Guy D. Lester, Consulting Editor Guy D. Lester • Jane E. Axon • Wendy E. Vaala Frequent monitoring of the ill newborn foal is essential because early recognition of subtle clinical changes can have a significant impact on outcome. This includes observations of behavior and repeated structured physical examinations. (Assessment of the foal is described in Chapter 16.) It is important that the clinician develop a routine method of examination that is consistently followed, irrespective of the perceived level of illness. This ensures that the assessment is complete and no abnormalities are missed. Delivering detailed and potentially expensive therapy to a foal with a significant congenital defect is potentially avoided by adopting this approach. By the time that many sick newborn foals are examined by veterinarians, they are critically ill and require emergency therapy to restore circulation, tissue perfusion, and oxygenation. This approach is conserved across many diseases. After fluid resuscitation, there can be an investigation into underlying diseases and directed therapy instituted. If high-volume fluid resuscitation is anticipated, a large-bore (14- or 16-gauge) catheter should be placed into the jugular vein. Identification of the vein can be facilitated by elevation of the hindquarters in lateral recumbency and by placing a rolled towel under the neck. Teflon catheters (Abbocath, Abbott Hospitals, North Chicago, Ill.) are more thrombogenic than silastic or polyurethane catheters, but they are usually easier and quicker to place. Short catheters placed in peripheral veins can be difficult to maintain and are not suitable for large-volume, rapid fluid replacement. Most referral hospitals use polyurethane catheters as they reduce the incidence of thrombophlebitis and eliminate the need for frequent catheter replacement. Using strict aseptic technique, the catheter is typically inserted using the “over-the-wire” (Seldinger) technique. Once positioned, the catheters can often be left in place for up to 3 weeks. A combination of superglue and sutures is very effective in keeping the catheters in place. Catheter sites are covered but monitored frequently for signs of infection. Insertion site swelling is not uncommon for 24 hours after placement of some catheters. Blood samples for culture, hematology, and biochemistry can be collected at the time of catheter placement if the condition of the foal is not highly critical. After placement of an intravenous catheter, warmed fluid can be delivered at a rate of 20 mL/kg over 15 to 20 minutes.1,2 Typically the initial fluid of choice is a multi-electrolyte replacement solution, such as lactated Ringer solution (LRS) or Hartmann’s solution. After the initial or subsequent fluid boluses the patient is reassessed, specifically noting changes in mucous membrane color, capillary refill time, pulse quality and rate, mentation, warmth of peripheral tissues, intestinal sounds, and urine production.3 If there is minimal or no improvement, the bolus can be repeated a further three times, to a maximum of 80 mL/kg, or 4 L for a 50-kg foal. During this time essential laboratory data can be collected, including the packed cell volume (PCV), total plasma protein (TPP), and blood glucose concentration. Ideally, an arterial or venous sample is obtained for blood gas, electrolyte, and lactate determination. Factors that influence the rate or total volume of fluid used during resuscitation include a low TPP concentration (<35 g/L); preexisting limb, body, or head edema; or spontaneous hemorrhage. Colloids are indicated in low protein states. Plasma is the most commonly used colloid in foal practice, but it is expensive and requires thawing and the use of an in-line filter. However, the benefits of fresh or fresh-frozen plasma are numerous, including improvement of osmotic pressure, provision of coagulation factors, to provide buffer base, and immunotherapy.2 The administration of plasma has been associated with anaphylactic or anaphylactoid reactions, which can be fatal. For this reason, plasma is not routinely used for acute fluid resuscitation. Plasma should be administered at an average rate of 10 mL/kg/h. Give the first 100 mL slowly and monitor the foal’s pulse, respiratory rate, and temperature. Synthetic colloids are less expensive, and they do not require special storage conditions nor use of a special filter, but they do not have the range of benefits of plasma. The use of synthetic colloids in foals has been questioned.1–3 Hydroxyethyl starch (e.g., Hetastarch), however, has been used successfully for rapid fluid resuscitation in foals with few adverse reactions (3 mL/kg at a rate of 10 mL/kg/h).4 Once critical hypovolemia has been rectified, assessment can be made of residual dehydration.3 Foals have a relatively greater proportion of extracellular fluid when compared with adults,5 and in contrast to adults can appear thin or emaciated when dehydrated. Traditional clinical parameters used to assess hydration in adult horses, such as moisture of oral mucus and skin tenting, are less reliable when used in foals. The volume of fluid is, however, calculated in a manner similar to that used in adults. A foal with moderately to severely sunken eyes is estimated to be 8% to 10% (of body weight) dehydrated. The estimated fluid deficit in a 40-kg animal that is 10% dehydrated would be approximately 4 L. The calculated deficit should be replaced over the following 12 hours, along with consideration of maintenance fluid requirements and any ongoing losses. If an animal is mildly dehydrated and the gastrointestinal tract is not seriously compromised, fluid requirements can be provided by the enteral route, using milk or commercially available dextrose and electrolyte mixtures. When intravenous fluid therapy is used for maintenance of hydration, the Holliday-Segar formula has been advocated for calculation of fluid delivery rates in foals with impaired renal function, or in foals less than 3 days of age where renal function is not known.2,3 The formula uses a principle that slight fluid deprivation is less critical than overhydration, particularly when sodium-rich fluids are used. For the first 10 kg of body weight, the foal receives 100 mL/kg/day; for 11 to 20 kg of body weight, the foal receives 50 mL/kg/day; and for each kilogram over 20 kg the foal is assigned 25 mL/kg/day.2 For a 50-kg foal this is calculated as 1000 mL/day for the first 10 kg, 500 mL/day for the second 10 kg, and 750 mL/day for the remaining 30 kg of body weight, for a total daily volume of 2250 mL or an hourly flow rate of 94 mL.2 For older foals, or foals with normal renal function, higher flow rates can be used, typically in the range of 2 to 5 mL/kg/h.3,6 If hypotension persists in the face of fluid resuscitation, inopressor therapy may be indicated. It has been recommended that the aim of therapy is to raise the mean arterial pressure (MAP) above 60 mm Hg, although this does not imply that tissue perfusion is adequate.7 Agents that are commonly used include dobutamine, dopamine, norepinephrine, and vasopressin.7 Dobutamine is a positive inotrope that improves cardiac output primarily by improving stroke volume. The drug is administered as a continuous rate infusion (CRI) with a wide dose range of 2 to 20 µg/kg/min, but it is recommended to commence therapy at the low end of the range.3 It is not uncommon to administer a vasopressor with dobutamine to improve tissue perfusion. Norepinephrine acts at α1– and α2-receptors to mediate vasoconstriction, as well as acting at β1-adrenergic receptors, causing positive inotropic and cardiotropic effects.7 The drug has a very short half-life and is given by CRI at a highly variable dose range, from 0.05 to 5 µg/kg/min. Again, starting at the low end of the range is recommended. Dopamine also has some combined α- and β-adrenergic effects, in addition to moderate to strong affinity for dopamine receptors (DA-1 and DA-2 receptors).7 Activity at dopaminergic receptors primarily mediates vasodilation, particularly in renal, cerebral, and splanchnic vascular beds. Lower infusion rates are used to improve renal and splanchnic perfusion (1 to 5 µg/kg/min), whereas higher infusion rates (10 to 25 µg/kg/min) will be required for patients in severe septic shock. Vasopressin (0.25 to 1 mU/kg/min) acts at V1a receptors in the peripheral circulation, causing vasoconstriction, and at V2 receptors in the kidney to facilitate water reposition.7 Low-dose vasopressin is commonly used in conjunction with norepinephrine infusion and is reported to be effective in the management of the hypotensive neonatal foal. Beneficial effects include an increase in MAP, a reduction in heart rate, and increased urine output.8 The fluid rate is in part dictated by the need to provide glucose supplementation. A rate of 5 mg/kg/min has been suggested as an initial CRI flow rate, based on the estimated placental supply in late gestation (6.8 mg/kg/min).3,9 This equates to an hourly flow rate of 3 mL/kg (150 mL/h for a 50-kg foal) of a 10% glucose solution. Blood glucose concentrations should be monitored closely and the rate adjusted as required. Boluses of glucose solutions should be avoided because the resultant glucosuria will lead to urinary losses of fluid, electrolytes, and glucose. It can produce a marked rebound hypoglycemia. In most foals, electrolyte concentrations will normalize over time assuming adequate renal function and milk intake. There are, however, several conditions in which specific electrolyte supplementation or avoidance is important. Foals with uroperitoneum can develop life-threatening hyperkalemia. Specific management is discussed later in this chapter in the section on distended/painful abdomen. Fluid therapy is typically based on 0.9% saline, supplemented with glucose. Potassium supplementation may be required for critically sick neonates, anorexic foals, foals with diarrhea, or those receiving diuretic therapy. If serum concentrations fall below 2.5 mEq/L, intravenous supplementation is recommended. Potassium requirements are difficult to estimate from serum concentrations, but the following equation has been recommended: Potassium can be added safely to fluids at a rate of 10 to 40 mEq /L, although when delivering fluids at a maintenance rate using the Holliday-Segar formula, concentrations up to 60 mEq/L may be used.2 At higher concentrations it is very important not to exceed recommended rates of administration. Potassium supplementation should not exceed 0.5 mEq/kg/h, or 25 mEq/h for a 50-kg foal. Sodium overload is a problem associated with prolonged use of salt-rich replacement solutions.2 The problem is avoided by using specially formulated low-sodium, high-potassium replacement solutions that are supplemented with glucose, or using 5% dextrose in water as the base solution for fluid therapy. The principles of hyponatremia or hypernatremia treatment are discussed in Chapter 22. Sodium bicarbonate is commonly given to foals that are acidemic, assuming that it is metabolic in origin. The initial approach to management should be rehydration and improvement in tissue perfusion. This is achieved using crystalloids, colloids, and inopressor agents. When bicarbonate is administered to foals where the acidosis has originated from poor tissue perfusion, the results are often disappointing. Bicarbonate supplementation is indicated in conditions in which there has been loss of buffer, such as diarrhea or renal tubular acidosis. If bicarbonate deficits are greater than 10 mEq/L (serum [HCO3−] <15 mEq/L) after rehydration, then supplementation can be given using the following equation: Isotonic bicarbonate can be made by adding 150 mL of 8.4% bicarbonate solution to 850 mL of sterile water, or 200 mL of 5% bicarbonate solution to 800 mL of sterile water. Bicarbonate solutions should be given slowly. Bicarbonate should not be combined with any calcium-containing solution or else precipitation will occur. The effect of the bicarbonate replacement therapy should be monitored closely and the dose adjusted accordingly. Neonates with severe diarrhea because of ongoing losses of bicarbonate through the feces may need considerably more than the calculated deficit to maintain an adequate blood pH until the diarrhea subsides. Plasma is administered through an aseptically placed catheter using a blood administration set with an in-line filter. The rule of thumb for plasma administration for FPT is 20 mL/kg, or approximately 1 L for a 45-kg foal. In healthy foals, 1 L of plasma with immunoglobulin G (IgG) of 1200 mg/dL (12 g/L) raises the serum IgG 200 to 250 mg/dL (2 to 2.5 g/L). For foals with total failure of passive transfer, it may be necessary to provide 2 or 3 L of commercial plasma if the goal is to reach a serum IgG concentration of 800 mg/dL (8 g/L). The same amount of plasma has less effect in septic foals. Ill foals require relatively more plasma because the serum half-life of IgG is less, IgG may be sequestered in intravascular spaces or at sites of inflammation, and IgG may catabolize more readily. The thermoneutral zone describes an ambient temperature range in which heat production and heat loss are at a minimum.10 The lower level of the range (Tlc) defines a temperature cutoff, below which the metabolic rate rises and the rate of evaporative heat loss increases. For 2-day-old pony foals, the Tlc was estimated to be 25° C,10 a value much higher than the typical ambient temperature that many foals experience. However, healthy foals are able to maintain their core body temperature down to an ambient temperature of 5° C, but premature or sick newborn foals are often unable to maintain their body temperature and frequently require external heat sources during treatment. Foals on phenobarbital therapy are also susceptible to a reduction in body temperature. It is critical to dry the foal and remove it from drafts to reduce convective heat loss. If the rectal temperature is less than 37.7° C (100° F), efforts should be made to warm the foal by raising the environmental temperature. Rewarming the periphery only with high external heat without simultaneously warming the body core can produce peripheral vasodilation and accentuate cardiovascular collapse. Warming can be achieved using forced air warmers (Bair Hugger, 3M, St. Paul, Minn.), radiant heat lamps, blankets, or careful use of heating pads. A heat pack can be made by placing a wet towel inside two rectal sleeves and microwaved to the desired temperature. It is important to address hypotension using warmed fluid therapy. It is important that the environment for housing the sick foal is clean. A temporary barrier between the mare and recumbent foal facilitates treatment of the foal, yet keeps the dam within sight, sound, touch, and smell of her foal, which facilitates bonding. Many of these foals are recumbent, making them further vulnerable to contagious or opportunistic pathogens. Recumbency increases the risk of pneumonia, predisposes to ileus and constipation, increases the risk of milk aspiration, and exacerbates musculoskeletal weakness. Recumbent foals are also at risk for the development of decubital ulcers and should be bedded on a soft and absorbent surface. They should be carefully turned and assisted to stand every 2 hours. Placement into custom-made V-pads (Dandy Products, Goshen, Ohio) can help the foal rest in sternal recumbency, improving oxygenation and reducing the risk of lung atelectasis and secondary pneumonia (Fig. 17-1). Differences between neonatal and adult animals in drug effects generally can be attributed to differences in drug distribution, metabolism, or excretion. Some general characteristics of the neonatal period include better absorption of drugs from the gastrointestinal tract, less drug binding to plasma proteins, increased apparent volume of distribution of drugs that are distributed in the extracellular fluid volume, increased permeability of the blood-brain barrier, and slower elimination (i.e., longer half-life) of many drugs.11 Some of these responses are attributed to differences in body water between neonates and adult horses. Total body water (TBW) in day-old foals was 744 ± 24 mL/kg, compared with 670 ± 60 mL/kg in adult horses.5,12 During growth, the intracellular fluid compartment remains relatively consistent relative to size, whereas the extracellular fluid (ECF) compartment decreases as a percentage of body weight, with an increasing body fat percentage. The ECF volume was estimated at 381 ± 18 and 394 ± 29 mL/kg, in 1- and 2-day-old foals, respectively.5,13 This compares with reported values in adult horses of 214 to 253 mL/kg. Plasma volume is around 96 mL/kg in neonates, which again is much greater on a per-kilogram body weight basis than reported values in adults of 53 to 63 mL/kg.5 Although the neonate has a higher percentage of TBW than the adult, it is more vulnerable to water loss because of increased basal metabolic rate, relatively greater surface area, predisposition to increased heat and water losses, and reduced urine concentrating ability. Provision of adequate nutritional support to the compromised neonate is an essential component of critical care. Common reasons for not providing adequate nutrition include failure to estimate the nutritional requirements of the sick neonate, the need to use alternative methods and routes of delivery, and a gastrointestinal tract that is often compromised and intolerant of nutrient intake. The nutritional requirements for optimum growth of the normal-term foal are poorly defined, let alone for the premature, growth-retarded, or debilitated animal whose caloric, protein, mineral, and vitamin requirements might be very different. Measurements of milk production of mares combined with data concerning the free-choice milk intake of normal orphan foals and premature and sick foals recovering from various illness suggest that a figure of 125 to 150 kcal/kg/day, or even higher, is close to the normal caloric intake.14 Healthy full-term foals nurse an average of 2 minutes, seven times an hour, and consume between 20% and 30% of their body weight in milk daily, resulting in weight gain of 1.3 to 1.7 kg/day.15–17 On this diet, a 50-kg foal would therefore consume 10 to 12.5 L of milk a day to provide 120 to 150 kcal/kg/day, 50% of which is used for growth and 50% for basal metabolism and thermoregulation. Factors that influence weight gain include caloric intake, the level of exercise, and the resting energy expenditure (RER). Weight gain is reduced in hospitalized newborn foals. RER is, however, less in ill foals, at approximately 44 to 49.5 kcal/kg/day, compared with 65 kcal/kg/day in healthy foals.18 The general recommendation is to provide a minimum of 50 kcal/kg/day and aim for a weight gain of 0.6 to 1.0 kg/day. If there is no medical contraindication for oral feeding, and the gastrointestinal tract is functional, then enteral nutrition is the preferred and most effective route of nutritional supplementation. Enteral feeding stimulates normal gut maturation, growth of intestinal villi, production of crypt cells, hepatic and biliary secretions, and brush border disaccharidase enzyme activity. Therefore, even in foals that must be fed parenterally, small volumes of enteral feeds are given to prevent gut atrophy. Foals that are not nursing from the mare can be fed by bottle, bucket, or nasogastric tube. If an effective swallow reflex is present, bottle feeding can be used. Bucket feeding allows the foal to drink with its head and neck in a flexed position and is helpful for foals with a weak swallow reflex or foals destined to be hand raised. Milk should be introduced in a shallow handheld bowl and the foal encouraged to suckle the finger or nipple as its head is lowered into the milk. Foals less than 7 days of age should ideally be fed every 1 to 2 hours. Nasogastric intubation is required if ineffectual swallowing and sucking are present. A styleted small-bore flexible silicone tube (5- to 7-mm internal diameter) with a weighted tungsten end is preferred. Individual choice dictates whether the tube is passed for each feeding or left indwelling. Indwelling tubes can be sutured to the nares using a Chinese finger-trap suture technique,19 or taped to half a tongue depressor, which is then taped to the foal’s muzzle and/or fleece halter. Tubes should be sealed between feedings to avoid aerophagia. Recumbent foals should be maintained in sternal recumbency using a V-pad immediately after tube feeding to reduce the risk of gastroesophageal reflux and aspiration. If the dam is available and milk production is adequate, free-choice nursing is optimal. A nurse mare is the next best substitute. Popular enteral formulas include mare’s milk, goat’s milk, and artificial milk replacers. Goat’s milk is acceptable and is higher in fat, total solids, and gross energy than mare’s milk. Foals raised on goat’s milk occasionally become constipated and can develop a mild metabolic alkalosis. Cow’s milk is not as digestible and can cause diarrhea, but it can be used if additional sugar is added and some of the fat is removed. This can be accomplished by using 2% fat milk and adding 20 g of dextrose per liter of milk. A variety of artificial milk replacers are available. The ideal replacer should have approximately 22% crude protein, 15% fat, and less than 0.5% fiber on a dry matter basis. Calf milk replacer can be used if it is mixed with mare’s milk. Complications associated with enteral feeding include colic, abdominal distention, diarrhea, constipation, flatulence, misplacement of the nasogastric tube, aerophagia, nasal and pharyngeal irritation from the tube, and aspiration pneumonia. Parenteral nutrition (PN) is used to supply at least a portion of the daily nutritional requirements to critically ill foals. PN is indicated whenever feeding via the gut is inadequate or contraindicated. Candidates for partial or total PN include those individuals with diarrhea, postsurgical patients, foals with botulism, premature and septic animals, and other individuals with gastrointestinal tracts poorly tolerant of enteral feedings. PN involves administration of hypertonic solutions containing dextrose, amino acids, lipids, vitamins, electrolytes, and trace minerals. These PN solutions must be administered continuously through a jugular catheter. Complications include metabolic disturbances such as hyperglycemia/hypoglycemia, glucosuria/osmotic diuresis, insulin resistance, hypertriglyceridemia, azotemia, and imbalances of trace minerals, vitamins, and electrolytes.20,21 Catheter-related problems include thrombosis, phlebitis, and sepsis. Commonly used stock solutions for PN include 50% dextrose, 8.5% or 10% amino acids, and 10% or 20% lipid emulsion. A variety of PN worksheets are available, some of which are complicated.22 See Chapter 50 for more details and a worksheet. A simplified formula for 50 kg that is well tolerated in most foals is 2.5 L of 8.5% amino acids, 1 L of 20% lipid, and 1.5 L of 50% dextrose added to an empty 5-L bag. This mixture contains 750 g of dextrose, 212.5 g of amino acids, and 200 g of lipid, and it provides 2550 kcal from dextrose (3.4 kcal/g), 850 kcal from amino acids (4.0 kcal/g), and 2000 kcal from lipids/glycerol (10.0 kcal/g). The PN solution has 47% of total calories from glucose, 16% from protein, and 37% from fat. It is recommended that foals should not receive more than 50% of non-protein calories from lipid. The solution can be run at 104 mL/h over 48 hours, providing approximately 54 kcal/kg/day. It is common to supplement the solution with multivitamin concentrate, trace minerals, and KCl (to a final concentration of 20 to 40 mEq/L). The PN solution is hypertonic (1233 mOsm/L), so it is common to run concurrently with low tonicity solutions or free water. Foals receiving PN should have their blood and urine glucose monitored. Serum glucose concentration should remain greater than 80 mg/dL (4.4 mmol/L) and less than 180 mg/dL (10 mmol/L). Serum should be checked for gross lipemia. Heparin can be administered at 10 U/kg as an IV bolus to treat lipemia. Foals must be weaned onto and off of PN slowly. All IV lines must be checked routinely for signs of infection. Most commonly, parenteral and enteral nutrition has been used in combination; parenteral nutrient delivery is used to supplement, not totally replace, oral intake. Enteral nutrition helps to maintain the intestinal mucosa. Details concerning the use of PN compounds can be found in Chapter 50. Wendy E. Vaala • Guy D. Lester Numerous conditions of the newborn foal can produce a primary complaint of weakness and/or somnolence. The gestational and postnatal age of the neonate should be established. If weakness has been present since birth, in utero–acquired bacterial or viral infections, birth asphyxia and trauma, chronic placental problems, and congenital anomalies should be placed higher on the list of differential diagnoses. Lethargy and loss of suckle are often the first signs of neonatal illness. A full udder on the dam accompanies poor nursing behavior in the neonate. If the neonate is somnolent and has injected mucous membranes and hyperemic coronary bands, sepsis is the primary differential and the most life threatening. If the neonate is relatively bright but is becoming progressively weaker, consider peripartum hypoxia and early signs of neonatal encephalopathy (NE). If the newborn shows signs of physical immaturity such as tendon laxity and silky hair coat, weakness may be due to fatigue, hypothermia, hypoxia, and/or hypoglycemia. Unfortunately, many weak foals begin to fade as a result of multiple problems. Glycogen branching enzyme deficiency in certain Quarter Horse and Paint lineages is an example of a genetic mutation associated with a range of abnormal signs that could include persistent recumbency.23 If weakness is present without accompanying somnolence, several other differentials should be considered, including trauma. If weakness is detected in one or more limbs immediately after birth, peripheral nerve and muscle damage associated with vigorous extraction from the birth canal should be ruled out. Affected limbs are weak and hyporeflexic, and there may be regions of cutaneous anesthesia.24 The diagnosis is based on history and neurologic assessment and confirmed using electrodiagnostics after 10 to 14 days.25 The prognosis for recovery is dependent on the severity of the neural lesion. Where axon continuity is preserved, the outlook is generally good with resolution within days to weeks; where there has been disruption to axons or whole nerves, the outlook is guarded. Regrowth and reinnervation of muscle is possible; axonal regrowth is estimated at 1 inch per month.25 Foals with rupture of the gastrocnemius muscle will be unable to rise or stand unsupported.26 The treatment includes limb stabilization and exercise restriction, with a guarded prognosis for an athletic career. Neuromuscular diseases causing weakness without somnolence include botulism, nutritional myodegeneration (NMD; white muscle disease), and congenital myopathies. Botulism is an infection acquired via the gastrointestinal tract or through wounds or the umbilicus. Consequently, signs appear in neonates that are typically 10 days or older. Most cases of NMD associated with selenium and/or vitamin E deficiency occur during the first year of life among rapidly growing large animal neonates, but an in utero form of NMD may occur, resulting in clinical signs in affected foals soon after birth. Clinical signs associated with NMD may include localized (dysphagia) or generalized paresis. Rhabdomyolysis in foals may be precipitated by stress such as sepsis or excessive periparturient hypoxia. Affected foals are reluctant to move, paretic, and occasionally dysphagic.27 Pelvic limb muscles are often palpably firm. Elevated serum creatine kinase and electrolyte disturbances of hyponatremia and hyperkalemia may be observed. It should be determined whether drugs or anesthetics were administered to the dam before or at the time of delivery, as many agents cross the placenta and exert depressive and other adverse effects on the fetus. Phenylbutazone administered to normal pregnant mares crosses the placenta and results in substantial concentrations of phenylbutazone, and its active metabolite oxyphenbutazone, in the foal. Although clinical signs of phenylbutazone toxicity were not noted in the foals postnatally,28 adverse effects are possible, particularly if other problems are present. Neonatal depression induced by drugs is particularly important following cesarean section deliveries. Maternally administered anesthetics and analgesics can suppress respiration and heart rate in the newborn. In horses, both xylazine and detomidine cause maternal and fetal bradycardia and reduced cardiac output.29,30 These effects will cause a reduction in placental perfusion and fetal oxygenation. If the newborn shows depression associated with maternal administration of these drugs, an α2-adrenergic antagonist, such as yohimbine, can be given. Adverse reactions to adrenergic antagonists in adult horses have been reported, some of which were fatal.31 Weakly basic drugs, when given to the mare, tend to concentrate in the fetus. Diazepam is an example of such a drug that crosses the placenta rapidly and accumulates in the fetal circulation, resulting in lethargy, hypotonia, and hypothermia in the neonate following delivery. Flumazenil has been used to reverse the sedative effects of benzodiazepines. Maternal systemic illness of various types may also result in a weak newborn. Many neonatal disorders are associated with severe electrolyte and metabolic derangements that may clinically manifest as weakness, including hypoglycemia, acidosis, hyponatremia, hypernatremia, or hyperkalemia. Such abnormalities may occur before or at the time of birth, and laboratory assessment of the weak newborn is essential for accurate diagnosis. Young foals with hypocalcemia can present with stiff gait, muscular tremor, tachycardia, sweating, muscular tremor, and recumbency.32 Profound weakness associated with metabolic acidosis is commonly observed in foals with diarrhea. Correction of the acidosis by rehydration or intravenous administration of bicarbonate usually produces rapid improvement. A number of congenital bacterial, fungal, and viral infections that cause abortions and stillbirths may also result in the birth of a live, weak neonate. Clinical manifestations of fetal infections depend on the age of the fetus and virulence and trophism of the infecting agent. Generally, weakness resulting from uroperitoneum, renal, and liver failure; postnatally acquired infections; and neonatal isoerythrolysis (NI) is not expected to appear during the first 24 hours of age. Rather, foals with NI are usually presented between 24 and 72 hours of age, foals with uroperitoneum at 2 to 5 days or older, and neonates with postnatally acquired infections most commonly at 2 to 5 days of age or older. Paraplegia and tetraplegia are commonly associated with spinal cord compression. Compression of the spinal cord in neonates most commonly results from vertebral body malformations, osteomyelitis, or fractures. Occipitoatlantoaxial malformations (OAAMs) involve the occipital condyles of the skull and the first two cervical vertebrae.33 Generally vertebral body malformations occur sporadically, with genetic, nutritional, and/or environmental factors being implicated.34 Osteomyelitis and vertebral body abscess may be a sequel to bacteremia following neonatal sepsis or pneumonia. Rhodococcus equi vertebral body osteomyelitis, with or without associated pulmonary infection, has been reported in foals. Leukocytosis and hyperfibrinogenemia are commonly observed in neonates with vertebral body abscesses. In most instances vertebral abscesses do not infiltrate the pachymeninges, so the cerebrospinal fluid (CSF) either is normal or has a mild elevation of protein and or a mild pleocytosis. A complete neurologic examination is an important component of the work-up of the weak neonate. In particular, it should be noted if the weakness is accompanied by signs of somnolence and diffuse cerebral disease. Limb reflexes should be tested to establish whether components of the spinal reflex pathways are involved in the disease process (sensory nerve, lower motor neuron, neuromuscular junction, muscle). For example, foals with severe spinal cord hemorrhage may have relatively normal mentation, but spinal reflexes may be greatly diminished, and profound weakness may be present. Animals with other types of spinal cord disease (e.g., trauma, vertebral malformations) may also show weakness and ataxia yet have normal cerebral function. Virtually any severe systemic disease such as generalized infection can cause both profound somnolence and weakness in a neonate without the presence of actual brain pathology. Primary neurologic disease in neonates is rare; commonly, neurologic dysfunction is associated with multisystemic disease. A thorough comprehensive physical examination and work-up is required to define a problem list and formulate an appropriate management plan. A complete blood count, blood cultures, and assessment of immunoglobulin status provide an indication of the likelihood of sepsis. Hypoxia and metabolic acidosis are ruled out by assessing arterial blood gas (ABG) status, and electrolyte disturbances and hypoglycemia are evaluated by measuring serum electrolytes and blood glucose concentration. Collection of CSF to assess the central nervous system is usually performed when disease in other organ systems that may account for the altered mental state has been ruled out and no improvement in the patient’s condition is observed following correction of electrolyte, blood gas, and metabolic derangements. L. Chris Sanchez Sepsis is the major cause of morbidity and mortality in the equine neonate.35,36 The response to microbial invasion of the bloodstream involves the systemic inflammatory response syndrome (SIRS), and it is this nonspecific inflammatory response, rather than the infectious organism, that is responsible for the classical signs of sepsis. Deterioration can occur rapidly, even in the face of aggressive treatment. A variety of terms have been used to describe the host response to infection and associated processes and syndromes. A set of definitions were published by the American College of Chest Physicians and the Society of Critical Care Medicine in 1992.37 Briefly, SIRS refers to a systemic response, regardless of the inciting cause, which results in at least two of the following four clinical features: fever; tachycardia; tachypnea or hyperventilation; or leukocytosis, leukopenia, or a relative increase of circulating immature neutrophils. When SIRS occurs in response to a confirmed infectious process, the process is termed sepsis. Infection refers to the invasion of normally sterile tissue by microorganisms or the inflammatory response generated in response to those invading organisms. Bacteremia is the presence of viable bacteria in the bloodstream, viremia describes the presence of viable virus particles in blood, fungemia is the presence of viable fungal elements, and so on. When sepsis is associated with organ dysfunction, hypoperfusion, or hypotension, the event is termed severe sepsis. Septic shock is defined by sepsis-induced hypotension that persists despite adequate fluid therapy and is accompanied by hypoperfusion abnormalities or organ dysfunction. Manifestations of organ dysfunction can include coagulopathy, in addition to classical sites of renal, gastrointestinal, hepatic, cardiovascular, or pulmonary dysfunction.38 The multiple organ dysfunction syndrome (MODS) describes alteration of organ function in an acutely ill patient such that homeostasis cannot be maintained. MODS can occur either as a primary event (e.g., as a direct result of trauma) or as a result of a host response. A syndrome of immunosuppression caused by an exaggerated systemic anti-inflammatory response with increased circulating levels of anti-inflammatory mediators, leukocyte anergy, or increased susceptibility to infection has been termed the compensatory anti-inflammatory response syndrome (CARS). The mixed anti-inflammatory response syndrome (MARS) applies to fluctuations between episodes of SIRS and CARS.39 Infection with gram-negative bacteria occurs commonly in neonatal foals.36,40–42 The pathophysiology of septic shock in gram-negative sepsis involves bacterial endotoxin and the subsequent release of pro- and anti-inflammatory cytokines.43,44 Septic foals can have decreased gene expression of TNF-α and TNF-β, and increased expression of IL-8 relative to healthy foals,45 or increased gene expression of IL-4 and TLR446 among other abnormalities. Factors that may predispose to infection are numerous and include maternal illness, increases or decreases in gestational length, partial or complete failure of passive transfer, poor environmental conditions, and inadequate or improper umbilical care. Maternal factors have been reported to play a central role in 24% of bacteremic foals.47 These include dystocia,48 premature placental separation, placentitis, and various other forms of maternal illness such as colic and respiratory disease. Many of these factors co-exist but some occur primarily, such as placentitis, whereas others are secondary, such as premature placental separation. In utero infection of the fetus typically occurs due to an ascending infection of the fetal membranes and often results in premature delivery.49 Because chronic placentitis often results in accelerated or precocious fetal maturation, a resultant premature foal born to such a mare likely has a greater chance of being septic but has a higher probability of survival than a foal born at a similar gestational age to a mare without placentitis or other chronic stimulation. Failure of transfer of passive immunity (FTPI) predisposes to an increased risk of postnatal infection. A number of studies have documented a close relationship between the foal serum IgG concentration and incidence of disease.50–53 Colostrum administration via nasogastric tube was associated with disease in one report,48 which supports the notion that route and timing of transfer are likely relevant, along with the potential for bacterial challenge. Farm management is important, including general hygiene, stocking density, exposure to disease, nutrition, and prepartum vaccination and deworming programs. Foals with partial failure of passive transfer were not at any greater risk of disease than those with adequate transfer on a well-managed Standardbred farm.54 Common postnatal routes of infection include umbilical remnants, gastrointestinal tract, and the respiratory tract. The umbilicus was traditionally regarded as the most important site for pathogen entry, but recently there has been recognition of the role of the gastrointestinal tract as an important portal for bacteria.55 The absorption of macromolecules in the proximal small intestine occurs through specialized cells via pinocytosis, with little discrimination between maternal immunoglobulin and other macromolecules. Absorption peaks shortly after birth and declines to less than 1% by 20 hours.56 Unlike other species, the absorption of immunoglobulins does not appear to be Fc-receptor mediated in the foal. The foal will selectively absorb IgG and IgM over IgA.57 In neonatal pigs and lambs, deprivation of milk or colostrum can extend intestinal permeability to immunoglobulins by up to 5 days.58 In contrast, intestinal permeability to immunoglobulins cannot be delayed through withholding of macromolecules to newborn foals.59 It is not known if closure can be hastened by the feeding of macromolecules immediately after birth in foals. The other main postnatal factors beyond FTPI include gestational age and environmental conditions. Foals with exceptionally short or long gestational lengths may be at a heightened risk for development of sepsis.60 Poor environmental conditions can result in an increased bacterial load to the gastrointestinal tract, especially during the initial periods of udder seeking.55
Manifestations and Management of Disease in Foals

General Principles of Treatment and Care of the Abnormal Foal
Catheter Selection and Placement
Fluid Resuscitation
Correction of Dehydration
Maintenance Fluid Therapy
Use of Inopressor Drugs
Glucose Supplementation
Electrolyte Management
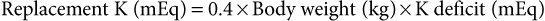
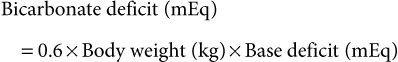
Plasma Transfusion for Failure of Passive Transfer of Immunoglobulin
Maintenance of Body Temperature
Bedding and General Environment
Special Considerations of Drug Therapy
Nutritional Support
Weakness and/or Somnolence
Sepsis
Predisposing Factors
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Manifestations and Management of Disease in Foals
Chapter 17

