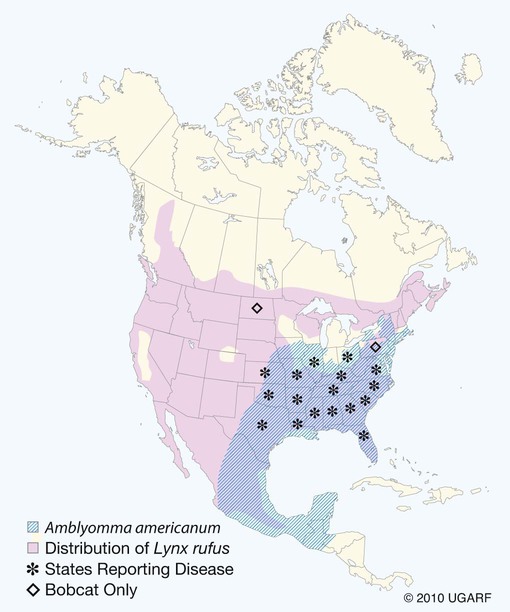
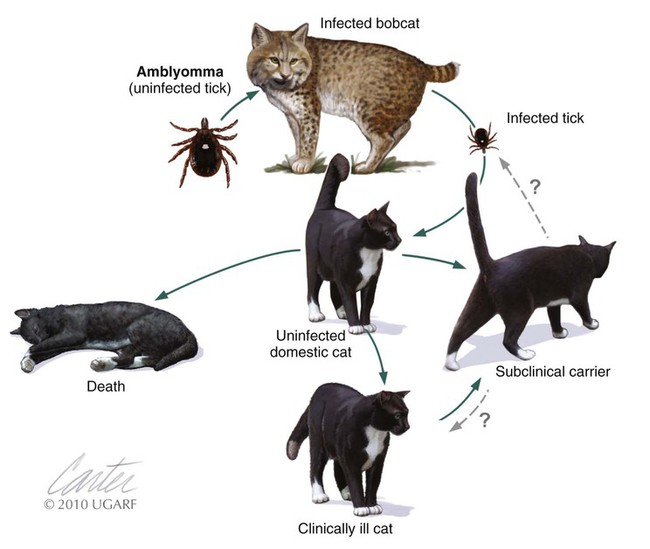
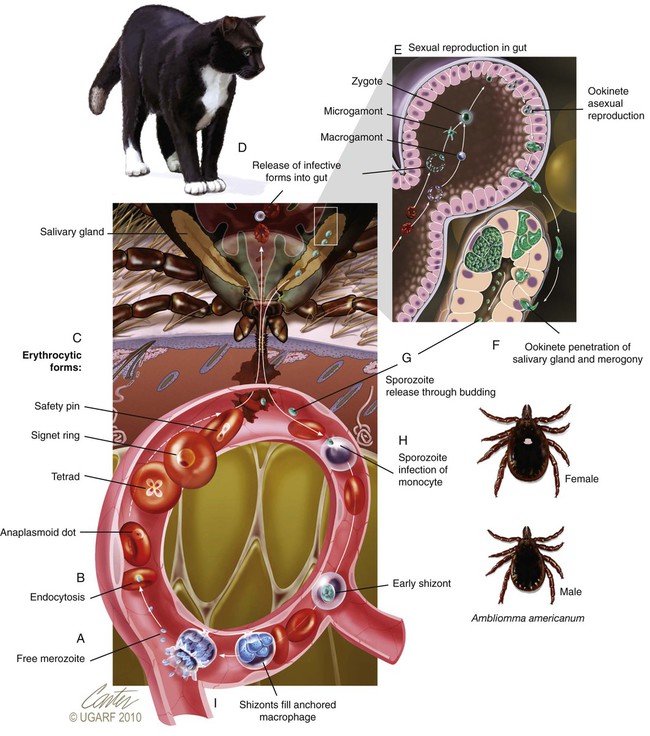
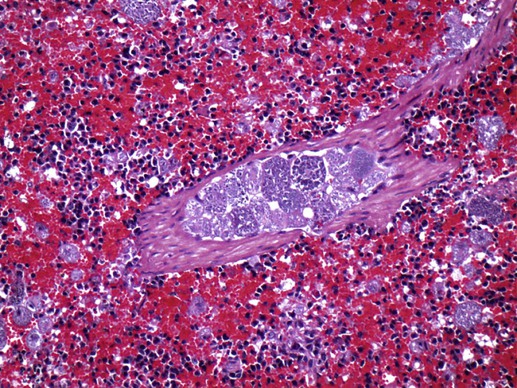
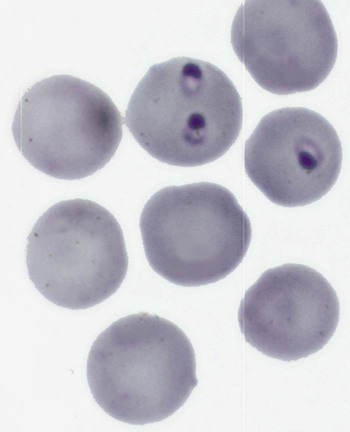
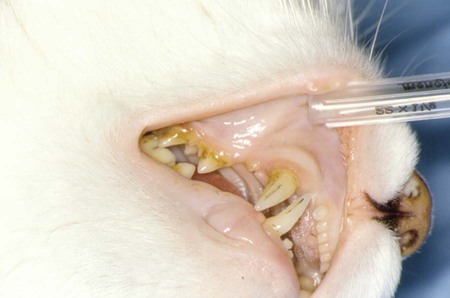
Although C. felis is the species most relevant to the health of domestic cats in the United States, it is only one of several species of the genus Cytauxzoon.20 A morphologically indistinguishable intraerythrocytic parasite has been identified in Pallas’s cats (Otocolobus manul) imported from Mongolia.38 It has been tentatively named Cytauxzoon manul. Sequencing results of the 18S ribosomal RNA (rRNA) genes (ribosomal DNA, rDNA) were that the organism is related to but distinct from C. felis.59 When injected into domestic cats, C. manul produced erythroparasitemia, but no disease, and was unable to prevent virulent disease when those cats were subsequently infected with C. felis.37 Another two similar but distinct pathogens have been detected in Iberian lynx (Lynx pardinus) from southern Spain.51a,52 Cytauxzoon parasites identified in domestic cats in South America may or may not be genetically identical to the C. felis found in the United States.51,64 Infection with C. felis or very closely related protozoa occurs in a wide variety of nondomesticated felid species, including the presumed reservoir host, which in the United States is the bobcat (Lynx rufus rufus) as well as captive and wild large cats.* In bobcats, infection is usually persistently subclinical erythroparasitemia. Extensive experimental transmission studies to any non-Felidae species, including immunodeficient mice, have failed.42 Cytauxzoonosis can occur in cats of any age and either gender, with no identified breed predilections. Immune suppression is not necessary for cats to become infected, and there is no evidence that infection is more likely in retrovirus-infected cats than in noninfected cats. Outdoor cats are more likely to become infected, especially during spring and summer, probably because of increased exposure to tick vectors.56 In retrospective studies, infection has rarely been identified from November through March.4,56 Similarly, 75% of infected cats identified by the University of Missouri Veterinary Medical Diagnostic laboratory over a several-year period were identified between May and July.15 For many years after its original description in Missouri during the mid-1970s,66 feline cytauxzoonosis was recognized only in the south-central United States. The geographic range has expanded subsequently, with infections now reported in cats throughout the southeastern and mid-Atlantic regions.5,34 Although not yet demonstrated in domestic cats, C. felis infection has been demonstrated in bobcats living as far north as Pennsylvania6 and North Dakota.61a Although it is possible that infections outside the south-central United States were simply missed in years past, the relative ease of recognition of the parasite on examination of blood smears or by finding organisms in tissues on necropsy exam makes this possibility seem unlikely. Instead, the parasite likely now occurs throughout a wider geographic region. The geographic range of reported C. felis infections in domestic cats in North America appears to overlap with the ranges inhabited by both bobcats and the Lone Star tick (Amblyomma americanum)61a (Fig. 75-1). In addition to disease in domestic and captive wildcats in the United States,14,25,35,69 C. felis infections in both domestic and captive wildcats have been reported in South America.2,51,51 Because the primary reservoir host for the pathogen in the United States is the bobcat, infected domestic cats are typically from rural or suburban rather than urban areas. Cats living close to wooded areas or less intensely managed land are more likely to become infected.56 It is common for multiple cats in a neighborhood, or even a household, to become infected within a single season, likely reflecting the presence of infected bobcats in the area. There are no large published epidemiologic studies of the prevalence of C. felis infection in domestic cats. In a single study of healthy cats presented to trap-neuter-return programs in Tennessee (n = 75), Florida (n = 494), and North Carolina (n = 392), prevalence of C. felis infection as detected by polymerase chain reaction (PCR) was 1.3%, 0.4%, and 0%, respectively.31 It is likely that prevalence rates are higher in regions traditionally found to have enzootic infection; the authors of this chapter are aware of several colonies of cats in Missouri and North Carolina with prevalence rates of up to 72%. Prevalence of infection in cats from Brazil with a hemoprotozoan parasite morphologically indistinguishable from C. felis was similarly high, up to 48.5%.51 Prevalence of infection in bobcats depends on geography, and estimates range from 7% in Pennsylvania to 33% in North Carolina to 62% in Oklahoma.6,28,28 In Florida, the prevalence of asymptomatic infection for transplanted Texas cougars was 39% and for Florida panthers was 35%.60 There are no published studies on incidence rates of infection in domestic cats. In a retrospective review, cytauxzoonosis accounted for approximately 1% of feline submissions to the Oklahoma Animal Disease Diagnostic Laboratory and 1.5% of feline admissions to the Oklahoma State University Veterinary Medical Teaching Hospital between 1995 and 2006 and between 1998 and 2006, respectively.56 Information from personal communications with the authors of this chapter suggests that veterinarians in enzootic “hot spots” see as many as 5 cases of cytauxzoonosis a week during the peak season, with many more veterinarians reporting that they see 5 to 10 cases per summer. Infection of domestic cats with C. felis in a natural setting requires a tick vector; ill and naïve cats can be cohoused without disease transmission.67 Experimentally, infection can be transmitted through inoculation of schizont-laden tissues or transfusion of blood collected during the acute illness.27,41,41 Transfusion of blood from recovered cats, which contains only erythrocytes containing piroplasms, rather than mononuclear-associated schizonts, does transmit the piroplasm stage of infection but does not result in schizogony or clinical illness in the recipient.7,27 A variety of ticks feed on both domestic and wild Felidae, and C. felis has been recovered from Dermacentor variabilis and A. americanum ticks.9 Although vector competence and transstadial transmission (nymphs to adults) were demonstrated for D. variabilis in a 1984 study,7 neither Dermacentor ticks nor Rhipicephalus sanguineus were able to transmit infection in a later investigation.58 In the later study and another subsequent trial, only A. americanum nymphs fed on infected cat and were able to transmit infection during subsequent feeding as adult ticks.57,58 There are no published studies investigating transovarial transmission or transstadial transmission during other tick life stages. Other ticks may also serve as competent vectors for transmission. Although neither D. variabilis nor A. americanum is found in Brazil, naturally occurring infection with cytauxzoonosis has been reported there.2,50,50 Presumably, Amblyomma cajennense or another ixodid tick may be the responsible vector.55 Unlike domestic cats (see next paragraph), when bobcats become infected with C. felis there is limited schizogenous replication associated with a typically mild to moderate acute clinical illness (although fatal infection of bobcats has been described).8,27,27 On recovery from illness, bobcats remain infected with the piroplasms in their erythrocytes. Ingestion of piroplasms contained in erythrocytes serves as the means of transmission of the parasite to the tick intermediate hosts. Naïve ticks pick up the pathogen after feeding on these persistently infected carriers. These ticks can then transmit sporozoites to the next felid host on which it feeds (Fig. 75-2). If the tick next feeds on another bobcat, the sylvan cycle is repeated. If the tick feeds on a domestic cat, infection often results in massive schizogenous replication and profound illness. For years, domestic cats were deemed terminal hosts because most died from cytauxzoonosis (see Fig. 75-2). Felidae that recover from acute infection can apparently harbor the piroplasms for months or even years without serious consequences.* In the past decade it has become clear that domestic cats can survive acute illness and can remain persistently parasitemic.5,30,49,58,68 Additionally, domestic cats that harbor piroplasms without any history of clinical illness are occasionally identified.31,49,49 In an experimental setting, it appears that domestic cats are capable of transmitting infection through the tick vector.44,58 Although these studies demonstrate reservoir competence, more extensive studies are necessary to establish the actual reservoir capacity of domestic cats in a natural setting. The existence of domestic cat colonies with high prevalence rates of C. felis infections combined with extensive exposure to competent tick vectors suggests that both the reservoir and vector capacities are likely to be high in select populations. If so, movement of domestic carrier cats (with their owners) might help account for the expansion in the endemic geographic region for this pathogen. In the life cycle of C. felis, the sexual phase of reproduction occurs in the tick gut (Fig. 75-3). Ookinetes leave the gut, enter the body cavity, and migrate to the salivary glands, where they replicate as infective sporozoites. After these sporozoites enter the host during tick feeding, they penetrate and develop, primarily within mononuclear phagocytes. They are visible first as indistinct vesicular structures within the cytoplasm of infected cells and later as large, distinct, nucleated schizonts that actively undergo division by schizogony and binary fission (see Fig. 75-3). Multiplication of schizonts within host cells is observed ultrastructurally to be true schizogony, without host cell division. Later in the course of the disease, schizonts develop buds (merozoites) that separate and eventually fill the entire host cell. The phagocytes line the lumens of vessels within almost every organ and become huge and numerous, often occluding the vessel similar to a thrombus. The host cell probably ruptures, releasing the merozoites into the blood or tissue fluid. Merozoites appear in macrophages 1 to 3 days before they are observed in erythrocytes. These organisms then invade uninfected erythrocytes and produce late-stage parasitemias that are detected on examination of blood films, usually 1 to 3 days before clinical illness. The phase of asexual schizogenous reproduction, within the host’s mononuclear phagocytic cells, is responsible for the pathologic processes resulting in clinical illness. It is unclear whether only sporozoites can enter macrophages and initiate schizogony or if early schizonts can spread from macrophage to macrophage. Schizonts distend mononuclear cells within the lumen of venous channels in the interstitium of most organs (Fig. 75-4), but may be especially severe in the lungs, liver, spleen, and other lymphoid tissues.40,67 Disseminated intravascular coagulation (DIC), presumably activated by vascular endothelial disruption, has been a complication based on laboratory findings in naturally infected cats.25,29 Vascular obstruction, anoxia, and release of damaging substances secondary to cell death and rupture may all contribute to multiple organ failure. Fission of the schizont within the mononuclear cells results in the formation of merozoites.62 Eventually, the merozoite-distended cell ruptures, thereby releasing merozoites. Erythrocytes take up merozoites via endocytosis, producing the classic piroplasms associated with infection (Fig. 75-5). Piroplasms (merozoites) reproduce through asexual binary fission only in erythrocytes. The presence of piroplasms is sometimes associated with hemolysis during the later stages of the acute illness, but piroplasms are not likely a major contributor to disease. The immunologic response to C. felis is unknown, and it is unclear why bobcats usually develop a more limited schizogenous replication than do domestic cats. Erythroparasitemia acquired via transfusion of piroplasm-containing erythrocytes does not protect domestic cats from subsequent fatal schizogony.27,53 Experimental studies suggest that when domestic cats survive the schizogenous phase of infection, they become immune to repeated infection and associated clinical illness.22,53 However, the authors of this chapter have spoken with veterinarians in endemic regions who have observed clinical illness indistinguishable from cytauxzoonosis in cats known to have survived cytauxzoonosis in previous years. No attempt has been made to look for schizonts in the cats experiencing a “second” illness, so it is unclear whether these cats have recrudescent illness, reinfection, or some other illness altogether. Less virulent strains of C. felis might result in a more limited schizogony with less associated pathology in domestic cats. The theoretical existence of less virulent strains is supported by a regional occurrence of survivors at a greater than expected rate.49 Using noncoding regions of rRNA known as internal transcribed spacer regions, variability in strains of C. felis from naturally infected cats in Georgia and Arkansas was demonstrated.10 Initially, a significant association was demonstrated between survivability and the particular strain of C. felis.10 A subsequent study found that asymptomatic cats were often infected with an organism possessing the same internal transcribed spacer region (ITS)-sequence type identified in cats that have succumbed to infection, concluding that ITS analysis cannot be used to discriminate between highly pathogenic and less pathogenic strains.12 Currently, it is not possible to prospectively screen clinical samples for prognostic purposes. Although historical and physical examination findings are nonspecific, an acute onset of anorexia, lethargy, and fever in cats within an endemic region (especially during spring and summer) should raise immediate suspicions for cytauxzoonosis. The onset of clinical disease occurs from 1 to 3 weeks after tick-transmitted infection.7,58 Initial clinical signs are vague and typically include anorexia and lethargy. Within hours to days, illness becomes more severe and owners may notice increased vocalization, weakness, icterus, dark yellow urine color, respiratory distress, obtunded mentation, or even seizures. The most consistent alteration on physical examination is pyrexia (often marked: 39.4° C to 41.6° C [103° F to 107° F), but hypothermia occurs in moribund animals. Mucus membranes may be icteric and/or pale. Evidence of dehydration and delayed capillary refill time or mucosal pallor may be observed (Fig. 75-6). Tachypnea and tachycardia are typical, with or without overt respiratory distress. Mild to moderate lymphadenomegaly, splenomegaly, and hepatomegaly are often detected. Sometimes, cats are hyperesthetic during muscle palpation. Cats may become comatose shortly before death. The entire disease course is usually rapid, and many cats will succumb within days.33,53
Cytauxzoonosis
Etiology And Epidemiology
Pathogenesis
Clinical Findings
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine