32 Developmental orthopedic disease
Introduction
In general, DOD incorporates the following spectrum of conditions (adapted from McIlwraith 2001, Jeffcott 2005):
• Acquired angular limb deformities
• Tarsal bone collapse (synonym: cuboidal bone malformation)
• Cervical vertebral malformation
Of these different conditions, osteochondrosis has been the main focus of scientific investigations in horses. The incidence of osteochondrosis in the growing foal can be high, with reported values, depending on the breed and study parameters, of between 10–50% (Stock et al 2005, van Weeren 2006, Wittwer et al 2006). Most breeds of horses are susceptible although ponies and feral horses seem to be much less commonly affected (Jeffcott 2005, Marshall 2007, Lepeule et al 2009). Nonetheless, a recent report documented osteochondrosis of the lateral trochlear ridge in four ponies in which the lesions were similar to those observed at the same site in horses (Voute et al 2011). The heritability of osteochondrosis has been reported to be in the range of (h2 =) 0.1 to 0.34 for Warmblood horses and between (h2 =) 0.17 and 0.52 for Standardbred horses (Komm 2010).
All of the conditions included under the DOD umbrella are likely to have a complex multifactorial etiopathogenesis (McIlwraith 2001); however, a number of key factors are commonly thought to be involved, including:
• Biomechanical stress or trauma on joint and growth cartilage
• Hormonal factors (e.g. hyperinsulinemia)
This multifactorial etiology explains why clear-cut studies showing the induction or prevention of DOD cannot be achieved with small sample populations. Genetic factors seem to be very important in the pathogenesis of osteochondrosis and the identification of so-called quantitative trait loci (QTL) in Hanoverian Warmblood and South German Coldblood horses has emphasized this (Komm 2010).
There are key predilection sites for each of the DOD conditions (Table 32-1). Clinical signs that can be associated with DOD include enlarged growth plates, joint heat, prolonged recumbency, stiffness, lameness, and reduced activity linked to lack of movement/playing (Jeffcott 2005, van Weeren 2006). Clinical signs typically become apparent between birth (although pathological changes may be present prenatally) and 24 months of age (Table 32-1). It is worth noting that whereas the clinical signs associated with angular limb deformities symptoms are external and obvious, for the other manifestations of DOD such as osteochondrosis, no obvious clinical signs may be present.
Table 32-1 Approximate Time of Onset of Key Clinical Signs and the Main Anatomical Sites Involved for Each Condition Within the DOD Complex
| Clinical condition | Typical time of onset of signs (months) | Main anatomical sites involved |
|---|---|---|
| Physitis | 4–12, >24 | Distal limbs and stifles joints |
| Osteochondrosis | 3–20 | Proximal and distal limbs and cervical spine |
| Flexural deformities | 1–4 | Distal forelimbs |
| Tarsal bone collapse | 1–18 | Tarsal joints |
| Cervical vertebral malformation | 6–24 | Cervical spine |
| Vertebral abnormalities | 6–9 | Thoracolumbar spine |
Data from Jeffcott 2005.
Etiology and pathology of DOD
A brief summary of the key conditions within the DOD cluster, and their suggested main etiological factors, is given below (for further information see Stashak 2001, Auer and Stick 2006).
Physitis (synonyms: epiphysitis, physiolysis)
Physitis is an abnormality of endochondral ossification in the metaphyseal growth plate (physis), where the structural integrity of the metaphyseal bone is weakened by the disturbance in maturation of the cartilage cells (Jeffcott 2005). Radiographically, physitis may manifest as an irregularity in the growth plate with sclerosis of adjacent bone and an altered metaphyseal contour (Williams et al 1982). Metaphyseal broadening or flaring of the ends of the long bones is a typical feature in the affected growing foal. The enlargement usually presents as a firm swelling and is caused by the formation of new bone. These processes are self-limiting and disappear after closure of the growth plates. Physitis is probably one of the most frequently seen conditions within the DOD complex in Thoroughbred foals (Gee et al 2005). Although the exact etiology of physitis is unknown, high energy intakes which promote rapid growth, traumatic injuries and a genetic predisposition are suggested to be involved (Thompson et al 1988, Gee et al 2005).
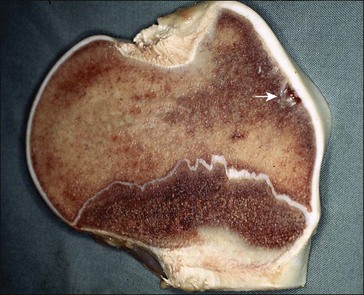
Osteochondrosis (OC, synonyms: dyschondroplasia, OCD: osteochondrosis dissecans)
OC is a common disorder of growth cartilage in domestic animals and humans (Komm 2010). In animals, the disorder has been described in pigs, dogs, horses, cattle, cats and rats (Ytrehus et al 2007). It is regarded as the most important cause of leg weakness in swine and it is a frequent cause of lameness in young athletic horses (Ytrehus et al 2007).
OC has been described as a disturbance in the process of endochondral differentiation, proliferation, maturation and eventual ossification in fast-growing animal species and humans (Ytrehus et al 2007). The first stage of OC development may be either an impairment of cellular differentiation in the growing cartilage or a failure of maturation and ossification by differentiated chondrocytes low down in the maturation zone (Ytrehus et al 2007, see Fig. 32.1). Either pathway can result in focal lesions that involve areas of cartilage along or just below the otherwise healthy articular cartilage (Fig. 32.2).
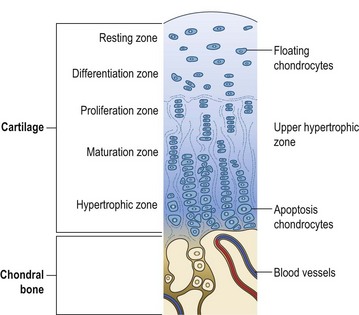
Figure 32.1 Zones of development from articular cartilage to subchondral bone along the growth plates.
Source: A.D. Ellis.
The second stage includes necrosis of the basal layers of the hypertropic cartilage with subsequent pressure and strain within the joint, giving rise to fissures in the damaged cartilage (Rejnö & Strömberg 1978, Jeffcott & Henson 1998). A third stage, which does not always occur, may be the flaking away or fully breaking loose of damaged cartilage fragments – described on X-rays as “joint mice” – and termed osteochondrosis dissecans (OCD – often wrongfully used synonymously with OC).
Osteochondrotic lesions have been reported in many equine joints, but they are most commonly found in the tibiotarsal and femoropatellar joints, and in the dorsal aspect of the distal metacarpus and metatarsus (McIlwraith 2001, van Weeren 2006). In horses, the period during which a lesion develops and possibly regresses is limited to the first months of life. New osteochondrotic lesions rarely develop after the fifth month of life, and lesions that developed in those first five months may be repaired in the following six months (van Weeren & Barneveld 1999, van Weeren 2006).
Several potential etiological factors have been suggested for equine osteochondrosis including rapid growth, nutritional imbalances (overfeeding, mineral imbalances and toxicity), endocrine factors, biomechanical stress, trauma or lack of exercise, failure of vascularization and a genetic predisposition (Jeffcott & Henson 1998, Ytrehus et al 2007). One hypothesis for example suggests that cessation of blood supply of the cartilage canal is the primary event in OC (Ytrehus et al 2007). However, recently there has been more focus on the importance of genetic factors (Komm 2010). Candidate genes that may be responsible for osteochondrosis in horses have been identified (e.g., Lampe et al 2009, Wittwer et al 2009).
Results from a study by Bruin and Creemers (1994) are interesting in that they highlight the complex multi-factorial etiology of OC lesions and their possible repair (Fig. 32.3): Dutch Warmblood foals were kept in stables (from 3 months of age) and either exercised on a 20 m diameter horse exerciser (high exercise) or let into a sand paddock (low exercise) for short daily periods. Beside the differences in the level of exercise, foals were also fed one of two feeding levels (high feed vs low feed). In this study the highest incidence of OC was associated with either a high feeding level with a low exercise intensity or a high intensity of exercise in combination with a low feeding level.
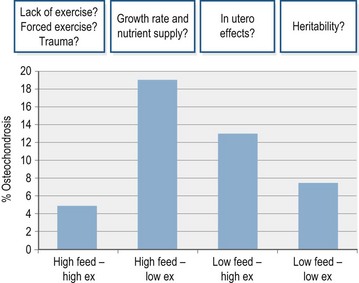
Figure 32.3 Proportion of OC occurrence (radiographic diagnosis) in Dutch Warmblood foals kept according to 4 management systems: high feed, high exercise (ex), low feed and low ex groups (n = 8 per treatment, adapted from Bruin and Creemers 1994) The gray horizontal overlay highlights factors which may have influenced results due to pre-experimental and experimental influences in such a small sample group.
There is often a discrepancy between the occurrence of osteochondrotic lesions produced under standardized conditions with a very small number of horses and those osteochondrotic lesions reported under field conditions. For example, osteochondrotic lesions in the growth plates, which were apparently produced through an excessive carbohydrate intake (Glade & Belling 1986), are generally very rare in clinical cases (van Weeren 2006).
Angular limb deformities (synonym: bent legs)
Angular limb deformities represent a deviation of the limb in the sagittal plane. The angulation can arise from any of the physes or the cuboidal bones of the carpus and tarsus. The most common physes involved are those of the distal radius, tibia, metacarpus and metatarsus. The most common deformity is the carpal valgus followed by the fetlock varus, which describes a lateral or medial deviation away from the midline of the carpus or cannon bone respectively. The etiology of angular limb deformities is again complex and thought to be multifactorial. Angular limb deformities are thought to be associated with growth imbalances induced by one of the following defects: joint laxity, irregularities of endochondral ossification of the bones in the carpus or tarsus, excessive exercise, poor confirmation, asynchronous longitudinal growth rate and trauma (Jeffcott 2005). The impact of nutrition has not been investigated.
Flexural deformities (synonyms: contracted tendons, ballerina syndrome)
Flexural deformities are expressed either as a hyperflexion or a hyperextension of a joint region. Flexural deformities are observed in the distal interphalangeal joint, the metacarpophalangeal or metatarsophalangeal joint, the carpal region and rarely the tarsal region (Auer 2006). The forelimbs are more frequently affected. Flexural deformities may be present at birth (congenital deformities) or acquired later in life (acquired deformities). Congenital deformities are thought to be the result of external environmental factors affecting the mare during gestation period (e.g., influenza outbreak, equine goiter, defects in cross-linking of elastin and collagen caused by lathyrism, etc) or other pathological causes such as intrauterine malpositioning (Auer 2006, Charman & Vasey 2008). However, the etiology in many cases remains speculative.
Acquired flexural deformities can be seen in rapidly growing foals, and in association with other painful conditions (Auer 2006). Affected foals are usually on an above-average level of nutrition (Lloyd-Bauer & Fretz 1989). However, the authors did not specify the energy intake. In addition, it has been postulated that an abrupt change from a previously inadequate energy intake to an excessive energy intake may trigger this problem (Auer 2006).
Tarsal bone collapse (synonyms: incomplete ossification of cuboidal bones, cuboidal disease, tarsal bone necrosis)
This condition is associated with collapse of the dorsal aspect of the central and third tarsal bones which causes a flexion deformity of the tarsus (Jeffcott 2005).
Cervical vertebral malformation (synonyms: Wobbler disease, equine spinal ataxia)
Cervical vertebral malformation is characterized by malformation or compression of the spinal cord which leads to spasticity, ataxia and incoordination. The two most common syndromes are cervical vertebral instability and cervical static stenosis (Auer & Stick 2006). Cervical vertebral instability is centered in the cranial vertebrae of the neck of young horses. It therefore causes dynamic spinal cord compression and typically affects horses between 4 and 12 months of age (Pool 1993). Cervical static stenosis is located caudally in the neck of horses and is characterized by a closing of the cervical canal causing compression on the spinal cord. Typically, horses start to show the first clinical signs between one and four years of age (Pool 1993). The etiopathogenesis is unknown; however, nutritional imbalances, rapid growth, exercise, gender (males > females), trauma and a genetic predisposition have all been suggested to be factors that could be involved (Auer & Stick 2006). At the present time, none of these possible causes have been confirmed or disproved. It has been demonstrated that dietary restriction and resting young horses with early signs of cervical vertebral malformation may help prevent more severe cervical vertebral abnormalities developing (Mayhew et al 1978, cited by Jeffcott 2005). In addition, proper nutritional balance in the pregnant and lactating mare has been suggested to decrease the occurrence of this disease in foals; and also is a major factor in the treatment of confirmed cases in young, growing horses (Mayhew et al 1978).
Acquired vertebral deformities (synonyms: lordosis, kyphosis)
These conditions describe varying degrees of lordosis and kyphosis, where the vertebral column deviates abnormally in a ventral or dorsal direction respectively. The most common abnormality is kyphosis in the rapidly growing foal which usually occurs post-weaning from 6 to 9 months of age (Jeffcott 2005). Lordosis is usually obvious at birth and the condition can deteriorate as the foal grows. These defects are thought to be the result of fetal malpositioning (Auer & Stick 2006).
Impact of nutrition on DOD
The potential impact of nutrition on DOD, particularly in the area of osteochondrosis research, has attracted much attention and discussion (e.g., Glade & Belling 1986, Ellis 2001, Gee et al 2007). Several epidemiological studies have shown that mares and foals under typical feeding conditions are often fed diets that do not meet the recommended nutritional minimum intake levels (e.g., Winkelsett et al 2005, Lepeule et al 2009).
Quantity and quality of energy intake
Rapid growth has been suggested to be a major factor in the development of certain types of DOD (Sandgren et al 1993, Jelan et al 1996, van Weeren & Barnefeld 1999). While final skeletal height is a genetically determined factor, the time period over which an individual achieves its predetermined adult height can be influenced by nutrition. For example, weight and girth gains for ad libitum-fed weanlings and yearlings were higher than those foals fed a restricted diet (Ott & Asquith 1986, Cymbaluk et al 1990). Dietary energy intake and its impact on the incidence of OC have been in the focus of interest for several decades. A high energy intake (150% of NRC level for digestible energy) increased body weight gains and growth rates in weanlings (Thompson et al 1988). It was speculated that skeletal development was impaired by the high growth rate, especially in those cases in which the protein intake was also low (Thompson et al 1988).
Oversupply of energy in the growing foal creates a mismatch between the rates of body weight increase, skeletal growth and micronutrient supply during particular periods of growth as reviewed by Ellis (2001) and Harris et al (2005). However, excess energy and rapid growth should not be considered as being synonymous. Rapid growth may occur due to a genetic predisposition and excess energy intake may not always lead to rapid growth. It is likely that OC develops only when several predisposing factors coexist. This probably accounts for some conflicting reports on the importance of overfeeding and growth (Ellis 2001).
A study by van Tilburg and Ellis (2002) analyzed growth data and radiographic OC scores recorded systematically at a research station between 1994 and 1999. These authors found no correlation between growth rates, withers height and OC scores in 144 Dutch Warmblood foals (30% OC positive) over the period from birth to 12 months. However, OC positive foals showed a higher average daily gain (possible growth spurt?) in the 3 months post weaning (4–6 months of age). Recent large scale epidemiological studies confirm that under typical feeding conditions, body weights and withers heights are not significantly different between OC affected or OC unaffected Warmblood foals (Vervuert et al 2002) or Thoroughbred foals (Jelan et al 1996). The intake of high energy levels (~130% of NRC level vs. 70% of NRC level for digestible energy) was apparently associated with disturbances in growth plate cartilage metabolism (e.g., decreased cartilage hexosamine and hydroxyproline contents) in Thoroughbred weanlings (Glade & Belling 1984, 1986) and widespread lesions, referred to as dyschondroplasia, were reported in growing, exercise restricted, foals (Savage et al 1993a). However, the high energy intake was not associated with an increase in average daily body weight gain (Savage et al 1993a) nor an increase in the daily increase in withers height (Glade & Belling 1986).
Nutritional influence on hormonal regulation of bone growth
Several studies provide evidence that hormonal factors, influenced by the nature of the energy intake (i.e. energy supply and source), may be involved in the etiopathogenesis of DOD (Ralston 1996, Henson et al 1997, Jeffcott & Henson 1998, Treiber et al 2005, Staniar et al 2007). The key hormones involved include insulin, insulin-like growth factors (IGF I and IGF II) and the thyroid hormones (Fig. 32.4).
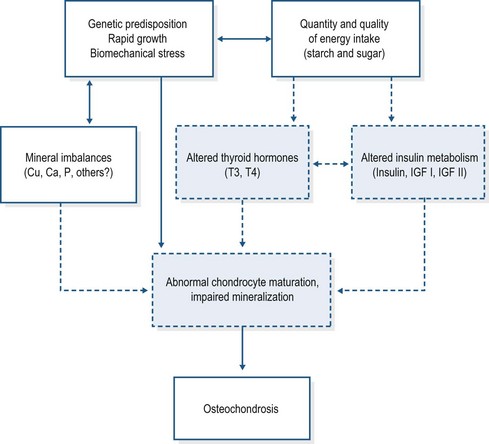
Figure 32.4 Etiological factors involved in equine osteochondrosis.
Adapted from Jeffcott & Henson 1998.
Insulin, IGF I and IGF II affect endochondral ossification under in vitro conditions in fetal and foal chondrocytes (Henson et al 1997), whereas the in vivo results remain inconclusive (Sloet van Oldruitenborgh-Oosterbaan et al 1999, Verwilghen et al 2009).
IGF-1 is an important factor for the stimulation of cartilage matrix synthesis by the chondrocytes (Henson et al 1997) and OC lesions in foals have been associated with lower plasma IGF 1 concentrations in comparison with unaffected foals (Sloet van Oldruitenborgh-Oosterbaan et al 1999). In contrast however, Verwilghen et al (2009) found higher plasma IGF 1 concentrations in horses (>15 months) affected with DOD lesions than in healthy horses. They considered this to reflect a repair mechanism within the affected cartilage. In conclusion, more research is needed to elucidate the role of IGF-1, especially the local effects of IGF-1 on bone and cartilage.
Insulin, in vitro, acts as a “survival factor” preventing chondrocyte apoptosis (Henson et al 1997). Diets containing high amounts of easily digestible carbohydrates induced higher postprandial glycemic and insulinemic responses in weanlings affected by OC than in healthy foals (Ralston 1996, Henson et al 1997, Pagan et al 2001, Williams et al 2001, Treiber et al 2005). For example, Treiber et al (2005) found that a group of 9-month-old weanlings that had been provided access from birth to a high sugar and starch feed had a significantly lower insulin sensitivity than the group provided a fat- and fiber-rich complementary feed. Taken together, these findings suggest that chronic elevation of plasma insulin (and/or associated effects) at key stages in the early growth period of horses might trigger imbalances in chondrocyte maturation, thereby delaying the rate of endochondral ossification. However, the Treiber et al (2005) weanling study did not evaluate the effect of diet on the incidence of OC, assessed either by clinical signs of lameness or radiographic evaluation. Data showing an effect on the clinical incidence of OC from feeding fat- and fiber-rich diets instead of traditional starch- and sugar-rich diets are still lacking. Ott et al (2005) did not find that feeding a high starch concentrate intake for 112 days (>30% starch vs. 0% starch) led to a higher incidence of OC in weanlings.
Insulin also shows a strong interaction with the thyroid hormones (T3 and T4). It has been reported, in weanlings, that insulin will stimulate the rapid removal of T4 from the circulation following the ingestion of a high-carbohydrate meal (Glade & Belling 1984, Glade et al 1984). The changes in the hormonal pattern were associated with disturbances in growth plate cartilage metabolism. These results may be of particular interest as thyroid hormones are involved in the final stages of chondrocyte differentiation and the invasion of growth cartilage by blood vessels.
As a result of the studies above and others, feeding diets high in soluble carbohydrates to growing horses have been implicated as a contributing factor to the development of DOD (Ralston 1996, Henson et al 1997, Jeffcott & Henson 1998, Treiber et al 2005, Staniar et al 2007). Therefore the replacement of rapidly digestible carbohydrates with fat and fiber has attracted some attention. The substitution of dietary fat for readily digestible carbohydrates like sugar and starch (SS) decreased postprandial glycemic and insulinemic responses in Thoroughbred weanlings (Staniar et al 2007), but the results on growth factors like IGF-1 are controversial. Staniar et al (2007) found a significant diet-related effect on plasma IGF-1 and average daily gain (both higher in SS foals with seasonal effect), whereas previously Ropp et al (2003)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree