye and the animal’s response to therapy. Potential causes for an ophthalmic or visual problem, including any possible relationship to neurologic or iatrogenic (e.g., drug-induced) disease, toxin exposure, or systemic illness, should be explored. A few basic instruments and materials facilitate an efficient and thorough examination. These include a focused light source (a Finoff transilluminator is preferred), direct ophthalmoscope with a cobalt blue filter, magnifying loupes or optivisor, and a small fixation forceps. Sterile fluorescein dye strips, tear test strips, culture swabs, physiologic saline solution (flushing solution), topical anesthetic (0.5% proparacaine), and mydriatic solution (1% tropicamide) are often also necessary. For fundic examination, a direct or Panoptic ophthalmoscope may be used or indirect ophthalmoscopy can be performed using a handheld lens and light source. To irrigate the nasolacrimal ducts, polyethylene tubing (5 French) should be available. Prior to manual restraint, the animal’s activities and movements in its normal environment should be observed. This provides insight into the general well-being of the patient and its visual status. The examiner should study the animal’s body condition, attitude, posture, coordination, and head carriage and ability to navigate in its normal surroundings. As the animal is approached, closer inspection reveals whether facial and ocular symmetry and normal eye movements are present. Signs of ocular pain (e.g., blepharospasm, apparent photophobia, or epiphora) are noted, as well as size and position of the globes and the presence of ocular or nasal discharge, opacities, or masses. Adequate restraint is essential to conducting a complete ophthalmic examination in large animals. Manual restraint of small ruminants and neonates is usually adequate. For cattle, restraint in a chute with a head catch or in a stanchion with the head pulled laterally by nose tongs or a halter is ideal; restraint of a horse in stocks with a halter is recommended. Chemical restraint may be necessary in cattle, and use of a nose twitch and/or chemical restraint is often needed for horses. The animal’s health and duration of the examination should be considered prior to the administration of chemical restraint. Chemical restraint may consist of a combination of injectable sedative (e.g., xylazine or detomidine for horses), with or without an injectable analgesic (e.g., butorphanol for horses). An auriculopalpebral (and occasionally frontal) nerve block using a local anesthetic agent such as 2% lidocaine facilitates examination, especially in patients with a painful ophthalmic condition. Menace responses and palpebral and pupillary light reflexes (PLRs) must be performed prior to administration of sedatives, analgesics, or local anesthetics. An evaluation of the integrity of cranial nerves associated with normal ocular function is conducted (see Chapter 8). This includes an assessment of the following prior to sedation: Detailed examination of ocular structures should then be performed in a predetermined pattern (i.e., anterior to posterior).1–3 A set pattern of examination is recommended to avoid interfering with diagnostic tests that may have to be performed (e.g., Schirmer tear test, culture). After evaluation of the animal in its normal environment, it is best to move the patient to an environment that is quiet and well-lit for extraocular examination and can be darkened for intraocular examination. Standing in front of the patient, the clinician should evaluate for alterations in the normal symmetrical appearance of the eyes and periocular structures (Color Plate 39-1). Attention should be paid to lash height, pupil size and position, and globe position. If lashes are pointed downward, this may indicate enophthalmos or ptosis of the lid; if lashes are directed upward, this may indicate exophthalmos (orbital disease) or buphthalmos. Evaluate the size, shape, and symmetry of the pupils by retro-illumination using a focal light source. Evaluate both direct and consensual PLRs in a darkened environment. At this point, the examiner determines whether ocular cultures or tear measurements are desired, because these procedures must be completed before further manipulations are performed and before topical pharmacologic agents are instilled.4 Depending on clinical signs, severity of ocular disease, and species being examined, viral, bacterial, or fungal cultures may be indicated. Sterile swabs moistened with saline and appropriate enrichment broth or transport media are applied in direct contact with the tissue surface of interest to be cultured. Palpation of the boundaries of the orbit for irregularities, asymmetry, masses, or fractures should then be performed. The globe is then retropulsed to assess for increased resistance (indicating a space-occupying mass) and to inspect the anterior aspect of the nictitating membrane (“third eyelid”). Retropulsion is performed by gently pressing the globe back into the orbit with a finger placed over the partially closed upper eyelid. Retropulsion should not be performed if the cornea is compromised by a deep ulcer or laceration. The eyelids are inspected for integrity, position, and movement. Each eyelid is digitally everted for inspection of the margins, meibomian gland openings, and palpebral conjunctiva. Paresis, malposition (entropion, ectropion [Color Plate 39-2]), defects, masses, inflammation (swelling, ulceration, exudate), alopecia, foreign bodies, and abnormal lashes are noted. The nictitating membranes are examined for normal position, integrity, degree of pigmentation, and the presence of follicles or masses (Color Plate 39-3). To inspect for foreign bodies, topical anesthetic solution (0.5% proparacaine) is instilled two to three times onto the ocular surface. The nictitating membrane is then grasped and manipulated with small fixation forceps, and both sides are examined for foreign bodies. Care should be taken not to damage the cartilage of the third eyelid with toothed forceps. Normally the conjunctiva appears moist, glistening, and semitransparent. Signs of conjunctivitis are chemosis (conjunctival edema), hyperemia, and ocular discharge. Color changes of the conjunctiva usually accompany anemia (blanched, pale) or icterus (yellow, amber). Chemosis may indicate severe hypoproteinemia. Conjunctival lesions noted include focal swellings, follicles, adhesions, or masses. The sclera underlying the bulbar conjunctiva is inspected for color, contour, swellings, masses, pigmented areas, or surface irregularities. Lacrimal system examination entails evaluation of both secretory and excretory components. Normal secretions result in a moist, glistening ocular surface. Although not typically performed in large animals, tear test strips may be used to quantify aqueous tear secretion (see Ancillary Diagnostic Procedures). Schirmer tear testing is indicated in cases with a lackluster corneal appearance or persistent keratitis. To examine the excretory components, the upper and lower puncta and nasal openings of the nasolacrimal system are identified (Color Plate 39-4). Any overflow of tears onto the face (epiphora) is noted. Causes of increased ocular secretions (e.g., frictional irritants, foreign bodies, corneal ulcers, ocular inflammation) must be ruled out. Causes for stimulation of lacrimal secretions must be differentiated from causes of outflow occlusion, such as congenital atresia and acquired obstruction of the nasolacrimal system. Application of fluorescein dye can detect corneal ulceration and aids in assessment of nasolacrimal system patency. Passage of dye from the nasal opening of the nasolacrimal duct within 5 minutes confirms patency (Jones test). Retrograde irrigation of the nasolacrimal duct by inserting a length of 5-French flexible tubing into the nasal punctum and flushing with saline may be necessary to differentiate insufficient drainage from excessive secretions. The rest of the ophthalmic examination is performed in a darkened area. Using a focal light source, obtain the fundic reflex to highlight opacities in the ocular media (aqueous, lens, or vitreous). Opacities in the ocular media will obstruct the fundic reflex (Color Plate 39-5); using this reflex, the examiner may also compare symmetry of the pupils. The cornea is then examined for irregularities and opacities. The cornea is an avascular structure that is smooth and transparent, with a moist reflective surface. Direct a focal light source across the cornea (parallel) to highlight corneal irregularities. Localization of opacities to the cornea can be conducted by using this technique or alternatively with a direct ophthalmoscope or handheld slit-lamp. Corneal edema appears as a hazy blue corneal opacity and should be characterized as localized or diffuse (Color Plate 39-6). With severe corneal edema, bullae (vesicles) may be noted. Infectious keratitis is characterized by suppuration and necrosis. The cornea becomes more densely opaque and acquires a beige, green, or milky appearance when infiltrate is present; the margins will be indistinct and the eye painful. Corneal abscesses occur as focal areas of suppuration within the stroma underlying a nonulcerated cornea (Color Plate 39-7). Corneal opacities may also result from focal or diffuse scarring (gray with distinct margins), areas of corneal degeneration or dystrophy (white, chalky to refractile with distinct margins), or stretching of Descemet’s membrane (Haab’s striae) from elevation of intraocular pressure or previous trauma (parallel lines, resemble “railroad tracks” [Color Plate 39-8]). Inflammatory products clustered on the corneal endothelium (keratic precipitates) appear as multiple beige or brown foci, usually on the ventral aspect of the corneal endothelium. This finding indicates the presence of anterior uveitis. Intraocular examination begins with evaluation of the clarity and depth of the anterior chamber. The anterior chamber can be evaluated using a direct ophthalmoscope set on the slit-beam setting (light perpendicular to the cornea and brought close until in focus), a handheld slit-lamp, or a transilluminator directed parallel to the cornea (Color Plate 39-9). An optivisor may be needed for magnification. Opacities within the anterior chamber include inflammatory products (cells and fibrin), proteins (flare), red blood cells (hyphema), or white blood cells (hypopyon). Suspended or clustered inflammatory materials in the anterior chamber indicate anterior uveitis. Besides the presence of exudates, loss of anterior chamber transparency may result from lens luxation, vitreal prolapse, anterior synechia, or intraocular masses (neoplasia or foreign bodies). Loss of normal anterior chamber depth may result from leakage of aqueous humor, iris prolapse, iris bombé (forward bulging of the iris caused by iris-lens adhesions), or anterior lens luxation. Increased depth of the anterior chamber may be caused by a protruding cornea (keratoconus), a hypermature (resorbing) cataract, or posterior lens luxation. The iris is inspected for altered contour, pigmentation, mobility, neovascularization, pupil size and shape, and the presence of uveal masses (including the normal granula iridica). Granula iridica (i.e., corpora nigra) are normal structures present along the pupil margin. These structures may atrophy with chronic uveitis or become cystic. Transillumination of uveal masses allows differentiation of solid structures (e.g., melanoma) from uveal cysts. In animals with lightly colored or spotted hair coats, multicolored irides should be recognized as normal variants. Although uncommon, congenital iris thinning (hypoplasia) may be noted as dark, flat, or translucent areas when transilluminated. The lens-iris interface is best evaluated when the pupil is dilated. Iris membranes, adhesions, or strands should be characterized as congenital (persistent pupillary membranes) or acquired (synechiae or remnants of iris atrophy) (Color Plate 39-10). Pupillary openings are evaluated for size, shape, symmetry, movements, and opacities. Direct and indirect (consensual) PLRs are assessed. The examiner must recall that PLRs are not a test of vision (i.e., abnormal responses may be observed in visual animals, and normal reflexes may occur in nonvisual animals). Pupillary abnormalities that should be noted are inequality in size (anisocoria), abnormal movements (hippus), abnormal location (corectopia), or abnormal shape (dyscoria) (Color Plate 39-11). Opacities of the pupil usually result from loss of lens transparency or from presence of intraocular exudates. Obscuration of the pupil space may occur with severe miosis (from acute anterior uveitis), anterior lens luxation, condensation of anterior chamber exudates, and synechiae formation (from chronic uveitis). In animals with normal PLRs, complete examination of the lens and structures posterior to the lens (i.e., vitreous and fundus) may be achieved only after dilation with a mydriatic agent such as 1% tropicamide, which usually occurs 20 to 30 minutes after instillation. Once dilated, repeat retro-illumination of the ocular media to evaluate for any opacities blocking view of the fundic reflex; fundic examination can then be performed. Using a focused light, the lens is inspected for a smooth, transparent, convex anterior capsule and normal position (no part of the equator should be visible). When evaluating for lens opacities (cataracts), the examiner should direct the focused light through the axial part of the lens to establish the presence of a fundic reflex. Cataractous changes are observed as dark areas seen within the area of reflected light. Cataracts may be classified according to the extent to which a fundic reflex is obstructed; a partial reflex indicates an incomplete cataract, whereas an absent reflex indicates a complete cataract. If the lens profile appears distorted (wrinkled) with numerous refractile particles present, this is typically indicative of a hypermature (resorbing) cataract. Evaluate the depth of the anterior chamber and for aqueous flare by using a small focal light source directed perpendicular to the cornea. This can be performed using the small spot of light or slit-beam on a direct ophthalmoscope. Alternatively, a handheld slit-lamp may be used. The anterior chamber is the space between the cornea and iris that contains aqueous humor. Breakdown of the blood-ocular barrier (anterior uveitis) (Color Plate 39-12) will result in increased protein in the anterior chamber and can be detected as aqueous flare. The anterior chamber will appear shallow in cases with phthisis bulbi, anterior synechiae, iris bombé, or anterior lens luxation. The anterior chamber will appear deep in cases with hypermature cataracts, buphthalmia, and posteriorly luxated lenses. Ophthalmoscopy must be used to examine the vitreous and fundus. The direct ophthalmoscope provides an upright image and high magnification, but it requires a close working distance and provides a small field of view, making it a poor screening tool. Using the direct ophthalmoscope, the vitreous should be in focus with a dioptric setting between +6 to +1, and the fundus is usually in focus between +1 and −2 diopters. Monocular indirect ophthalmoscopy (Panoptic) provides an upright image; moderate magnification and field of view are provided. Indirect ophthalmoscopy provides an inverted virtual image with a less magnified view, making it a better screening tool. The vitreous is first examined for congenital remnants (retained hyaloid structures) and opacities, including degenerative materials or exudates. Examination of the fundus begins with identifying the optic disc (papilla) and studying its size and shape. The shape, location, and vascular pattern of the optic disc and the appearance of the fundus vary considerably among species. In ruminants the optic disc margin typically appears irregular and fluffy, indicating myelination of axons entering the optic disc. However, it tends to be horizontally elliptical or kidney shaped and located in the tapetal portion of the fundus.5 An optic disc with extensive myelination may be elevated above the surface of the fundus (sometimes called pseudopapilledema). In ruminants the major retinal arterioles are large and are accompanied by venules that anastomose on the surface of the optic disc. The dorsal arteriole and venule usually intertwine as they course away from the disc over the midtapetum (Fig. 39-1). By contrast, the equine fundus is characterized by a large pink or salmon-colored horizontally elliptical or oval disc located in the nontapetum5 (Fig. 39-2). Horses are paurangiotic, meaning they have vessels that only extend a short distance from the optic nerve head. The small retinal blood vessels extend radially from the margin of the disc, and no anastomotic venules are visible over the optic disc. In both ruminants and horses the fibrous tapetum is present in the dorsal two thirds of the eye and is penetrated by choroidal capillaries; thus the fundus in these species is typified by dark, stippled foci termed stars of Winslow (end-on capillaries). Coloration of the tapeta of large animals also varies considerably and may range from gold to bluish green. In animals with heterochromia irides, areas of the fundi may characteristically be devoid of pigmentation and may lack a tapetum. These areas may appear orange or red because of direct visualization of the choroidal vasculature. Abnormalities of the optic disc include hypoplasia (micropapilla), elevation (papilledema), depression (cupping), degeneration (atrophy [Fig. 39-3]), and vascular changes (e.g., congestion, attenuation, hemorrhage). The tapetal fundus is evaluated for clarity, coloration, pigmentation (Fig. 39-4), and integrity of the retinal vessels (Fig. 39-5). The nontapetal fundus is evaluated for uniformity of pigmentation. Both tapetal and nontapetal areas are assessed for retinal elevations or separations (Fig. 39-6), hemorrhages, degenerations, disorganization (dysplasia), or scleral defects (colobomas).5 Several additional procedures may form important supplements to the complete ophthalmic examination. Although some ancillary procedures require specialized equipment and expertise, many may be performed in general practice. Fluorescein and rose bengal are ocular surface stains most often used as aids in diagnosing conjunctival and corneal diseases. The tip of a sterile dye strip is moistened with saline or eye-irrigating solution, and a drop of the stain is instilled onto the eye. Fluorescein is a water-soluble dye used to detect exposed corneal stroma resulting from an epithelial defect (erosion), stromal ulceration, or descemetocele and is viewed with cobalt blue light. The pattern of fluorescein staining for a descemetocele is characterized by a donut-shaped area of positive fluorescence, with the perimeter retaining stain and the center or deepest area (Descemet’s membrane) not retaining stain. Fluorescein may also be used to evaluate the patency of nasolacrimal ducts, because an open duct allows the transmission of stain, which may be observed exiting the duct system at the nasal orifice (Jones test). Rose bengal is retained by devitalized surface cells and is therefore useful in detection of subtle abnormalities like hyperplastic or desquamating cells associated with ocular surface drying, herpetic infection, keratomycosis, or squamous cell carcinoma. Stain uptake appears pink when illuminated with white light. Although tear deficiencies are uncommon in large animals, tear test strips may be beneficial to quantify aqueous tear production in selected cases. A sterile filter paper strip (40 × 5 mm with notched end) is inserted into the lower conjunctival fornix. In large animals it is sufficient to measure the amount of wetting in 30 seconds (≥20 mm is normal). Tear testing is indicated in patients with a dry, lackluster corneal surface, persistent ocular discharge, or keratitis. Cytologic evaluation of ocular surface scrapings or intraocular aspirates may differentiate between inflammatory and neoplastic diseases or in some cases may provide a definitive diagnosis. Orbital aspirates may be diagnostic in cases of exophthalmos caused by neoplasia (e.g., lymphosarcoma). Immunofluorescent or polymerase chain reaction (PCR) testing of cytologic specimens may confirm viral (e.g., infectious bovine rhinotracheitis) or Chlamydophila infections.3 Bacterial cultures taken from the ocular surface or from ocular aspirates, with subsequent antimicrobial susceptibility testing, may be necessary for definitive diagnosis and appropriate treatment of ocular infections. The diagnostic laboratory performing ocular cultures may offer suggestions on culture procedures, including preferred transport media and handling of samples. It is especially important to consult with the laboratory in advance when anticipating culturing for fungi, Mycoplasma, Chlamydophila, or viral agents. Tonometry, a means of measuring the intraocular pressure (IOP), is useful in diagnosing glaucoma (elevated IOP) and uveitis (low IOP) and in assessing response to therapy for these conditions. Tonometry is indicated in cases with corneal edema, redness, lens luxation, abnormal PLR, and cases with vision loss or a history of glaucoma. Digital tonometry (gently indenting the globe through the upper eyelid) is not considered an accurate measure of IOP; Schiøtz tonometry is not applicable to large domestic species. Applanation tonometry using instrumentation such as the TonoPen provides accurate and reproducible IOP readings in large animals and is routinely performed. Additionally, use of the rebound tonometer, TonoVet, has been used successfully and can be calibrated for use in equine patients. The clinician should be aware that eyelid blocks, sedation, and head position can all impact IOP readings. Biomicroscopy using a portable handheld slit-lamp is useful for identifying the location and nature of anterior ocular opacities. Focal irritants (e.g., ectopic cilia, small foreign bodies) and small lesions may only be visible with the magnification provided using biomicroscopy. Alternatively, use of a focal light source with magnification in the form of an otoscope or optivisor may be helpful in providing magnification. Funduscopic examination may be performed relatively quickly and easily using the technique of indirect ophthalmoscopy. Monocular indirect ophthalmoscopy is performed using a handheld light source and a separate 20- or 14-diopter focusing lens for equine and bovine patients; a 28-diopter lens may be utilized for smaller ruminants. Other ancillary diagnostic procedures include electroretinography (to evaluate retinal function), visual evoked potentials (to evaluate visual pathways), and imaging procedures (radiography, ultrasonography, computed tomography [CT]). Although plain skull radiographs and some contrast studies (e.g., dacryocystorhinography) may be performed in a general practice setting, the remaining procedures require techniques and equipment usually available only at referral centers. Renee T. Carter† Symptoms of ocular disease can be grouped into 5 primary categories at the time of presentation: Patients may present with one or a combination of these clinical signs. This section provides a general description of the signs and examples of ocular diseases in which a particular sign predominates. Table 39-1 summarizes common signs of ocular disease in large animals. TABLE 39-1 Causes of Important Ocular Signs in Large Animals B, Bovine; C, caprine; ERU, equine recurrent uveitis; IBK, infectious bovine keratoconjunctivitis; IBR, infectious bovine rhinotracheitis; O, ovine. Ocular or periocular asymmetry results from unilateral changes in facial soft tissue or orbital structures, orbital volume, globe size, eyelid position, or pupil size and position. Changes in symmetry often involve a reduction or increase in volume of a certain tissue. Reduction in tissue volume occurs with congenital hypoplasia, cicatricial shrinkage, atrophy, or dehydration. Increase in tissue volume may involve the whole globe (buphthalmos) or be characterized by irregular enlargement, as seen with inflammatory or neoplastic lesions involving the globe, orbit, or eyelids. Asymmetry may also result from a neurologic dysfunction. Common examples include reduced palpebral fissure size (secondary to facial nerve paralysis), strabismus, third eyelid protrusion, and anisocoria (see Chapter 8). This section describes the common signs of ocular disease. It is not the intent to describe in detail each of the diseases that may be noted; these disorders are covered in other sections of this chapter. Changes in globe position affect symmetry and include exophthalmos, enophthalmos, and strabismus. Forward displacement of the eye (exophthalmos) (Fig. 39-7) is often associated with a space-occupying orbital lesion (neoplasia, inflammatory, vascular, cystic) or, less often, a congenitally shallow, underdeveloped orbit. Posterior malposition of the globe (enophthalmos) may result from active globe retraction caused by pain or from loss of supporting retrobulbar soft tissues as seen in cases of muscle atrophy, loss of orbital fat, or denervation. Congenital strabismus is a developmental abnormality that results in ocular asymmetry due to deviation of normal globe position and has been reported in Jersey, Shorthorn, Holstein, and German Brown Swiss cattle.1,2 Strabismus can occur with exophthalmos and has also been associated with systemic disorders such as polioencephalomalacia and listeriosis.2 Unequal orbit volume resulting in displacement of the globe or globe malposition may also occur with traumatic orbital fractures. Unequal globe size can also account for ocular asymmetry. A congenitally small globe (microphthalmia) occurs as a genetic defect in cattle and horses1,3 and has also been associated with exposure to infectious agents and teratogens. Microphthalmia is frequently accompanied by multiple ocular anomalies and may be associated with multiple organ involvement or vertebral abnormalities.2 Acquired variations in globe size usually result from shrinkage of the globe (phthisis bulbi [Color Plate 39-13]) secondary to chronic uveitis; stretching or enlargement of the globe (megaloglobus, buphthalmos) occurs with glaucoma. Asymmetry of the upper or lower eyelid may be due to entropion, ectropion, blepharitis, conjunctivitis, or facial nerve paralysis (ptosis). Entropion has been reported in Simmentals1 but most commonly occurs secondary to microphthalmos or enophthalmos (retraction of the eye with pain, reduction in orbital volume, denervation). Trauma with secondary scar formation may result in either entropion or ectropion. Nictitating membrane (third eyelid) protrusion is frequently seen secondary to active retraction of the globe in response to ocular pain, enophthalmos caused by loss of orbital contents (dehydration, malnutrition, atrophy), presence of third eyelid masses, orbital space-occupying masses, or neurologic disorders (e.g., Horner syndrome, tetanus). Pupillary asymmetry, or anisocoria, may develop for a variety of reasons that can be grouped into (1) primary iridal lesions, including congenital iridal defects (hypoplasia, aniridia, coloboma, anterior segment dysgenesis, synechia, iris atrophy), (2) neurologic disorders (Horner syndrome, oculomotor nerve dysfunction), (3) intraocular diseases (uveitis, glaucoma, unilateral retinal lesions), (4) diseases involving the optic nerve or brainstem, and (5) previous use of pharmacologic agents like atropine that alter iris smooth muscle function. The presence of an ocular mass may be the primary cause of ocular asymmetry. Ocular surface neoplasms are relatively common in horses and cattle. Ocular squamous cell carcinomas usually arise from nonpigmented tissues of the nictitating membrane, the lateral limbal region, or the eyelid margin (where UV light exposure is highest).4 They may appear as irregularly raised, papillary surface masses or, less often, as smooth, vascularized lesions that invade the globe. Ulceration, exudation, and mucopurulent ocular discharge are frequent concurrent findings (see Ocular Neoplasia). Periocular sarcoids are also common in horses; their appearance is variable, ranging from flat foci of alopecia to fleshy or nodular nonulcerative lesions.5,6 Other ocular tumors occur in large domestic animals but are relatively uncommon. Dermoids and orbital cysts are congenital masses involving the periocular tissues, eye, or orbit. Other nonneoplastic ocular masses seen in large animals include firm parasitic and foreign body granulomas and soft, fluctuant subconjunctival swelling characteristic of prolapsed periorbital fat. Ocular and orbital pseudotumors have also been described in the horse.7 Changes in the color of ocular or periocular tissues or the presence of opacities in the clear ocular media (cornea, aqueous humor, lens, or vitreous) are important features of ocular disease. Such changes must be differentiated from normal congenital differences in ocular pigmentation. Developmental color dilution or absence of ocular pigmentation results in light or multicolored irides (heterochromia iridis). Examples of abnormal coloration include hyperemia of conjunctival (superficial) or episcleral (deep) blood vessels associated with ocular inflammation (see Red Eyes in Table 39-1), hemorrhage secondary to trauma or coagulopathies, pallor of the conjunctiva (which reflects severe anemia), and yellowing of the sclera and sometimes iris, indicating icterus. A change in the color of the iris may also occur with anterior uveitis. Opacities of the ocular media may occur either as surface (corneal) or intraocular (anterior chamber, lens, or vitreous) phenomena. Brown discolorations of the cornea are the result of pigmentation, iris prolapse, embedded foreign body, or pigmented fungal plaques. Grayish scars occur from previous episodes of keratitis; infectious keratitis will present with cream- to white-colored infiltrate; corneal degeneration will appear as white deposits of lipid or mineral; vascularization occurs secondary to chronic inflammation or neoplasia; and bluish discoloration is caused by corneal edema. These color changes frequently occur in various combinations in more severe keratitis, especially those of infectious origin such as chronic keratoconjunctivitis caused by Chlamydophila spp., Mycoplasma spp., or Moraxella bovis. Cataracts are perhaps the most obvious cause of intraocular opacities in large animals (Color Plate 39-14). Inherited cataracts have been reported in Thoroughbreds, Quarter Horses, Morgans, and Rocky Mountain horses.8 However, the presence of exudates within the aqueous humor or vitreous, congenital vascular remnants in the vitreous, degeneration of the vitreous, or retinal detachment may also account for intraocular opacities (see Table 39-1). Ocular discharge can be characterized as serous (epiphora), mucoid (catarrhal), purulent, or hemorrhagic (sanguineous). The type of discharge may assist in determining the severity and chronicity of the eye disease. Serous discharge generally indicates mild eye disease, whereas mucopurulent or hemorrhagic discharge indicates more serious disorders. A notable exception to this generalization is equine recurrent uveitis (ERU), which is associated with serous discharge (see Immune-Mediated Ocular Diseases). The nature of ocular discharge tends to change as the disease progresses or improves. This is most notable in inflammatory or infectious ocular diseases. Initially the discharge is predominantly serous, but it tends to become mucopurulent with chronicity (see Table 39-1). Epiphora describes facial wetting and results from overflow of tears over the eyelid margin. This may result from excessive secretion of tears (e.g., painful stimulus) or from physical or functional obstruction of the nasolacrimal system. In large animals, reflex lacrimation with an associated overabundance of tears is the typical response to ocular irritation or inflammation (e.g., foreign body, lash disorders, conjunctivitis, keratitis, uveitis). When epiphora is noted, careful digital and visual examination for foreign bodies within the conjunctival fornix or under the third eyelid is indicated. Epiphora is generally one of the earliest signs of conjunctivitis, ulcerative keratitis, or anterior uveitis. In cattle with keratoconjunctivitis caused by Moraxella bovis, epiphora is present several days before visible corneal ulceration occurs9 (see Infectious Bovine Keratoconjunctivitis). Developmental defects or malformations of the nasolacrimal duct system (e.g., imperforate puncta) results in epiphora secondary to impaired outflow in neonates. In these cases, the presence of epiphora may be misinterpreted as overproduction of tears. Previously undiagnosed congenital defects may also be the cause of persistent ocular discharge in adult animals. Acquired obstructions of the nasolacrimal ducts may result from infections, foreign bodies, facial trauma, nasal tumors, or sinusitis that involve the duct system. Whether congenital or acquired, simple nonseptic obstructions are characterized by epiphora; septic occlusions result in mucopurulent discharge from the eye or nostril on the affected side. Excessive mucus production is a feature of follicular conjunctivitis, possibly as a result of the rubbing of elevated lymphoid follicles on apposing conjunctival surfaces. Lymphoid follicles are noted in subacute or chronic forms of chlamydial conjunctivitis in sheep and with Onchocerca larval migration in horses. Mucoid ocular discharge may be observed concurrently with epiphora in acute ocular surface infections caused by viral or chlamydial agents. Excessive tenacious mucus may also result from inadequate secretion of the aqueous component of tears (i.e., keratoconjunctivitis sicca). Although keratoconjunctivitis sicca is not diagnosed as commonly in large animals as it is in dogs, it has been reported in horses, usually as a complication of guttural pouch pathology.10 Purulent to mucopurulent material is characteristic when bacterial organisms are involved in the ocular disease process. Bacterial conjunctivitis occurs frequently in large domestic species and manifests as red eyes with copious mucopurulent ocular exudate. Ocular foreign bodies and surface masses (e.g., squamous cell tumors) typically have associated bacterial infections. Mucopurulent discharge in the absence of ocular inflammation suggests infection of the nasolacrimal sac (dacryocystitis) or ducts, with reflux of exudate from the lacrimal puncta. Sanguineous or hemorrhagic discharge most often occurs after blunt or penetrating trauma to the eye (see Ocular Trauma). Foreign body penetration may damage the eyelid, conjunctiva, or globe, resulting in bleeding onto the ocular surface. Corneal ulcers may rupture and result in uveal prolapse and subsequent hemorrhage on the ocular surface. Patients with conjunctivitis or ocular surface tumors may develop ulceration resulting in bloody ocular discharge. Whenever blood is noted on the surface of the eye, it is imperative that a thorough ophthalmic examination be performed to determine the cause and evaluate integrity of the globe. Ocular pain can be manifested as blepharospasm, epiphora, photophobia, and periocular hyperesthesia. Animals with severe ocular pain usually resist manipulation of the eyelids or any form of ocular examination by persistently jerking the head away from the examiner and by closing the eyelids tightly. In cases of persistent ocular inflammation, discomfort and pruritus may be manifested by rubbing and self-trauma to ocular or periocular structures. Ocular pain may result from conjunctivitis, trauma, keratitis, uveitis, and glaucoma. Other causes of ocular pain include mechanical irritation from entropion, trichiasis, distichia, ectopic cilia, or foreign material.11 Foreign bodies causing ocular irritation in large animals are typically plant materials like seeds, hay stems, straw, twigs, bark, or thorns, although particles of sand or soil can also cause severe ocular irritation. Non-embedded particulate matter is usually entrapped by mucus and washed out of the eye by reflex tearing; it typically results only in transient discomfort. By contrast, embedded foreign material (i.e., between ocular surface layers or within ocular tissues) causes persistent ocular pain. Visual deficits in large animals manifest in a variety of ways. Obvious signs include bumping into objects and lack of response to visual stimuli such as light or hand motions. Another sign of blindness is reliance on stationary objects (e.g., fences, railings, other animals) to maneuver within the environment. Behavioral changes include reluctance to move or to venture into unfamiliar areas, nervousness, and spooking in response to sudden noises or movements.12 The nonvisual animal is frequently found standing isolated from the group and may experience injury by dominant individuals in the herd and suffer from weight loss.12 Searching nystagmus is also seen in some animals with congenital blindness. Nonvisual animals attempt to compensate for loss of vision with their other senses, which may result in an elevated head position or a head tilt. Patients may also exhibit snorting or intensive sniffing associated with nervousness, and maximum neck extension is observed. Frequently, blind animals will show exaggerated elevation of the limbs while walking. This must be differentiated from true hypermetria (see Chapter 8). Partial loss of vision may be difficult to determine, and detection depends on observing more subtle behavioral changes such as slight head cocking or tilting, difficulty maneuvering in dim light, or shying and startling from objects on one side or objects present in some specific part of the visual field. Conduction of a maze test will assist the clinician in the detection of partial vision loss. Animals may effectively compensate for congenital blindness or slow diminution of vision, particularly when they remain with other unaffected animals in a familiar environment. Visual disturbance may not be apparent until an affected animal is isolated or moved to an unfamiliar area. There are numerous causes of blindness in large animals, including those that involve only the visual system and some that involve other nervous system tissues or are multisystemic (Box 39-1). A functional approach to blindness involves anatomically classifying the cause as one of the following:
Diseases of the Eye

Ophthalmic History and Examination
Ophthalmic History
Instruments and Materials
Ophthalmic Examination Procedures
General Inspection
Restraint
Neuro-ophthalmic Assessment
Detailed Examination
Ancillary Diagnostic Procedures
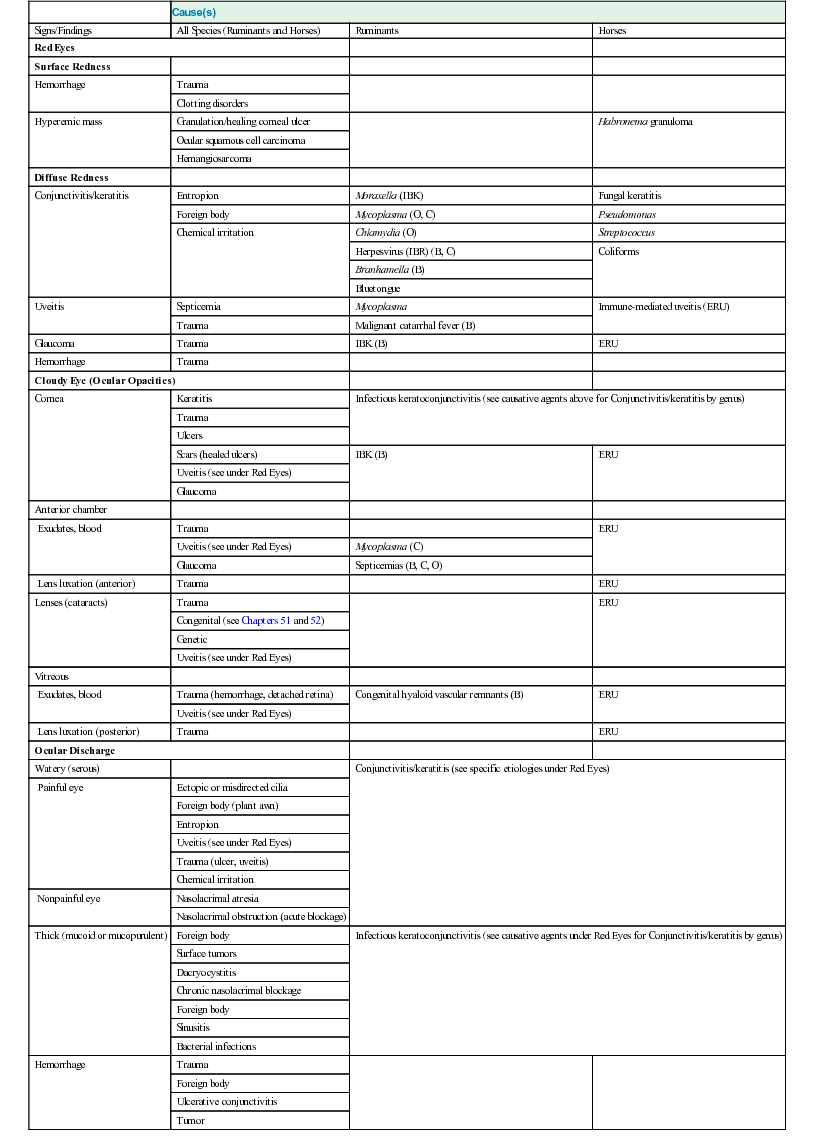
Signs of Ocular Disease
Cause(s)
Signs/Findings
All Species (Ruminants and Horses)
Ruminants
Horses
Red Eyes
Surface Redness
Hemorrhage
Trauma
Clotting disorders
Hyperemic mass
Granulation/healing corneal ulcer
Habronema granuloma
Ocular squamous cell carcinoma
Hemangiosarcoma
Diffuse Redness
Conjunctivitis/keratitis
Entropion
Moraxella (IBK)
Fungal keratitis
Foreign body
Mycoplasma (O, C)
Pseudomonas
Chemical irritation
Chlamydia (O)
Streptococcus
Herpesvirus (IBR) (B, C)
Coliforms
Branhamella (B)
Bluetongue
Uveitis
Septicemia
Mycoplasma
Immune-mediated uveitis (ERU)
Trauma
Malignant catarrhal fever (B)
Glaucoma
Trauma
IBK (B)
ERU
Hemorrhage
Trauma
Cloudy Eye (Ocular Opacities)
Cornea
Keratitis
Infectious keratoconjunctivitis (see causative agents above for Conjunctivitis/keratitis by genus)
Trauma
Ulcers
Scars (healed ulcers)
IBK (B)
ERU
Uveitis (see under Red Eyes)
Glaucoma
Anterior chamber
Exudates, blood
Trauma
ERU
Uveitis (see under Red Eyes)
Mycoplasma (C)
Glaucoma
Septicemias (B, C, O)
Lens luxation (anterior)
Trauma
ERU
Lenses (cataracts)
Trauma
ERU
Congenital (see Chapters 51 and 52)
Genetic
Uveitis (see under Red Eyes)
Vitreous
Exudates, blood
Trauma (hemorrhage, detached retina)
Congenital hyaloid vascular remnants (B)
ERU
Uveitis (see under Red Eyes)
Lens luxation (posterior)
Trauma
ERU
Ocular Discharge
Watery (serous)
Conjunctivitis/keratitis (see specific etiologies under Red Eyes)
Painful eye
Ectopic or misdirected cilia
Foreign body (plant awn)
Entropion
Uveitis (see under Red Eyes)
Trauma (ulcer, uveitis)
Chemical irritation
Nonpainful eye
Nasolacrimal atresia
Nasolacrimal obstruction (acute blockage)
Thick (mucoid or mucopurulent)
Foreign body
Infectious keratoconjunctivitis (see causative agents under Red Eyes for Conjunctivitis/keratitis by genus)
Surface tumors
Dacryocystitis
Chronic nasolacrimal blockage
Foreign body
Sinusitis
Bacterial infections
Hemorrhage
Trauma
Foreign body
Ulcerative conjunctivitis
Tumor
Ocular or Periocular Asymmetry
Change in Globe Position
Change in Globe Size
Changes in Eyelid Conformation
Anisocoria
Changes in Tissue Volume
Ocular Color Change
Ocular Discharge
Ocular Pain
Blindness

Diseases of the Eye
Chapter 39