Chapter 26 Diagnostic Imaging of the Gastrointestinal Tract
Radiography
Kari L. Anderson and Daniel A. Feeney
Technical Considerations
There are several reviews on specific radiographic techniques published elsewhere.1–6 Several decisions need to be made before considering any imaging procedure, particularly those that are invasive or more costly. Specifically, what is the aim of the radiographic study? Is it to investigate organ geometry (i.e., size, shape, location, surface character, number or degree of organ involvement), organ opacity (i.e., gas, fluid, soft-tissue parenchyma, mineral, or metal), organ function (i.e., motility, breakdown, secretion, absorption, or excretion), organ leakage, organ obstruction, organ wall anatomy, organ wall thickness or pliability, etiology of dilation (segmental or diffuse), gas patterns, or effect of a diseased adjacent organ? Once the intent of the imaging procedure is established, it should then be determined whether survey radiographs alone are adequate to make the diagnosis or whether additional imaging procedures will be necessary. Horizontal beam or contrast procedures could be a necessary part of the additional medical workup. If a contrast procedure is indicated, the type of contrast medium should also be considered. Considerations for a contrast radiographic procedure should include the goal of the study (e.g., assessment of organ distention, motility, leakage, or foreign bodies), potential for body cavity or pulmonary contamination, options for contrast medium administration (i.e., oral, gastric, nasoesophageal, or nasogastric intubation), potential for air embolism from negative contrast medium, and the best choice for the contrast agent. Micropulverized barium sulfate suspensions are utilized in the assessment of motility and mucosal morphology, but can cause inflammation if the suspensions gain entry into the lungs or body cavities. Ionic iodinated oral preparations are associated with rapid propulsion, but are less useful for evaluating mucosal morphology, and side effects because of airway aspiration may be significant. Nonionic iodinated media are useful for the assessment of motility but not mucosal morphology, and fewer side effects have been reported if the media gains entry into lungs or body cavities. Another consideration is whether the positive contrast medium should be used as constituted or administered with food. In general, contrast agents should be administered without food for evaluation of mucosal morphology, detection of leakage, and timing of organ transit (e.g., stomach and small bowel) unless the goal is to assess the solid phase of gastrointestinal transit. Once these questions have been answered, other considerations related to the patient may be addressed.
Preprocedural Considerations
Patients with alimentary disease can be nauseous, weak, or dehydrated as well as having complications of alimentary tract disease, including aspiration pneumonia, sepsis, electrolyte and acid–base abnormalities, and abdominal distention. A balance between what is optimal for the patient and what is optimal for the imaging procedure should take these complications into account. Dyspnea and acute pain, for example, could be significant factors to consider in delaying the decision to perform an imaging procedure. Patient attitude and preparation are equally important patient parameters to take into account. Fractious patients may harm staff or themselves and must be adequately restrained or sedated. The authors prefer light phenothiazine sedation if sedation is needed.7,8 Generally, no specific preparation is necessary for survey radiographs. In fact, unnecessary preparations or patient alterations may interfere with image quality. For example, sedation may lead to slight accumulation of air in the esophagus that may be confused with megaesophagus. No specific preparation is required for an esophagram, but both the upper gastrointestinal (GI) series and the barium enema require cleansing enemas and/or cathartics, and, unless imaging is considered an emergency, food should be withheld for at least 18 to 24 hours.
Positioning and Views
Standard Views
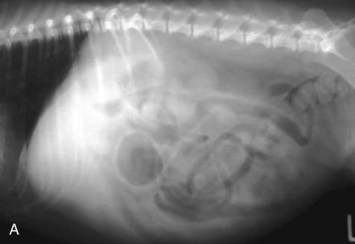
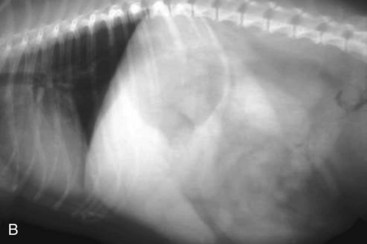
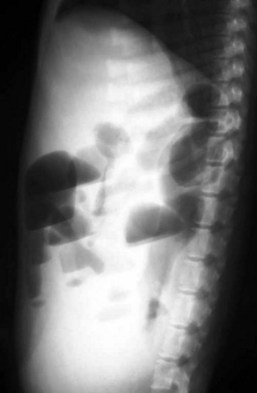
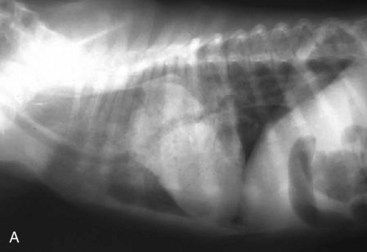
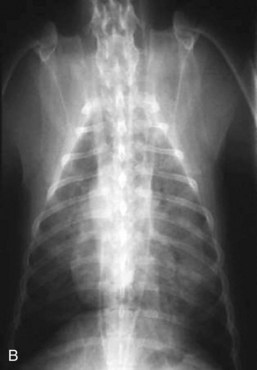
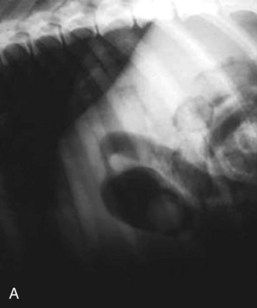
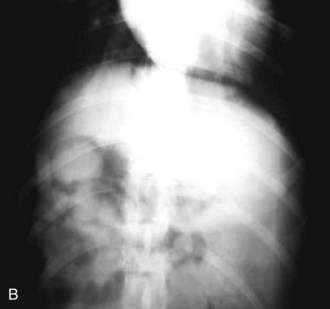
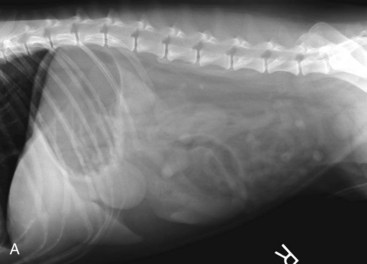
Standard, vertical–beam radiography (i.e., recumbent views taken with the patient on the x-ray table) with variable positioning can be used to characterize gas and fluid distribution within organs. The authors recommend right lateral and ventrodorsal (VD) recumbent views as a starting point for routine survey radiographs. the pylorus can appear like a ball-shaped foreign body in these views (Fig. 26-1).
Horizontal-beam Views
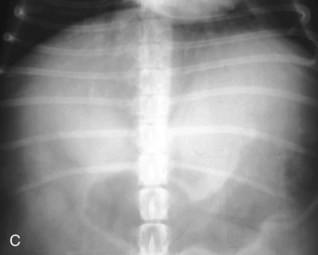
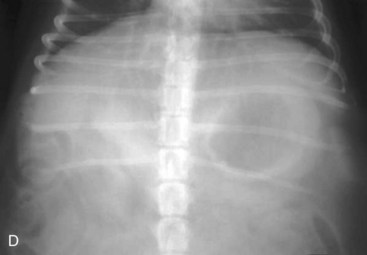
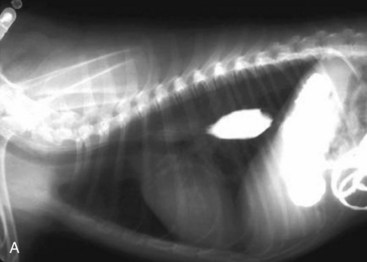
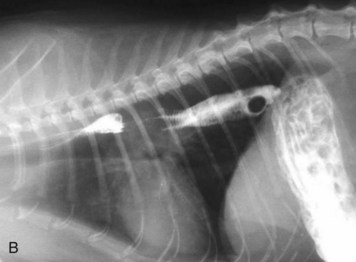
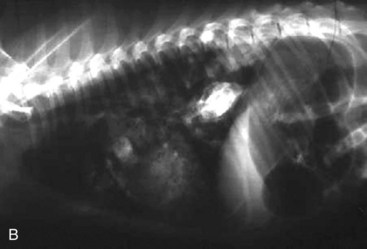
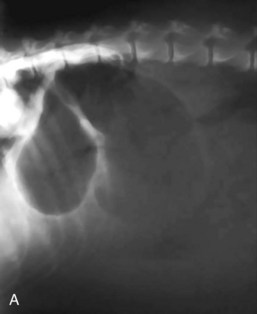
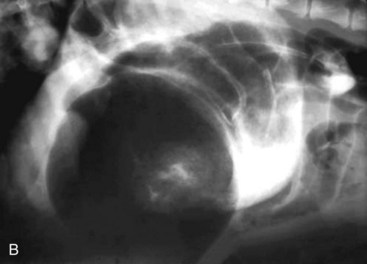
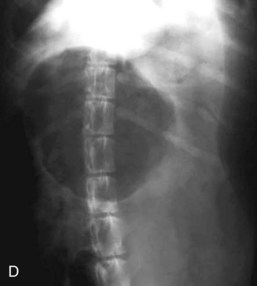
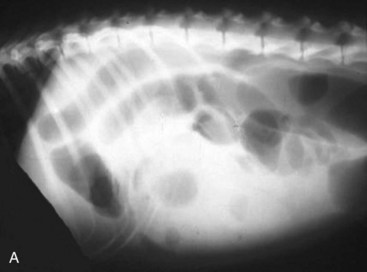
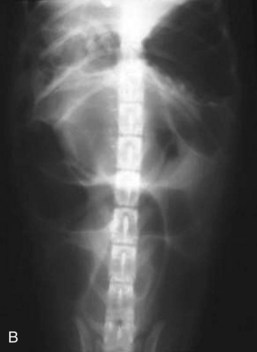
The objective of horizontal-beam radiography (i.e., the x-ray beam is directed parallel to the x-ray table) is to use the influence of gravity on gastrointestinal fluid distribution to clarify findings suspected on routine survey radiographs. The most common uses of horizontal-beam radiography are (a) to position the patient such that any freely movable (free) fluid can be shifted away from the area of interest; (b) to position the patient such that free fluid can be serially quantified; (c) to position the patient to permit differentiation of free and trapped fluid, which is often associated with inflammatory and neoplastic conditions; and (d) to position the patient such that air observed in the abdominal cavity is freely movable or trapped within normal viscera. A horizontal fluid line indicates the presence of both air and fluid in the abdominal cavity (Fig. 26-2).
Interpretation
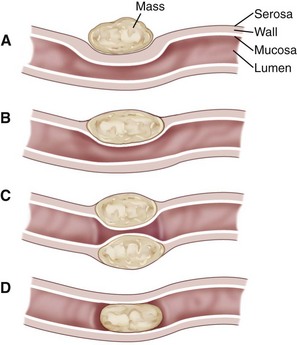
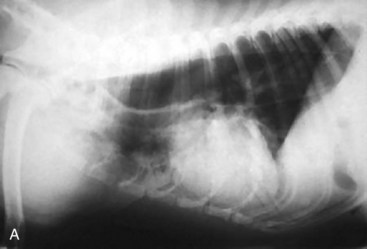
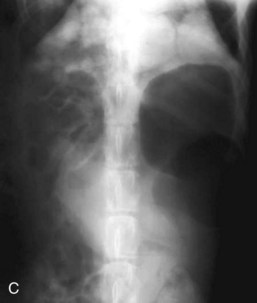
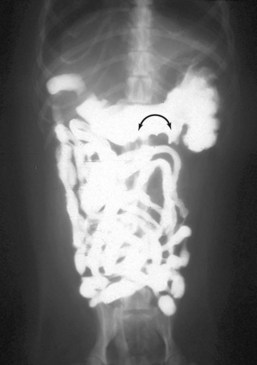
Alimentary disease should be classified as luminal, mural, extramural, or transmural (e.g., perforation [Fig. 26-3]). The intent of this classification is to define the general likelihood of various differential diagnoses. An understanding of basic principles of pathology makes this less complicated. For example, extramural lesions affect alimentary organ position and dimensions (e.g., compression of the colorectal junction by an enlarged prostate gland), but do not originate in the alimentary organ itself. Intraluminal lesions, on the other hand, are generally completely surrounded by the administered contrast medium. Finally, mural lesions, such as tumors or strictures, are generally only partially surrounded by the administered contrast medium. When considering mural lesions, wall thickness measurements play an important role. The normal wall thickness is 2 to 4 mm for the esophagus, 4 to 5 mm for the stomach (interrugal), and 3 to 4 mm for the small bowel and the colon.1,3–6 Any deviation from these measurements is usually a result of local or regional infiltrates causing wall thickening that can range from diffuse infiltrative bowel disease to focal/multifocal mural infiltrates such as tumors and strictures. Annular lesions are a unique subset of mural lesions and resemble either a napkin ring or an apple core because of their circumferential distribution and concentric luminal narrowing. Although possibly seen with strictures, this pattern is most commonly seen with intestinal neoplasia.
Considerations for Alimentary Tract Imaging
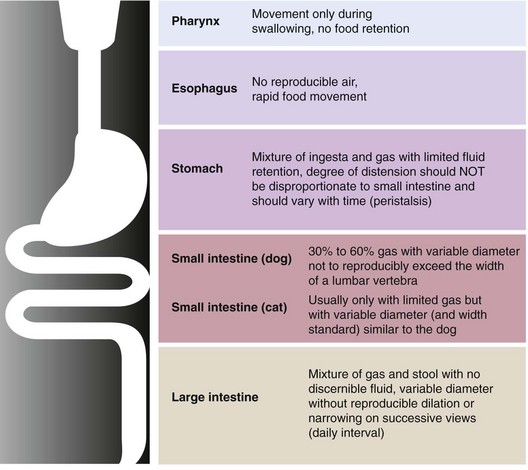
Of particular value is an assessment of the fluid and gas volumes along the gastrointestinal tract. First, any reproducible distention of the esophagus is abnormal. As a general rule, approximately 30% to 60% of the small bowel in dogs contains air, which is spread evenly throughout the bowel and relates in some reasonable proportion to the gas and fluid in the stomach. By comparison, most of the feline alimentary tract is devoid of gas, except in aged cats. This is a consequence of both anatomy and physiology (Fig. 26-4). The gastrointestinal tract is one continuous tubular lumen and there should be an orderly flow of ingesta from proximal to distal. There should be no disproportionate accumulation of fluid or solute or lack of any of these contents along the course of the GI tract. If such changes are identified on survey radiographs, this is an indication for further imaging investigation. The alimentary organs can be affected by regional and systemic influences as well as the local effects of the ingesta. The fluid/gas balance coupled with the organ size or diameter and distribution of a perceived abnormality can provide clinically useful insights into the pathophysiology of the disease. For example, a focal or multifocal distribution is associated with foreign bodies, tumors, strictures, and small incarcerations. In comparison, a diffuse distribution is associated with widespread intramural disease (e.g., infiltrative bowel disease), as well as widely differing luminal osmolarities related to bacterial proliferation, digestion, or absorption. A diffuse distribution can also result from disease involving other components of the digestive system, especially the liver (e.g., portal hypertension causing fluid buildup in the small bowel). Regional effects can be the result of intraluminal, intramural, or serosal irritation or inflammation (e.g., caused by peritonitis, pancreatitis, or a migrating foreign body).
Gas volume and its distribution can be key components in the interpretation of alimentary tract disorders. In omnivores, most alimentary gas derives from swallowed air. From low-grade impedance of gastrointestinal flow to complete obstruction, the likelihood of intraluminal buildup is air, fluid, digestible solids, and nondigestible solids (e.g., small bone fragments or small foreign objects). Unless an obstruction is nearly complete, only limited amounts of air will accumulate proximal to it because air can move past a partial obstruction more readily than fluid, food, or mobile fragments suspended in the partially digested chyme. The relationship of relative air versus fluid in a tubular organ segment and the influence of gravity are relevant variables to the assessment of gut wall thickness using survey radiography. As illustrated in Figure 26-5, the appearance of the wall of a hollow alimentary organ is based on the optical perception of the serosal and mucosal surfaces with the distance between them presumed to represent the wall thickness on routine vertical-beam radiographs. Overestimation occurs when the lumen is more than half full, which is common on alimentary survey radiography and must be factored into every attempt to assess wall thickness and may prompt a decision to perform additional imaging, including contrast or ultrasonographic procedures.
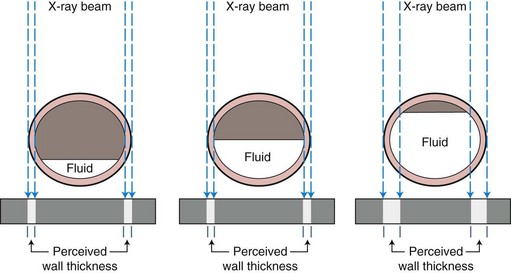
Figure 26-5 Perception of wall thickness.
Illustration of perception of bowel wall thickness caused by fluid content of the stomach.
There are some useful clinical clues to the underlying etiology of an alimentary tract disease.1,3–6 The buildup of a grainy opacity can indicate a chronic partial obstruction in either the stomach or the small intestine. A “thumbprinted” bowel, which describes a segment of the bowel that looks similar to a tubular piece of clay into which a thumb has been pressed, is suggestive of segmental or diffuse intramural infiltration because of neoplasia or pyogranulomatous infiltration. The thumb-printing effect comes from loss of wall pliability and variability in wall thickness. Another finding that can be observed is the so-called onion-skin or coil-spring appearance caused by contrast medium spreading between contiguous intestinal loops involved in an intussusception. The so-called washboard effect can be caused by regional mesenteric ischemia. The appearance of mural or wall layering may also be noted during radiography, which can be a result of infiltration of the wall with gas or minerals. Layering of luminal content may also be seen on horizontal-beam views, as different types of bowel content occupy different “layers” relative to gravity. Finally, the appearance of tubular plication, which appears as an eccentric accumulation of air, is caused by intestinal bunching occasioned by ingestion of linear foreign bodies (e.g., cloth, string).
The authors advocate two approaches to interpreting radiographs. The first is the gamut approach9 in which the radiographic signs are listed and the pathophysiologic or pathoanatomic causes are considered. The findings are then ranked in descending order of likelihood based on risk modifiers including clinical history, age, gender, breed, and species. Another helpful approach is the use of the so-called DAMNIT system, which adopts a pathogenesis approach to diagnosing radiographic findings. The disease processes considered in the DAMNIT system are degenerative, drug-induced, and developmental (D); anatomic, allergic, autoimmune, and anomalous (A); metabolic and mechanical (M); nutritional and neoplastic (N); infectious (bacterial, fungal, viral, parasitic), inflammatory, and immune-mediated (I); and, toxic, traumatic, and teratogenic (T). Judicious use of the DAMNIT approach limits the likelihood of a diagnosis being missed because it was not included in the gamut approach.
Specific Considerations for Different Organs of the Gastrointestinal Tract
Oral Cavity and Pharynx
Anatomic considerations
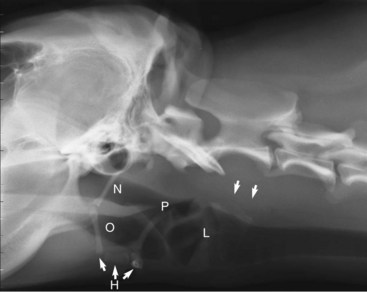
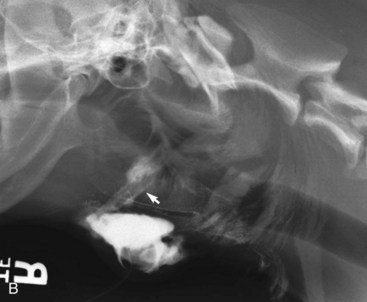
Coordinated prehension, mastication, bolus formation, and swallowing are all necessary to facilitate coordinated aborad propulsion of food and water into the esophagus and beyond. Any morphologic or functional abnormality can be detrimental to the process of ingestion (see Chapter 54). The anatomic structures important for ingestion include the tongue, teeth, supporting bones of the maxilla and mandible, oropharynx, hyoid apparatus, and larynx. The tongue is important for complex movements that allow for prehension, mastication, and swallowing. The teeth and the supporting bones of the maxilla and the mandible are needed for mastication, and the oropharynx, pharynx, and larynx are needed for propulsion, swallowing, and protecting the airway during swallowing, respectively. The hyoid apparatus acts as a suspensory mechanism for tongue and larynx during swallowing. Positioning is crucial for radiographs of the aforementioned structures to avoid erroneous interpretation. A lateral view provides the most information, but orthogonal views should always be obtained.10 The size and shape of the air-filled oropharynx varies with the position of the tongue, but the rectangular air-filled pharynx should maintain its size and shape independent of tongue position and phase of respiration (Fig. 26-6).
Abnormal Radiographic Signs
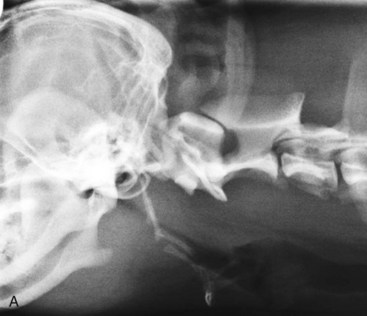
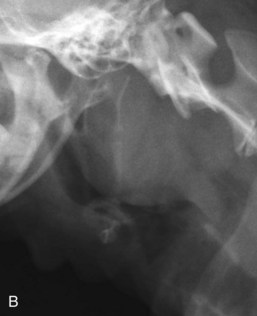
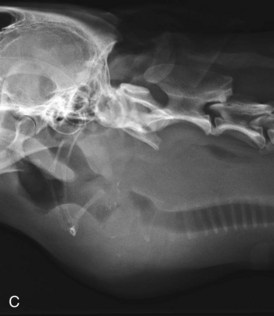
Close inspection of lateral and DV or VD views will elucidate some of the diseases of the pharynx, but most will require further investigation by visual inspection, special procedures (e.g., contrast studies), and alternate imaging studies (e.g., fluoroscopy, ultrasound, computed tomography [CT], or magnetic resonance imaging [MRI]). It is important to closely evaluate the surrounding soft tissues for air, which can be caused by penetrating foreign bodies, lacerations, or abscessation. Poor pharyngeal tone, pharyngeal collapse, mural infiltrates, edema, and mural or extrinsic masses may lead to an abnormal size and shape of the pharynx (Fig. 26-7). Extrinsic masses often lead to displacement of the pharynx, larynx, and/or the trachea and are also often associated with accompanying compressions of these structures (Fig. 26-7C). The hyoid apparatus may become displaced or may contain fractures in association with dysfunction (Fig. 26-7A and B).
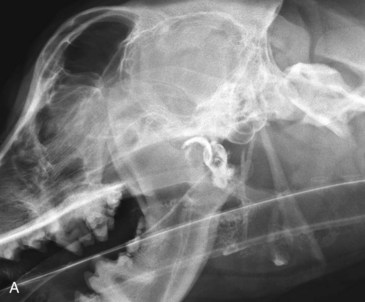
A contrast procedure utilizing a nonionic iodinated contrast agent can be helpful when evaluating the pharyngeal region for draining tracts. It is important to determine the extent of tissue involvement, especially as it is not uncommon for foreign bodies to penetrate the oropharynx or pharynx. Interpretation of the study involves determining the direction and extent of the tract, communication of the tract with the oropharynx, pharynx, esophagus, or other regional structures, and the cause of the draining tract (Fig. 26-8).
Dysfunction
Pharyngeal function is best observed fluoroscopically using liquid barium and barium-coated material (dynamic fluoroscopy).11 Sometimes an impression regarding pharyngeal function can be gained using static survey radiographs obtained during inspiration and expiration. Survey radiographic findings suggesting pharyngeal dysfunction include caudal migration of the hyoid apparatus during inspiration, hyperinflation of the pharynx during expiration, collapse of the pharynx during inspiration (see Fig. 26-7A), and persistent gaseous distention of the proximal esophagus. These findings should be further evaluated with the use of pharyngeal fluoroscopy. Diseases associated with pharyngeal dysfunction include myasthenia gravis and other muscular disorders; cricopharyngeal achalasia, which is characterized by cricopharyngeal hypertension and inability of the sphincter to relax; and cricopharyngeal dyssynchrony, which is characterized by an incoordination of pharyngeal contraction and cranial esophageal sphincter relaxation.11,12
Pharyngeal Diseases
The location and shape of the larynx may be governed by similar and additional factors. As with pharyngeal disease, downstream resistance may be a result of laryngeal paralysis, laryngeal neoplasia, laryngeal pyogranulomatous inflammation, or various causes of tracheal obstruction, including foreign bodies. Extralaryngeal lesions, such as retropharyngeal lymphadenopathy, neoplasia, abscess, granuloma, foreign body, or failure of the supporting structures such as hyoid luxation or hyoid fracture can all lead to radiographic changes of the larynx. An additional important consideration is that of regional opacity. Radiographic signs noted may include extrapharyngeal or laryngeal radiolucency caused by regional emphysema from pharyngeal or laryngeal perforation. Regional mineralization due to neoplasia, chronic hematoma, abscess, pyogranulomatous inflammation, or foreign body may also be observed. Penetrating pharyngeal foreign bodies can be potentially serious events in the dog and cat.13 Metal foreign bodies are usually easily identified, but less-dense objects may be easily missed. The most frequent radiographic abnormality identified in patients with a pharyngeal foreign body is subcutaneous gas accumulation or gas in fascial planes or gas accumulating subcutaneously.13 In some cases, a foreign body may be misinterpreted as a soft-tissue mass caused by regional inflammation or edema.10
Esophagus
Anatomic Considerations
The canine and feline esophagus is usually not visible on survey radiographs. There are occasional exceptions to this rule, including the increased likelihood of gastroesophageal reflux in left lateral recumbency and stress-induced or spontaneous reflux. These instances usually only involve the distal half of the esophagus and are transient. When visible, particularly when coated with barium, the normal esophagus exhibits bolus propulsion with interbolus linear stranding of the collapsed portion of the esophagus (Fig. 26-9A). There are some important species differences. The distal one-half to two-thirds of the feline esophagus normally has a “herringbone” appearance in the mucosal surface due to the presence of smooth muscle (Fig. 26-9B).
Abnormal Radiographic Signs
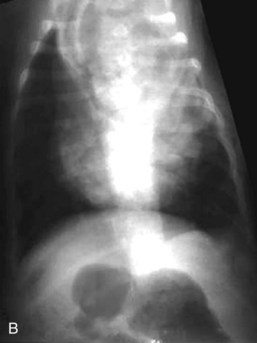
The normal esophagus is not visible on survey radiographs and needs to be rendered visible by contrast agents. The esophagus originates dorsal to the larynx and the proximal cervical trachea, courses gradually to the left of the trachea at the thoracic inlet to again become dorsal to the trachea in the cranial mediastinum, and track caudally dorsal to the heart to the esophageal hiatus of the diaphragm (Fig. 26-10). When rendered visible by positive contrast media, the location, morphology, and distribution of esophageal dysfunction can be identified (Figs. 26-11 and 26-12).6,14 In addition, when surface-coating agents such as barium are being used, the mucosal surface can be assessed. In normal patients the esophageal mucosal surface should be smooth and regular. The most common cause of ventrally displaced trachea is esophageal dilation.
Esophageal Diseases
As with other parts of the digestive system, a systematic approach should be used to arrive at a list of differential diagnoses based on radiographic findings. The peristaltic capacity of the esophagus can be altered by idiopathic megaesophagus, dysautonomia, esophagitis, overt electrolyte imbalance, heavy metal toxicity, congenital or acquired diverticula, and thyroid status (see Chapter 55). Downstream resistance can be the result of a stricture, tumor, anomalous constrictions such as vascular ring anomalies, or any intraluminal obstruction, such as a foreign body. Wall thickness and pliability can also be altered because of fibrosis, inflammatory reactions, or neoplastic infiltrates. The upstream delivery of ingested materials is governed by a primary esophageal peristaltic wave that takes less than 10 seconds to move from the pharynx to the stomach. Luminal distention triggers a secondary peristaltic wave that originates at the site of the distention and proceeds toward the stomach in less than 5 to 10 seconds. Esophageal abnormalities should be further classified as intraluminal, intramural, transmural, or extramural. Examples of intraluminal abnormalities are foreign material or retained ingesta because of ineffective peristalsis or increased downstream resistance. Intramural abnormalities can be a consequence of strictures or tumors. Transmural abnormalities can be caused by penetrating objects or by bronchoesophageal fistulae. Extramural abnormalities can be caused by compression, distortion, or displacement by a regional organ or mass. Finally, the esophageal mucosa should be evaluated. Mucosal ulceration can be caused by erosion from gastric or gastrointestinal reflux, trauma from lodged or migrating foreign bodies, tumors that disrupt the mucosal surface, or the ingestion of corrosive chemicals such as sodium hydroxide. Esophageal leakage because of traumatic or ulcerative lesions can lead to septic mediastinitis and should be carefully considered. In the authors’ experience, pneumomediastinum is a rare finding with esophageal penetrating lesions. The material that leaks into the mediastinum is usually of insufficient volume to noticeably distend the mediastinum. This makes the positive contrast esophagram all the more important in the assessment of a suspected esophageal perforation. This is usually initially performed with an iodine-based contrast agent, as these agents are isotonic and do not cause irritation if there is a tract involving the airway or lung. If the initial study is negative, it is followed by a barium study, which is more useful for detecting and localizing small leaks. Any barium contamination of the mediastinum should be surgically irrigated as soon as is practicable, but definitely in less than 12 hours.
Stomach
Anatomic Considerations
The canine and feline stomach usually contains modest amounts of fluid, but generally not enough to cause the appearance of distention on any radiographic view or recumbency. If the stomach begins to appear rounded rather than elongated in the transverse plane in a fasted animal, it should probably be considered to be abnormal. The cardiac region is more or less anchored behind the left diaphragmatic crus as a result of the distal esophagus passing through the esophageal hiatus. The stomach position is influenced by the liver, and to a lesser degree by the spleen, regional masses, the morphologic and functional integrity of the diaphragm, as well as the degree of luminal distention. In general, the long axis (i.e., fundus to pylorus) of the stomach is parallel to the last few ribs in lateral recumbency and perpendicular to the spine in sternal or dorsal recumbency. The cardiofundic region of the stomach is in the left craniodorsal abdomen behind the left diaphragmatic crus and the gastroesophageal junction is located on the right medial aspect of the cardia. The pylorus is in the right cranioventral abdomen behind the liver near the right lateral abdominal wall in the dog, and just to the right of the midline in the cat. Cats usually have little gastric gas, whereas dogs often have equal amounts of fluid and gas in the stomach. The position of the fluid and gas as a function of gravity and recumbency can influence the appearance of the stomach, giving the illusion of a thickened wall (see Fig. 26-5) or a ball-shaped foreign body, which can be a result of fluid accumulating in the pylorus in right lateral recumbency. Depending on whether the patient is fed or unfed, the stomach may contain varying amounts of food. Unfortunately, many ingested foreign materials cannot be distinguished from food except by recheck radiographs after 18 to 24 hours. Digestible solid food will exit the stomach by 14 to 18 hours after eating, unless there is an outflow problem (see Chapter 26). In contrast, nondigestible foreign material will persist for many hours. Gaseous distention of the stomach has many causes including aerophagia and atony, which can be pathologic, but can also be caused by sedative or anesthetic drug effects. By comparison, gastric distention caused by an outflow obstruction is usually associated with a mixture of fluid and gas and is often accompanied by the retention of grainy material. This tends to accumulate proximal to the outlet obstruction and may gravitate within the lumen depending on patient recumbency. These opacities may be an indication for a foreign body or they may simply indicate the buildup of materials normally dispersed in food (e.g., bone fragments).
Functional Considerations
The stomach has many functions, including storage, digestion, propulsion of solids and liquids, and secretion of gastric acid, pepsins, and mucus (see Chapters 1 and 56). A disease process involving any one of these functions can result in gastrointestinal signs ranging from anorexia to vomiting and can affect the size of the stomach as well as the makeup of the luminal contents. Because of pyloric resistance, fluid may remain in the stomach for up to 2 to 3 hours after ingestion, whereas solid food requiring greater breakdown can remain in the stomach for up to 18 hours. From a practical standpoint, the least invasive and most cost-effective radiographic procedure to determine whether gastric outflow is decreased is to repeat survey radiographs the following day or to obtain an alternate radiographic view. However retention of material in the stomach can be secondary because of reflex inhibition of gastric emptying by various intestinal (see Chapter 1) and/or peritoneal conditions. However retention can also be pathologic as a result of pyloric stenosis, foreign body, or tumor. Considerations of the various causes of gastric retention may help to facilitate the decision to use endoscopy, upper GI, laparoscopy, or exploratory laparotomy to further investigate the problem. To further characterize stomach wall conditions, including those of the pyloric sphincter, contrast gastric radiographic studies should be considered. The most practical and useful is a barium-based upper GI study.
Abnormal Radiographic Signs
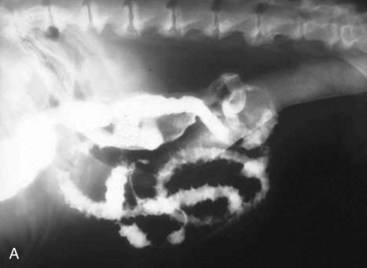
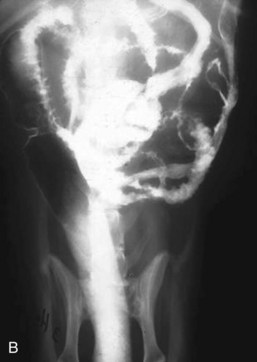
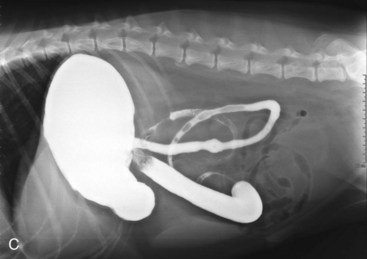
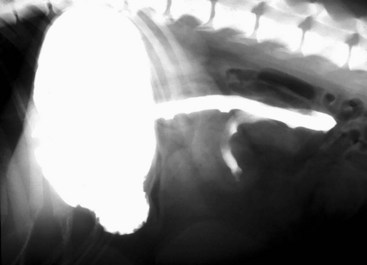
The normal and abnormal stomach are both visible on survey radiographs. Consequently, it is important to differentiate transient physiologic conditions, such as postprandial gastric distention from disorders that may cause alterations of gastric size, shape, and position. The localization of gas and fluid in the stomach depend on the position of the patient during radiography. Before arriving at a definitive diagnosis based on one radiographic view, additional views may provide helpful clues and limit the likelihood of premature or inaccurate diagnosis. Identification of discrete cardiofundic, gastroesophageal, and pyloric regions is clinically important and is usually accomplished by shifting the gas–fluid interface with changes in patient positioning (Fig. 26-13). Once an assessment of physiologic versus pathologic distention has been made, an assessment of gastric contents and presence or absence of segmental mural variations can be carried out. Other important parameters such as mucosal integrity, which may be altered because of ulceration, luminal status that may indicate the presence of a foreign body (Fig. 26-14), and wall thickness, which may be altered as a consequence of hypertrophy or tumor (Fig. 26-15), are best assessed by use of contrast studies.15–19 Similarly, the position of the gastroesophageal junction, and integrity of the gastric wall can be best assessed by contrast studies. Finally, the initiation and continuity of the peristaltic wave (normally there are two to five peristaltic waves per minute) are best assessed by gastric contrast studies. The choice between barium and an iodinated contrast medium should be made based on the goals of the study, such as mucosal coating, assessment of peristaltic capacity and pyloric function, or the identification of a leak.
Small Intestine
Anatomic Considerations
The amount of gas normally contained in the small intestine differs between dogs and cats. In dogs, typically 30% to 60% of the small bowel volume is gas unless the space is occupied by ingesta. In contrast, the normal adult cat usually has almost no gas in the small bowel unless ingesta are moving through it. In aged cats, there may be some stress-induced excess small intestinal gas, which must be taken into account during interpretation. Excessive, but evenly distributed, gas in the small bowel has been reported in association with mesenteric volvulus. The small intestine is usually evenly distributed along the ventral abdominal wall caudal to the stomach and liver and cranial to the urinary bladder. On VD views of obese cats, the left perirenal fat may give the illusion of right displacement of the small intestine. Both species have some degree of diffuse mucosal surface irregularity as can be identified using positive contrast materials. These irregularities in normal patients are a result of the normal mucosal villi.20,21 In both species small intestinal loops should appear homogeneous throughout. That said, active peristalsis will influence the perceived external diameter of small bowel loops and some variation of bowel loop diameter may be observed. The best approach to assess effects of peristalsis is to repeat survey radiographic views. This assures that patterns are not reproducible and that changes of loop content do occur.
Abnormal Radiographic Signs
In most patients, the normal or abnormal small bowel is readily visible on survey radiographs. The key to interpretation of survey radiographs is to be sure to differentiate transient physiologic changes such as postprandial luminal distention from pathologic changes in small bowel size, shape, and position. The localization of gas and fluid in the small bowel depends on the position of the patient during radiography. Several survey radiographic views may help to differentiate pathologic from physiologic findings. As discussed previously, the type and distribution of luminal contents may provide clues to the duration and severity of a disease process. In general, large-diameter intestinal loops are associated with greater duration, and high gas-to-fluid ratios are associated with greater severity. It should be kept in mind that bowel dilation has four specific causes: obstruction, inflammation, trauma or irritation, and ischemia. Large quantities of gas involving many or all small bowel segments may be a result of ischemia or severe serosal inflammation (Fig. 26-16), and not necessarily distal small bowel obstruction. There is also a continuum of bowel luminal compromise ranging from partial to complete obstruction. The recognition of these differences is paramount to the diagnostic process. Partial obstructions are usually segmental (i.e., two distinct populations of bowel can be recognized) and are characterized by greater fluid accumulation (than gas) whereas a complete or high-grade obstruction is characterized by equal amounts of gas and fluid or a clear predominance of gas. Partial obstructions can be due to mural lesions, such as tumors, strictures, or other intraluminal abnormalities like foreign bodies. The relative likelihood of these vary with age, behavior, and medical history (Figs. 26-17 and 26-18). As a general rule, conditions associated with large gas accumulations in the small intestine tend to be surgical diseases. Those conditions with proportionally more fluid than gas are more of a diagnostic and prognostic dilemma.22–25 Assessment of small bowel mucosal changes (e.g., ulcerations), luminal abnormalities (e.g., foreign bodies), wall thickness, peristaltic capacity and continuity, as well as wall integrity is best achieved by positive contrast studies. However the choice of barium versus an iodinated contrast medium should be made based on the goal of the study, including mucosal coating, assessment of peristaltic capacity, or the identification of a leak. The role of abdominal ultrasonography in relation to contrast radiography has not been systematically studied and needs to be determined in large prospective studies. Concerns persist regarding the predictability of the sonographic diagnosis in the hands of inexperienced individuals and problems associated with user-dependent techniques such as ultrasound.23–25
Small Bowel Diseases
Small intestinal mucosal ulceration is uncommon, except when associated with neoplasia. Smoothly marginated rectangular outpouchings of the duodenal lumen are occasionally seen in normal dogs, and have been termed pseudoulcers (Fig. 26-19). Although identification of small bowel surface erosion is possible by radiographic and ultrasonographic techniques, these lesions are best assessed by endoscopic techniques bearing in mind that only the descending duodenum and the ileum are accessible using this technique. Moreover, visual assessment of the mucosal brush-border to diagnose small intestinal disease or to differentiate between different disease processes has not been shown to be clinically useful.21 As with gastric pathology, the unexplained presence of free peritoneal air is usually associated with alimentary tract perforation. If there is any question about the presence or absence of free air, horizontal-beam radiography is a very useful technique (Fig. 26-20). The assessment of free peritoneal air as discussed for gastric disease is equally applicable for diseases of the small bowel.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree